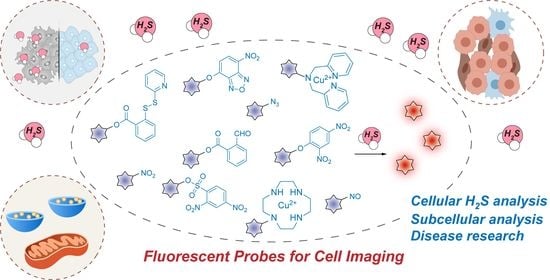
Recent Progress in Fluorescent Probes for the Detection and Research of Hydrogen Sulfide in Cells
Abstract
:1. Introduction
2. H2S Probe
2.1. Probes Based on H2S Reduction Reactions
2.1.1. Azide-Based H2S Probes
2.1.2. Nitro-/Nitroso-Based H2S Probe
2.2. Probes Based on H2S Nucleophilic Reactions
2.2.1. 2,4-Dinitrophenyl (DNP)-Based H2S Probe

2.2.2. 7-Nitro-1,2,3-benzoxadiazole (NBD)-Based H2S Probe
2.2.3. Disulfide Bond-Based H2S Probe
2.2.4. Other Nucleophilic-Based of H2S Probes
2.3. Probes Based on Metal and H2S Reactions
3. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Gadalla, M.M.; Snyder, S.H. Hydrogen sulfide as a gasotransmitter. J. Neurochem. 2010, 113, 14–26. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.W.; Moore, P.K. H2S Synthesizing Enzymes: Biochemistry and Molecular Aspects. In Chemistry, Biochemistry and Pharmacology of Hydrogen Sulfide; Moore, P.K., Whiteman, M., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 3–25. [Google Scholar]
- Singh, S.; Padovani, D.; Leslie, R.A.; Chiku, T.; Banerjee, R. Relative Contributions of Cystathionine β-Synthase and γ-Cystathionase to H2S Biogenesis via Alternative Trans-sulfuration Reactions. J. Biol. Chem. 2009, 284, 22457–22466. [Google Scholar] [CrossRef] [Green Version]
- Shibuya, N.; Tanaka, M.; Yoshida, M.; Ogasawara, Y.; Togawa, T.; Ishii, K.; Kimura, H. 3-Mercaptopyruvate Sulfurtransferase Produces Hydrogen Sulfide and Bound Sulfane Sulfur in the Brain. Antioxid. Redox Signal. 2008, 11, 703–714. [Google Scholar] [CrossRef]
- Madden, J.A.; Ahlf, S.B.; Dantuma, M.W.; Olson, K.R.; Roerig, D.L. Precursors and inhibitors of hydrogen sulfide synthesis affect acute hypoxic pulmonary vasoconstriction in the intact lung. J. Appl. Physiol. 2011, 112, 411–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, H.-J.; Wu, Z.-Y.; Nie, X.-W.; Wang, X.-Y.; Bian, J.-S. Implications of hydrogen sulfide in liver pathophysiology: Mechanistic insights and therapeutic potential. J. Adv. Res. 2021, 27, 127–135. [Google Scholar] [CrossRef]
- Li, L.; Bhatia, M.; Zhu, Y.Z.; Zhu, Y.C.; Ramnath, R.D.; Wang, Z.J.; Anuar, F.B.M.; Whiteman, M.; Salto-Tellez, M.; Moore, P.K. Hydrogen sulfide is a novel mediator of lipopolysaccharide-induced inflammation in the mouse. FASEB J. 2005, 19, 1196–1198. [Google Scholar] [CrossRef] [PubMed]
- Vincenzo, B.; Emma, M.; Roderick, J.F.; Giuseppe, C.; Mauro, P. Annexin A1 Mediates Hydrogen Sulfide Properties in the Control of Inflammation. J. Pharmacol. Exp. Ther. 2014, 351, 96. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Zhong, Y.; Su, Y.; Zhao, L.; Peng, J. Real-Time Imaging of Hepatic Inflammation Using Hydrogen Sulfide-Activatable Second Near-Infrared Luminescent Nanoprobes. Nano Lett. 2021, 21, 4606–4614. [Google Scholar] [CrossRef]
- Giuliani, D.; Ottani, A.; Zaffe, D.; Galantucci, M.; Strinati, F.; Lodi, R.; Guarini, S. Hydrogen sulfide slows down progression of experimental Alzheimer’s disease by targeting multiple pathophysiological mechanisms. Neurobiol. Learn. Mem. 2013, 104, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Kamoun, P.; Belardinelli, M.-C.; Chabli, A.; Lallouchi, K.; Chadefaux-Vekemans, B. Endogenous hydrogen sulfide overproduction in Down syndrome. Am. J. Med. Genet. Part A 2003, 116A, 310–311. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhu, J.; Pan, Y.; Dong, J.; Zhang, L.; Zhang, X.; Zhang, L. Hydrogen sulfide functions as a neuromodulator to regulate striatal neurotransmission in a mouse model of Parkinson’s disease. J. Neurosci. Res. 2015, 93, 487–494. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, G.; Wondimu, T.; Ross, B.; Wang, R. Hydrogen sulfide and asthma. Exp. Physiol. 2011, 96, 847–852. [Google Scholar] [CrossRef]
- Magierowski, M.; Jasnos, K.; Kwiecien, S.; Drozdowicz, D.; Surmiak, M.; Strzalka, M.; Ptak-Belowska, A.; Wallace, J.L.; Brzozowski, T. Endogenous Prostaglandins and Afferent Sensory Nerves in Gastroprotective Effect of Hydrogen Sulfide against Stress-Induced Gastric Lesions. PLoS ONE 2015, 10, e0118972. [Google Scholar] [CrossRef] [Green Version]
- Jha, S.; Calvert, J.W.; Duranski, M.R.; Ramachandran, A.; Lefer, D.J. Hydrogen sulfide attenuates hepatic ischemia-reperfusion injury: Role of antioxidant and antiapoptotic signaling. Am. J. Physiol.-Heart Circ. Physiol. 2008, 295, H801–H806. [Google Scholar] [CrossRef] [Green Version]
- Satori, C.P.; Henderson, M.M.; Krautkramer, E.A.; Kostal, V.; Distefano, M.M.; Arriaga, E.A. Bioanalysis of Eukaryotic Organelles. Chem. Rev. 2013, 113, 2733–2811. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Zeng, Z.; Jiang, J.-H.; Chang, Y.-T.; Yuan, L. Discerning the Chemistry in Individual Organelles with Small-Molecule Fluorescent Probes. Angew. Chem. Int. Ed. 2016, 55, 13658–13699. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Zhang, W.; Wu, L.; Yang, G.; Li, H.; Wang, R. Hydrogen sulfide (H2S) metabolism in mitochondria and its regulatory role in energy production. Proc. Natl. Acad. Sci. USA 2012, 109, 2943–2948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheung, N.S.; Peng, Z.F.; Chen, M.J.; Moore, P.K.; Whiteman, M. Hydrogen sulfide induced neuronal death occurs via glutamate receptor and is associated with calpain activation and lysosomal rupture in mouse primary cortical neurons. Neuropharmacology 2007, 53, 505–514. [Google Scholar] [CrossRef]
- Wei, L.; Kan, L.-Y.; Zeng, H.-Y.; Tang, Y.-Y.; Huang, H.-L.; Xie, M.; Zou, W.; Wang, C.-Y.; Zhang, P.; Tang, X.-Q. BDNF/TrkB Pathway Mediates the Antidepressant-Like Role of H2S in CUMS-Exposed Rats by Inhibition of Hippocampal ER Stress. Neuro Mol. Med. 2018, 20, 252–261. [Google Scholar] [CrossRef]
- Abe, K.; Kimura, H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J. Neurosci. 1996, 16, 1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filipovic, M.R.; Zivanovic, J.; Alvarez, B.; Banerjee, R. Chemical Biology of H2S Signaling through Persulfidation. Chem. Rev. 2018, 118, 1253–1337. [Google Scholar] [CrossRef]
- Vitvitsky, V.; Banerjee, R. Chapter Seven—H2S Analysis in Biological Samples Using Gas Chromatography with Sulfur Chemiluminescence Detection. In Methods in Enzymology; Cadenas, E., Packer, L., Eds.; Academic Press: Cambridge, MA, USA, 2015; Volume 554, pp. 111–123. [Google Scholar]
- Radford-Knoery, J.; Cutter, G.A. Determination of carbonyl sulfide and hydrogen sulfide species in natural waters using specialized collection procedures and gas chromatography with flame photometric detection. Anal. Chem. 1993, 65, 976–982. [Google Scholar] [CrossRef]
- Bae, J.; Choi, M.G.; Choi, J.; Chang, S.-K. Colorimetric signaling of hydrogen sulfide by reduction of a phenylseleno-nitrobenzoxadiazole derivative. Dye. Pigment. 2013, 99, 748–752. [Google Scholar] [CrossRef]
- Kahyarian, A.; Nesic, S. H2S corrosion of mild steel: A quantitative analysis of the mechanism of the cathodic reaction. Electrochim. Acta 2019, 297, 676–684. [Google Scholar] [CrossRef]
- Li, H.; Fang, Y.; Yan, J.; Ren, X.; Zheng, C.; Wu, B.; Wang, S.; Li, Z.; Hua, H.; Wang, P.; et al. Small-molecule fluorescent probes for H2S detection: Advances and perspectives. TrAC Trends Anal. Chem. 2021, 134, 116117. [Google Scholar] [CrossRef]
- Ibrahim, H.; Serag, A.; Farag, M.A. Emerging analytical tools for the detection of the third gasotransmitter H2S, a comprehensive review. J. Adv. Res. 2021, 27, 137–153. [Google Scholar] [CrossRef] [PubMed]
- Bezner, B.J.; Ryan, L.S.; Lippert, A.R. Reaction-Based Luminescent Probes for Reactive Sulfur, Oxygen, and Nitrogen Species: Analytical Techniques and Recent Progress. Anal. Chem. 2020, 92, 309–326. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Fan, J.; Du, J.; Peng, X. Small-molecule fluorescent probes for imaging gaseous signaling molecules: Current progress and future implications. Chem. Sci. 2020, 11, 5127–5141. [Google Scholar] [CrossRef] [Green Version]
- Jiao, X.; Li, Y.; Niu, J.; Xie, X.; Wang, X.; Tang, B. Small-Molecule Fluorescent Probes for Imaging and Detection of Reactive Oxygen, Nitrogen, and Sulfur Species in Biological Systems. Anal. Chem. 2018, 90, 533–555. [Google Scholar] [CrossRef]
- Guo, L.-Y.; Zhao, J.-S.; Peng, H.-S. Fluorescent Probes for Sensing and Imaging Biological Hydrogen Sulfide. Anal. Sens. 2022, 2, e202200025. [Google Scholar] [CrossRef]
- Li, J.; Su, Z.; Yu, C.; Yuan, Y.; Wu, Q.; Liu, J.; Peng, B.; Hu, W.; Lu, X.; Yu, H.; et al. Recent progress in the development of sensing systems for in vivo detection of biological hydrogen sulfide. Dye. Pigment. 2021, 192, 109451. [Google Scholar] [CrossRef]
- Gilbert, A.K.; Pluth, M.D. Subcellular Delivery of Hydrogen Sulfide Using Small Molecule Donors Impacts Organelle Stress. J. Am. Chem. Soc. 2022, 144, 17651–17660. [Google Scholar] [CrossRef] [PubMed]
- Montoya, L.A.; Pluth, M.D. Organelle-Targeted H2S Probes Enable Visualization of the Subcellular Distribution of H2S Donors. Anal. Chem. 2016, 88, 5769–5774. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Chen, Y.; Shao, B.; Cheng, J.; Li, X. Recent advances of small-molecule fluorescent probes for detecting biological hydrogen sulfide. Front. Chem. Sci. Eng. 2022, 16, 34–63. [Google Scholar] [CrossRef]
- Luo, Y.; Zuo, Y.; Shi, G.; Xiang, H.; Gu, H. Progress on the reaction-based methods for detection of endogenous hydrogen sulfide. Anal. Bioanal. Chem. 2022, 414, 2809–2839. [Google Scholar] [CrossRef]
- Cuevasanta, E.; Lange, M.; Bonanata, J.; Coitiño, E.L.; Ferrer-Sueta, G.; Filipovic, M.R.; Alvarez, B. Reaction of Hydrogen Sulfide with Disulfide and Sulfenic Acid to Form the Strongly Nucleophilic Persulfide*♦. J. Biol. Chem. 2015, 290, 26866–26880. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, P.; Jos, S.; Chakrapani, H. Reactive Oxygen Species-Triggered Tunable Hydrogen Sulfide Release. Org. Lett. 2018, 20, 3766–3770. [Google Scholar] [CrossRef]
- Fu, Y.-J.; Yao, H.-W.; Zhu, X.-Y.; Guo, X.-F.; Wang, H. A cell surface specific two-photon fluorescent probe for monitoring intercellular transmission of hydrogen sulfide. Anal. Chim. Acta 2017, 994, 1–9. [Google Scholar] [CrossRef]
- Deng, B.; Ren, M.; Wang, J.-Y.; Zhou, K.; Lin, W. A mitochondrial-targeted two-photon fluorescent probe for imaging hydrogen sulfide in the living cells and mouse liver tissues. Sens. Actuators B Chem. 2017, 248, 50–56. [Google Scholar] [CrossRef]
- Shen, D.; Liu, J.; Sheng, L.; Lv, Y.; Wu, G.; Wang, P.; Du, K. Design, synthesis and evaluation of a novel fluorescent probe to accurately detect H2S in hepatocytes and natural waters. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 228, 117690. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhao, M.; Jiang, X.; Sizovs, A.; Wang, M.C.; Provost, C.R.; Huang, J.; Wang, J. Genetically anchored fluorescent probes for subcellular specific imaging of hydrogen sulfide. Analyst 2016, 141, 1209–1213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, V.S.; Lippert, A.R.; Chang, C.J. Cell-trappable fluorescent probes for endogenous hydrogen sulfide signaling and imaging H2O2-dependent H2S production. Proc. Natl. Acad. Sci. USA 2013, 110, 7131–7135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bae, S.K.; Heo, C.H.; Choi, D.J.; Sen, D.; Joe, E.-H.; Cho, B.R.; Kim, H.M. A Ratiometric Two-Photon Fluorescent Probe Reveals Reduction in Mitochondrial H2S Production in Parkinson’s Disease Gene Knockout Astrocytes. J. Am. Chem. Soc. 2013, 135, 9915–9923. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Mao, Z.; Liu, L.; Liu, Z. A ratiometric two-photon fluorescent probe for imaging hydrogen sulfide in lysosomes. Talanta 2017, 167, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Song, B.; Yuan, J. A Golgi Apparatus-Targetable Probe Based on Lanthanide Complexes for Ratiometric Time-Gated Luminescence Detection of Hydrogen Sulfide. Eur. J. Inorg. Chem. 2023, 26, e202200642. [Google Scholar] [CrossRef]
- Li, S.-J.; Li, Y.-F.; Liu, H.-W.; Zhou, D.-Y.; Jiang, W.-L.; Ou-Yang, J.; Li, C.-Y. A Dual-Response Fluorescent Probe for the Detection of Viscosity and H2S and Its Application in Studying Their Cross-Talk Influence in Mitochondria. Anal. Chem. 2018, 90, 9418–9425. [Google Scholar] [CrossRef]
- Wu, Q.; Yin, C.; Wen, Y.; Zhang, Y.; Huo, F. An ICT lighten ratiometric and NIR fluorogenic probe to visualize endogenous/exogenous hydrogen sulphide and imaging in mice. Sens. Actuators B Chem. 2019, 288, 507–511. [Google Scholar] [CrossRef]
- Zhang, X.; Tan, H.; Yan, Y.; Hang, Y.; Yu, F.; Qu, X.; Hua, J. Targetable N-annulated perylene-based colorimetric and ratiometric near-infrared fluorescent probes for the selective detection of hydrogen sulfide in mitochondria, lysosomes, and serum. J. Mater. Chem. B 2017, 5, 2172–2180. [Google Scholar] [CrossRef]
- Chen, H.; Gong, X.; Liu, X.; Li, Z.; Zhang, J.; Yang, X.-F. A nitroso-based fluorogenic probe for rapid detection of hydrogen sulfide in living cells. Sens. Actuators B Chem. 2019, 281, 542–548. [Google Scholar] [CrossRef]
- Yuan, L.; Zuo, Q.-P. FRET-Based Mitochondria-Targetable Dual-Excitation Ratiometric Fluorescent Probe for Monitoring Hydrogen Sulfide in Living Cells. Chem.–Asian J. 2014, 9, 1544–1549. [Google Scholar] [CrossRef]
- Zhao, X.-j.; Li, Y.-t.; Jiang, Y.-r.; Yang, B.-q.; Liu, C.; Liu, Z.-h. A novel “turn-on” mitochondria-targeting near-infrared fluorescent probe for H2S detection and in living cells imaging. Talanta 2019, 197, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hu, L.; Xu, B.; Chen, H.; Cai, F.; Yang, N.; Wu, Q.; Uvdal, K.; Hu, Z.; Li, L. Endoplasmic reticulum-targeted fluorogenic probe based on pyrimidine derivative for visualizing exogenous/endogenous H2S in living cells. Dye. Pigment. 2020, 179, 108390. [Google Scholar] [CrossRef]
- Lv, J.; Wang, F.; Qiang, J.; Ren, X.; Chen, Y.; Zhang, Z.; Wang, Y.; Zhang, W.; Chen, X. Enhanced response speed and selectivity of fluorescein-based H2S probe via the cleavage of nitrobenzene sulfonyl ester assisted by ortho aldehyde groups. Biosens. Bioelectron. 2017, 87, 96–100. [Google Scholar] [CrossRef]
- Yang, B.; Su, M.-M.; Xue, Y.-S.; He, Z.-X.; Xu, C.; Zhu, H.-L. A selective fluorescence probe for H2S from biothiols with a significant regioselective turn-on response and its application for H2S detection in living cells and in living Caenorhabditis elegans. Sens. Actuators B Chem. 2018, 276, 456–465. [Google Scholar] [CrossRef]
- Li, B.; Mei, H.; Wang, M.; Gu, X.; Hao, J.; Xie, X.; Xu, K. A near-infrared fluorescent probe for imaging of endogenous hydrogen sulfide in living cells and mice. Dye. Pigment. 2021, 189, 109231. [Google Scholar] [CrossRef]
- Lu, G.; Gao, Y.; Wang, X.; Zhang, D.; Meng, S.; Yu, S.; Zhuang, Y.; Duan, L. A near-infrared fluorescent probe based on corrole derivative with large Stokes shift for detection of hydrogen sulfide in water and living cells. Dye. Pigment. 2022, 204, 110445. [Google Scholar] [CrossRef]
- Jia, X.; Li, W.; Guo, Z.; Guo, Z.; Li, Y.; Zhang, P.; Wei, C.; Li, X. An NBD-Based Mitochondrial Targeting Ratiometric Fluorescent Probe for Hydrogen Sulfide Detection. ChemistrySelect 2019, 4, 8671–8675. [Google Scholar] [CrossRef]
- Pak, Y.L.; Li, J.; Ko, K.C.; Kim, G.; Lee, J.Y.; Yoon, J. Mitochondria-Targeted Reaction-Based Fluorescent Probe for Hydrogen Sulfide. Anal. Chem. 2016, 88, 5476–5481. [Google Scholar] [CrossRef] [PubMed]
- Ismail, I.; Chen, Z.; Ji, X.; Sun, L.; Yi, L.; Xi, Z. A Fast-Response Red Shifted Fluorescent Probe for Detection of H2S in Living Cells. Molecules 2020, 25, 437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ou-Yang, J.; Jiang, W.-L.; Tan, K.-Y.; Liu, H.-W.; Li, S.-J.; Liu, J.; Li, Y.-F.; Li, C.-Y. Two-photon fluorescence probe for precisely detecting endogenous H2S in lysosome by employing a dual lock system. Sens. Actuators B Chem. 2018, 260, 264–273. [Google Scholar] [CrossRef]
- Ding, S.; Feng, W.; Feng, G. Rapid and highly selective detection of H2S by nitrobenzofurazan (NBD) ether-based fluorescent probes with an aldehyde group. Sens. Actuators B Chem. 2017, 238, 619–625. [Google Scholar] [CrossRef]
- Li, G.; Ma, S.; Tang, J.; Ye, Y. Lysosome-targeted two-photon fluorescent probes for rapid detection of H2S in live cells. New J. Chem. 2019, 43, 1267–1274. [Google Scholar] [CrossRef]
- Cao, T.; Teng, Z.; Gong, D.; Qian, J.; Liu, W.; Iqbal, K.; Qin, W.; Guo, H. A ratiometric fluorescent probe for detection of endogenous and exogenous hydrogen sulfide in living cells. Talanta 2019, 198, 185–192. [Google Scholar] [CrossRef]
- Chen, W.; Xu, S.; Day, J.J.; Wang, D.; Xian, M. A General Strategy for Development of Near-Infrared Fluorescent Probes for Bioimaging. Angew. Chem. Int. Ed. 2017, 56, 16611–16615. [Google Scholar] [CrossRef]
- Kang, J.; Huo, F.; Yin, C. A novel ratiometric fluorescent H2S probe based on tandem nucleophilic substitution/cyclization reaction and its bioimaging. Dye. Pigment. 2017, 146, 287–292. [Google Scholar] [CrossRef]
- Wu, Q.; Huo, F.; Wang, J.; Yin, C. Fluorescent probe for detecting hydrogen sulfide based on disulfide nucleophilic substitution-addition. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 238, 118437. [Google Scholar] [CrossRef]
- Wu, X.; Shi, J.; Yang, L.; Han, J.; Han, S. A near-infrared fluorescence dye for sensitive detection of hydrogen sulfide in serum. Bioorganic Med. Chem. Lett. 2014, 24, 314–316. [Google Scholar] [CrossRef]
- Wang, X.; Sun, J.; Zhang, W.; Ma, X.; Lv, J.; Tang, B. A near-infrared ratiometric fluorescent probe for rapid and highly sensitive imaging of endogenous hydrogen sulfide in living cells. Chem. Sci. 2013, 4, 2551–2556. [Google Scholar] [CrossRef]
- Shu, W.; Zang, S.; Wang, C.; Gao, M.; Jing, J.; Zhang, X. An Endoplasmic Reticulum-Targeted Ratiometric Fluorescent Probe for the Sensing of Hydrogen Sulfide in Living Cells and Zebrafish. Anal. Chem. 2020, 92, 9982–9988. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Ren, N.; Liu, X.; Dong, Y.; Wang, R.; Gao, J.; Sun, J.; Zhu, Y.; Wang, L.; Fan, C.; et al. Probing the Intracellular Dynamics of Nitric Oxide and Hydrogen Sulfide Using an Activatable NIR II Fluorescence Reporter. Angew. Chem. Int. Ed. 2021, 60, 8450–8454. [Google Scholar] [CrossRef]
- Sasakura, K.; Hanaoka, K.; Shibuya, N.; Mikami, Y.; Kimura, Y.; Komatsu, T.; Ueno, T.; Terai, T.; Kimura, H.; Nagano, T. Development of a Highly Selective Fluorescence Probe for Hydrogen Sulfide. J. Am. Chem. Soc. 2011, 133, 18003–18005. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Guo, X.; Hu, R.; Liu, X.; Wang, S.; Li, S.; Li, Y.; Yang, G. Molecular Engineering of Aqueous Soluble Triarylboron-Compound-Based Two-Photon Fluorescent Probe for Mitochondria H2S with Analyte-Induced Finite Aggregation and Excellent Membrane Permeability. Anal. Chem. 2016, 88, 1052–1057. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Krishnakumar, S.; Yu, J.; Liang, D.; Qi, H.; Lee, Z.-W.; Deng, L.-W.; Huang, D. Highly Selective and Sensitive Near-Infrared-Fluorescent Probes for the Detection of Cellular Hydrogen Sulfide and the Imaging of H2S in Mice. Chem.–Asian J. 2014, 9, 3604–3611. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Song, B.; Ma, H.; Shi, Y.; Yuan, J. A ratiometric time-gated luminescence probe for hydrogen sulfide based on copper(II)-coupled lanthanide complexes. Anal. Chim. Acta 2019, 1049, 152–160. [Google Scholar] [CrossRef]
















Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, W.; Zhang, Y.; Xiong, S.; Su, D. Recent Progress in Fluorescent Probes for the Detection and Research of Hydrogen Sulfide in Cells. Chemosensors 2023, 11, 333. https://doi.org/10.3390/chemosensors11060333
Liang W, Zhang Y, Xiong S, Su D. Recent Progress in Fluorescent Probes for the Detection and Research of Hydrogen Sulfide in Cells. Chemosensors. 2023; 11(6):333. https://doi.org/10.3390/chemosensors11060333
Chicago/Turabian StyleLiang, Weier, Yong Zhang, Shaoqing Xiong, and Dongdong Su. 2023. "Recent Progress in Fluorescent Probes for the Detection and Research of Hydrogen Sulfide in Cells" Chemosensors 11, no. 6: 333. https://doi.org/10.3390/chemosensors11060333
APA StyleLiang, W., Zhang, Y., Xiong, S., & Su, D. (2023). Recent Progress in Fluorescent Probes for the Detection and Research of Hydrogen Sulfide in Cells. Chemosensors, 11(6), 333. https://doi.org/10.3390/chemosensors11060333