Simultaneous Determination of the Main Peanut Allergens in Foods Using Disposable Amperometric Magnetic Beads-Based Immunosensing Platforms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
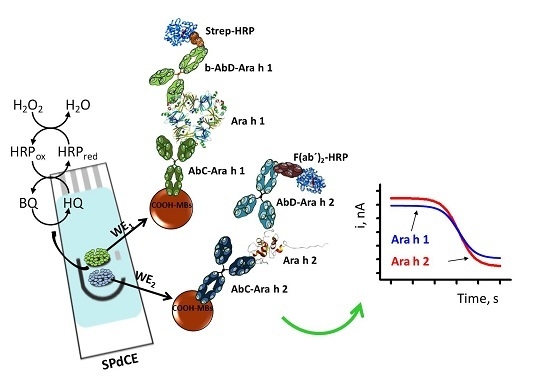
2.2. Modification of MBs
2.3. Amperometric Measurements
2.4. Analysis of Real Samples
3. Results
3.1. Analytical Characteristics
3.2. Selectivity of the Dual Magnetoimmunosensor
3.3. Simultaneous Determination of Ara h 1 and Ara h 2 in Food Samples
4. Discussion
Total Detection of Both Major Peanut Allergenic Proteins in Food Samples
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ELISAs | Enzyme-linked immunosorbent assays |
| HQ | Hydroquinone |
| LOD | Limit of detection |
| MBs | Magnetic beads |
| PCR | Polymerase chain reaction |
| SPdCEs | Screen-printed dual carbon electrodes |
References
- Walker, M.J.; Burns, D.T.; Elliott, C.T.; Gowland, M.H.; Clare Mills, E.N. Is food allergen analysis flawed? Health and supply chain risks and a proposed framework to address urgent analytical needs. Analyst 2016, 141, 24–35. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. Consumers-Food Allergies: What You Need to Know. 2015. Available online: http://www.fda.gov/Food/ResourcesForYou/Consumers/ucm079311.htm (accessed on 25 August 2015). [Google Scholar]
- Van Hengel, A.J. Food allergen detection methods and the challenge to protect food-allergic consumers. Anal. Bioanal. Chem. 2007, 389, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Montserrat, M.; Sanz, D.; Juan, T.; Herrero, A.; Sánchez, L.; Calvo, M.; Pérez, M.D. Detection of peanut (Arachis hypogaea) allergens in processed foods by immunoassay: Influence of selected target protein and ELISA format applied. Food Control 2015, 54, 300–307. [Google Scholar] [CrossRef]
- Ng, E.; Nadeau, K.; Wang, S.X. Giant magneto resistive sensor array for sensitive and specific multiplexed food allergen detection. Biosens. Bioelectron. 2016, 80, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Food Allergen Labelling and Information Requirements under the EU Food Information for Consumers, Regulation No. 1169/2011: Technical Guidance. Available online: http://www.food.gov.uk/sites/default/files/food-allergen-labelling-technical-guidance.pdf (accessed on 27 June 2016).
- Poms, R.E.; Agazzi, M.E.; Bau, A.; Brohee, M.; Capelletti, C.; Nørgaard, J.V.; Anklam, E. Inter-laboratory validation study of five commercial ELISA test kits for the determination of peanut proteins in biscuits and dark chocolate. Food Addit. Contam. 2005, 22, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Pedreschi, R.; Nørgaard, J.; Maquet, A. Current Challenges in Detecting Food Allergens by Shotgun and Targeted Proteomic Approaches: A Case Study on Traces of Peanut Allergens in Baked Cookies. Nutrients 2012, 4, 132–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Calleja, I.M.; de la Cruz, S.; Pegels, N.; González, I.; García, T.; Martín, R. Development of a real time PCR assay for detection of allergenic trace amounts of peanut (Arachis hypogaea) in processed foods. Food Control 2013, 30, 480–490. [Google Scholar] [CrossRef]
- Holzhauser, T.; Kleiner, K.; Janise, A.; Röder, M. Matrix-normalised quantification of species by threshold-calibrated competitive real-time PCR: Allergenic peanut in food as one example. Food Chem. 2014, 163, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.Y.; Nowatzke, W.; Oliver, K.; Garber, E.A.E. Multiplex detection of food allergens and gluten. Anal. Bioanal. Chem. 2015, 407, 4195–4206. [Google Scholar] [CrossRef] [PubMed]
- Alves, R.C.; Pimentel, F.B.; Nouws, H.P.A.; Marques, R.C.B.; González-García, M.B.; Oliveira, M.B.P.P.; Delerue-Matos, C. Detection of Ara h 1 (a major peanut allergen) in food using an electrochemical gold nanoparticle-coated screen-printed immunosensor. Biosens. Bioelectron. 2015, 64, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Alves, R.C.; Pimentel, F.B.; Nouws, H.P.A.; Correr, W.; González-García, M.B.; Oliveira, M.B.P.P.; Delerue-Matos, C. Detection of the peanut allergen Ara h 6 in foodstuffs using a voltammetric biosensing approach. Anal. Bioanal. Chem. 2015, 407, 7157–7163. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Valdepeñas Montiel, V.; Campuzano, S.; Pellicanò, A.; Torrente-Rodríguez, R.M.; Reviejo, A.J.; Cosio, M.S.; Pingarrón, J.M. Sensitive and selective magnetoimmunosensing platform for determination of the food allergen Ara h 1. Anal. Chim. Acta 2015, 880, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Valdepeñas Montiel, V.; Campuzano, S.; Pellicanò, A.; Torrente-Rodríguez, R.M.; Reviejo, A.J.; Cosio, M.S.; Pingarrón, J.M. Electrochemical detection of peanuts at trace levels in foods using a magnetoimmunosensor for the allergenic protein Ara h 2. Sens. Actuator B Chem. 2016. [Google Scholar] [CrossRef]
- Koppelman, S.J.; Vlooswijk, R.A.; Knippels, L.M.; Hessing, M.; Knol, E.F.; Van Reijsen, F.C.; Bruijnzeel-Koomen, C.A. Quantification of major peanut allergens Ara h 1 and Ara h 2 in the peanut varieties Runner, Spanish, Virginia, and Valencia, bred in different parts of the world. Allergy 2001, 56, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Pele, M.; Brohée, M.; Anklam, E.; Van Hengel, A. Presence of peanut and hazelnut in cookies and chocolates: The relationship between analytical results and the declaration of food allergens on product labels. Food Addit. Contam. 2007, 24, 1334–1344. [Google Scholar] [CrossRef] [PubMed]
- Jollet, P.; Delport, F.; Janssen, K.P.F.; Tran, D.T.; Wouters, J.; Verbiest, T.; Lammertyn, J. Fast and accurate peanut allergen detection with nanobead enhanced optical fiber SPR biosensor. Talanta 2011, 83, 1436–1441. [Google Scholar]
- Gamella, M.; Campuzano, S.; Conzuelo, F.; Reviejo, A.J.; Pingarrón, J.M. Amperometric magnetoimmunosensor for direct determination of D-dimer in human serum. Electroanal. 2012, 24, 2235–2243. [Google Scholar] [CrossRef]
- Escamilla-Gómez, V.; Hernández-Santos, D.; González-García, M.B.; Pingarrón-Carrazón, J.M.; Costa-García, A. Simultaneous detection of free and total prostate specific antigen on a screen-printed electrochemical dual sensor. Biosens. Bioelectron. 2009, 24, 2678–2683. [Google Scholar] [CrossRef] [PubMed]
- Bublin, M.; Breiteneder, H. Cross-reactivity of peanut allergens. Curr. Allergy Asthma Rep. 2014, 14. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Akiyama, H.; Maleki, S.; Yamakawa, H.; Iijima, K.; Yamazaki, F.; Matsumoto, T.; Futo, S.; Arakawa, F.; Watai, M.; et al. A specific qualitative detection method for peanut (Arachis hypogaea) in foods using polymerase chain reaction. J. Food Biochem. 2006, 30, 215–233. [Google Scholar] [CrossRef]
- Scaravelli, E.; Brohee, M.; Marchelli, R.; Van Hengel, A. Development of three real-time PCR assays to detect peanut allergen residue in processed food products. Eur. Food Res. Technol. 2008, 227, 857–869. [Google Scholar] [CrossRef]
- Köppel, R.; Dvorak, V.; Zimmerli, F.; Breitenmoser, A.; Eugster, A.; Waiblinger, H.U. Two tetraplex real-time PCR for the detection and quantification of DNA from eight allergens in food. Eur. Food Res. Technol. 2010, 230, 367–374. [Google Scholar] [CrossRef]
- Koppelman, S.J.; Wensing, M.; Ertmann, M.; Knulst, A.C.; Knol, E.F. Relevance of Ara h 1, Ara h 2 and Ara h 3 in peanut-allergic patients, as determined by immunoglobulin E Western blotting, basophil-histamine release and intracutaneous testing: Ara h 2 is the most important peanut allergen. Clin. Exp. Allergy 2004, 34, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, D.C. Peanut allergy diagnosis: As simple as Ara h 1, 2, and 3. Consultant for pediatricians. Consult. Pediatr. 2013, 12, 347–350. [Google Scholar]
- Maleki, S.J.; Viquez, O.; Jacks, T.; Dodo, H.; Champagne, E.T.; Chung, S.-Y.; Landry, S.J. The major peanut allergen, Ara h 2, functions as a trypsin inhibitor, and roasting enhances this function. J. Allergy Clin. Immunol. 2003, 112, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, J.-S.; Yang, X.-J.; Lin, D.-H.; Gao, Y.-F.; Su, Y.-J.; Yang, S.; Zhang, Y.-J.; Zheng, J.-J. Peanut allergy, allergen composition, and methods of reducing allergenicity: A review. Int. J. Food Sci. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]







| Ara h 1 | Ara h 2 | |
|---|---|---|
| Linear range (LR), ng/mL | 60–1000 | 0.25–5 |
| r | 0.996 | 0.999 |
| Sensitivity, nAmL/ng | 0.79 ± 0.05 | 115 ± 2 |
| LOD, ng/mL * | 18 | 0.07 |
| Limit of determination (LQ), ng/mL ** | 60 | 0.25 |
| [Ara h 1], mg/g | [Ara h 2], mg/g | |||||
|---|---|---|---|---|---|---|
| Extract | Dilution Factor | Dual Platform | ELISA | Dilution Factor | Dual Platform | ELISA |
| Fried peanuts | 1/1000 | (7 ± 2) | (7.3 ± 0.6) | 1/250,000 | (3.6 ± 0.8) | (3.4 ± 0.6) |
| Raw peanuts | 1/1000 | (2.3 ± 0.4) | (2.8 ± 0.3) | 1/250,000 | (3.8 ± 0.3) | (4.1 ± 0.5) |
| Chocolate bars with roasted peanuts | 1/50 | (0.18 ± 0.01) | (0.18 ± 0.03) | 1/25,000 | (0.23 ± 0.05) | (0.30 ± 0.08) |
| Peanut cream | 1/50 | (1.9 ± 0.2) | (1.8 ± 0.4) | 1/500,000 | (4.5 ± 0.8) | (4.3 ± 0.8) |
| Raw hazelnuts | — | ND | ND | — | ND | ND |
| Wheat flour | — | ND | ND | — | ND | ND |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Valdepeñas Montiel, V.; Torrente-Rodríguez, R.M.; Campuzano, S.; Pellicanò, A.; Reviejo, Á.J.; Cosio, M.S.; Pingarrón, J.M. Simultaneous Determination of the Main Peanut Allergens in Foods Using Disposable Amperometric Magnetic Beads-Based Immunosensing Platforms. Chemosensors 2016, 4, 11. https://doi.org/10.3390/chemosensors4030011
Ruiz-Valdepeñas Montiel V, Torrente-Rodríguez RM, Campuzano S, Pellicanò A, Reviejo ÁJ, Cosio MS, Pingarrón JM. Simultaneous Determination of the Main Peanut Allergens in Foods Using Disposable Amperometric Magnetic Beads-Based Immunosensing Platforms. Chemosensors. 2016; 4(3):11. https://doi.org/10.3390/chemosensors4030011
Chicago/Turabian StyleRuiz-Valdepeñas Montiel, Víctor, Rebeca Magnolia Torrente-Rodríguez, Susana Campuzano, Alessandro Pellicanò, Ángel Julio Reviejo, Maria Stella Cosio, and José Manuel Pingarrón. 2016. "Simultaneous Determination of the Main Peanut Allergens in Foods Using Disposable Amperometric Magnetic Beads-Based Immunosensing Platforms" Chemosensors 4, no. 3: 11. https://doi.org/10.3390/chemosensors4030011
APA StyleRuiz-Valdepeñas Montiel, V., Torrente-Rodríguez, R. M., Campuzano, S., Pellicanò, A., Reviejo, Á. J., Cosio, M. S., & Pingarrón, J. M. (2016). Simultaneous Determination of the Main Peanut Allergens in Foods Using Disposable Amperometric Magnetic Beads-Based Immunosensing Platforms. Chemosensors, 4(3), 11. https://doi.org/10.3390/chemosensors4030011