Guanine Quadruplex Electrochemical Aptasensors
Abstract
:1. Introduction
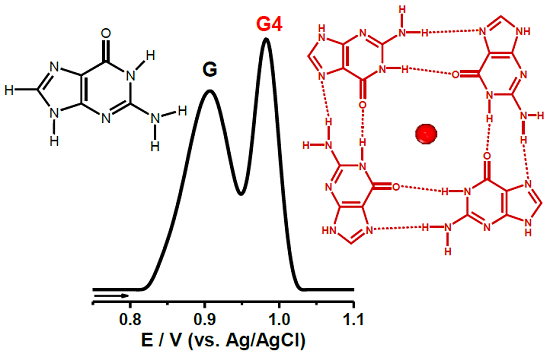
2. G4 Electrochemistry
3. G4 Electrochemical Biosensors
3.1. G4 Electrochemical Aptasensors
3.1.1. Sandwich-Type G4 Electrochemical Aptasensor
3.1.2. Structure-Switching G4 Electrochemical Aptasensor
3.2. Hemin/G4 DNAzyme Electrochemical Biosensor
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Mergny, J.-L.; De Cian, A.; Ghelab, A.; Saccà, B.; Lacroix, L. Kinetics of Tetramolecular Quadruplexes. Nucleic Acids Res. 2005, 33, 81–94. [Google Scholar]
- Neidle, S. Therapeutic Applications of Quadruplex Nucleic Acids; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Simonsson, T. G-quadruplex DNA structures-variations on a theme. Biol. Chem. 2001, 382, 621–628. [Google Scholar]
- Tran, P.L.T.; De Cian, A.; Gros, J.; Moriyama, R.; Mergny, J.-L. Tetramolecular Quadruplex stability and assembly. Top. Curr. Chem. 2013, 330, 243–273. [Google Scholar]
- Zhang, S.; Wu, Y.; Zhang, W. G-quadruplex structures and their interaction diversity with ligands. ChemMedChem 2014, 9, 899–911. [Google Scholar]
- Keniry, M.A. Quadruplex Structures in nucleic acids. Biopolymers 2000–2001, 56, 123–146. [Google Scholar]
- Chambers, V.S.; Marsico, G.; Boutell, J.M.; Di Antonio, M.; Smith, G.P.; Balasubramanian, S. High-Throughput sequencing of DNA G-quadruplex structures in the human genome. Nat. Biotechnol. 2015, 33, 877–881. [Google Scholar]
- Murat, P.; Balasubramanian, S. Existence and consequences of G-quadruplex structures in DNA. Curr. Opin. Genet. Dev. 2014, 25, 22–29. [Google Scholar]
- Henderson, A.; Wu, Y.; Huang, Y.C.; Chavez, E.A.; Platt, J.; Johnson, F.B.; Brosh, R.M.; Sen, D.; Lansdorp, P.M. Detection of G-quadruplex DNA in mammalian cells. Nucleic Acids Res. 2014, 42, 860–869. [Google Scholar] [CrossRef] [PubMed]
- Dailey, M.M.; Hait, C.; Holt, P.A.; Maguire, J.M.; Meier, J.B.; Miller, M.C.; Petraccone, L.; Trent, J.O. Structure-Based drug design: From Nucleic acid to membrane protein targets. Exp. Mol. Pathol. 2009, 86, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Todd, A.K.; Johnston, M.; Neidle, S. Highly prevalent putative quadruplex sequence motifs in human DNA. Nucleic Acids Res. 2005, 33, 2901–2907. [Google Scholar] [CrossRef] [PubMed]
- Huppert, J.L. Hunting G-quadruplexes. Biochimie 2008, 90, 1140–1148. [Google Scholar] [CrossRef] [PubMed]
- Huppert, J.L.; Balasubramanian, S. Prevalence of quadruplexes in the human genome. Nucleic Acids Res. 2005, 33, 2908–2916. [Google Scholar] [CrossRef] [PubMed]
- Chiorcea-Paquim, A.-M.; Oliveira-Brett, A.M. Redox behaviour of G-quadruplexes. Electrochim. Acta 2014, 126, 162–170. [Google Scholar] [CrossRef]
- Artandi, S.E.; DePinho, R.A. Telomeres and Telomerase in Cancer; Hiyama, K., Ed.; Humana Press: Totowa, NJ, USA, 2009. [Google Scholar]
- Neidle, S. The structures of quadruplex nucleic acids and their drug complexes. Curr. Opin. Struct. Biol. 2009, 19, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Borovok, N.; Iram, N.; Zikich, D.; Ghabboun, J.; Livshits, G.I.; Porath, D.; Kotlyar, A.B. Assembling of G-strands into novel tetra-molecular parallel g4-dna nanostructures using avidin-biotin recognition. Nucleic Acids Res. 2008, 36, 5050–5060. [Google Scholar] [CrossRef] [PubMed]
- Borovok, N.; Molotsky, T.; Ghabboun, J.; Porath, D.; Kotlyar, A. Efficient procedure of preparation and properties of long uniform G4-dna nanowires. Anal. Biochem. 2008, 374, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Gray, R.D.; Trent, J.O.; Chaires, J.B. Folding and Unfolding pathways of the human telomeric G-quadruplex. J. Mol. Biol. 2014, 426, 1629–1650. [Google Scholar] [CrossRef] [PubMed]
- Karsisiotis, A.I.; Webba da Silva, M. Structural probes in quadruplex nucleic acid structure determination by NMR. Molecules 2012, 17, 13073–13086. [Google Scholar] [CrossRef] [PubMed]
- Adrian, M.; Heddi, B.; Phan, A.T. NMR spectroscopy of G-quadruplexes. Methods 2012, 57, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Hurley, L.H. Biochemical Techniques for the characterization of G-quadruplex structures: EMSA, DMS footprinting, and DNA polymerase stop assay. Methods Mol. Biol. 2010, 608, 65–79. [Google Scholar] [PubMed]
- Jaumot, J.; Gargallo, R. Experimental methods for studying the interactions between G-quadruplex structures and ligands. Curr. Pharm. Des. 2012, 18, 1900–1916. [Google Scholar] [CrossRef] [PubMed]
- Oliveira Brett, A.M.; Serrano, S.H.P.; Piedade, A.J.P. Applications of Kinetic Modelling; Comprehensive Chemical Kinetics; Elsevier: Amsterdam, The Netherlands, 1999; Volume 37. [Google Scholar]
- Oliveira Brett, A.M. Biosensors and Modern Biospecific Analytical Techniques; Comprehensive Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2005; Volume 44. [Google Scholar]
- Brett, A.M.O. Electrochemistry for probing DNA damage. In Encyclopedia of Sensors; Grimes, C.A., Dickey, E.C., Pishko, M.V., Eds.; American Scientific Publishers: Valencia, CA, USA, 2006; p. 301. [Google Scholar]
- Oliveira-Brett, A.M.; Paquim, A.M.C.; Diculescu, V.C.; Piedade, J.A.P. Electrochemistry of nanoscale DNA surface films on carbon. Med. Eng. Phys. 2006, 28, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Oliveira Brett, A.M.; Diculescu, V.C.; Chiorcea-Paquim, A.M.; Serrano, S.H.P. Electrochemical Sensor Analysis; Comprehensive Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2007; Volume 49. [Google Scholar]
- Oliveira Brett, A.M. Electrochemical DNA assays. In Bioelectrochemistry: Fundamentals, Experimental Techniques and Applications; Bartlett, P.N., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2008; p. 411. [Google Scholar]
- Oliveira-Brett, A.-M. Nanobioelectrochemistry. In Electrochemistry at the Nanoscale, Nanostrutures Science and Technology; Schmuki, P., Virtanen, S., Eds.; Nanostructure Science and Technology; Springer New York: New York, NY, USA, 2009; p. 407. [Google Scholar]
- Diculescu, V.C.; Chiorcea-Paquim, A.-M.; Oliveira-Brett, A.M. Applications of a DNA-electrochemical biosensor. TrAC Trends Anal. Chem. 2016, 79, 23–36. [Google Scholar] [CrossRef]
- Chiorcea–Paquim, A.-M.; Santos, P.; Diculescu, V.C.; Eritja, R.; Oliveira-Brett, A.M. Guanine Quartets. In Guanine Quartets: Structure and Application; Spindler, L., Fritzsche, W., Eds.; Royal Society of Chemistry: Cambridge, UK, 2012; pp. 100–109. [Google Scholar]
- Hianik, T.; Wang, J. Electrochemical aptasensors—Recent achievements and perspectives. Electroanalysis 2009, 21, 1223–1235. [Google Scholar] [CrossRef]
- Musumeci, D.; Montesarchio, D. Polyvalent nucleic acid aptamers and modulation of their activity: A focus on the thrombin binding aptamer. Pharmacol. Ther. 2012, 136, 202–215. [Google Scholar] [CrossRef] [PubMed]
- Tucker, W.O.; Shum, K.T.; Tanner, J.A. G-quadruplex DNA aptamers and their ligands: Structure, function and application. Curr. Pharm. Des. 2012, 18, 2014–2026. [Google Scholar] [CrossRef] [PubMed]
- Ni, X.; Castanares, M.; Mukherjee, A.; Lupold, S.E. Nucleic Acid Aptamers: Clinical Applications and Promising New Horizons. Curr. Med. Chem. 2011, 18, 4206–4214. [Google Scholar] [CrossRef] [PubMed]
- Diculescu, V.C.; Chiorcea-Paquim, A.-M.; Eritja, R.; Oliveira-Brett, A.M. Thrombin-binding Aptamer quadruplex formation: Afm and voltammetric characterization. J. Nucleic Acids 2010, 2010, 841932. [Google Scholar] [CrossRef] [PubMed]
- Diculescu, V.C.; Chiorcea-Paquim, A.-M.; Eritja, R.; Oliveira-Brett, A.M. Evaluation of the Structure–activity Relationship of Thrombin with Thrombin Binding Aptamers by Voltammetry and Atomic Force Microscopy. J. Electroanal. Chem. 2011, 656, 159–166. [Google Scholar] [CrossRef]
- Chiorcea-Paquim, A.-M.; Santos, P.V.; Oliveira-Brett, A.M. Atomic force microscopy and voltammetric characterisation of synthetic homo-oligodeoxynucleotides. Electrochim. Acta 2013, 110, 599–607. [Google Scholar] [CrossRef]
- Chiorcea-Paquim, A.-M.; Santos, P.V.; Eritja, R.; Oliveira-Brett, A.M. Self-Assembled G-Quadruplex Nanostructures: AFM and Voltammetric Characterization. Phys. Chem. Chem. Phys. 2013, 15, 9117–9124. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues Pontinha, A.D.; Chiorcea-Paquim, A.-M.; Eritja, R.; Oliveira-Brett, A.M. Quadruplex Nanostructures of d(TGGGGT): Influence of Sodium and Potassium Ions. Anal. Chem. 2014, 86, 5851–5857. [Google Scholar] [CrossRef] [PubMed]
- Chiorcea-Paquim, A.-M.; Pontinha, A.D.R.; Oliveira-Brett, A.M. Time-Dependent Polyguanylic Acid Structural Modifications. Electrochem. Commun. 2014, 45, 71–74. [Google Scholar] [CrossRef]
- Chiorcea-Paquim, A.-M.; Rodrigues Pontinha, A.D.; Oliveira-Brett, A.M. Quadruplex-Targeting Anticancer Drug BRACO-19 Voltammetric and AFM Characterization. Electrochim. Acta 2015, 174, 155–163. [Google Scholar] [CrossRef]
- Pontinha, A.D.R.; Sparapani, S.; Neidle, S.; Oliveira-Brett, A.M. Triazole-Acridine Conjugates: Redox Mechanisms and in Situ Electrochemical Evaluation of Interaction with Double-Stranded DNA. Bioelectrochemistry 2013, 89, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Chiorcea-Paquim, A.-M.; Pontinha, A.D.R.; Eritja, R.; Lucarelli, G.; Sparapani, S.; Neidle, S.; Oliveira-Brett, A.M. Atomic Force Microscopy and Voltammetric Investigation of Quadruplex Formation between a Triazole-Acridine Conjugate and Guanine-Containing Repeat DNA Sequences. Anal. Chem. 2015, 87, 6141–6149. [Google Scholar] [CrossRef] [PubMed]
- Oliveira Brett, A.M.; Diculescu, V.C.; Chiorcea Paquim, A.-M.; Serrano, S. Chapter 20 DNA-Electrochemical Biosensors for Investigating DNA Damage. Compr. Anal. Chem. 2007, 49, 413–437. [Google Scholar]
- Diculescu, V.C.; Chiorcea Paquim, A.-M.; Oliveira Brett, A.M. Electrochemical DNA Sensors for Detection of DNA Damage. Sensors 2005, 5, 377–393. [Google Scholar] [CrossRef]
- Vasilescu, A.; Marty, J.-L. Electrochemical Aptasensors for the Assessment of Food Quality and Safety. TrAC Trends Anal. Chem. 2016, 79, 60–70. [Google Scholar] [CrossRef]
- Willner, I.; Zayats, M. Electronic Aptamer-Based Sensors. Angew. Chem. Int. Ed. 2007, 46, 6408–6418. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.K.H.; Sen, D.; Yu, H.-Z. Design and Testing of Aptamer-Based Electrochemical Biosensors for Proteins and Small Molecules. Bioelectrochemistry 2009, 77, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Velasco-Garcia, M.; Missailidis, S. New Trends in Aptamer-Based Electrochemical Biosensors. Gene Ther. Mol. Biol. 2009, 13, 1–10. [Google Scholar]
- Yin, X.-B. Functional Nucleic Acids for Electrochemical and Electrochemiluminescent Sensing Applications. TrAC Trends Anal. Chem. 2012, 33, 81–94. [Google Scholar] [CrossRef]
- Hianik, T.; Ostatná, V.; Sonlajtnerova, M.; Grman, I. Influence of Ionic Strength, pH and Aptamer Configuration for Binding Affinity to Thrombin. Bioelectrochemistry 2007, 70, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Cao, Z.; Lu, Y. Functional Nucleic Acid Sensors. Chem. Rev. 2009, 109, 1948–1998. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Wang, L.; Li, J.; Fan, C.; Zhao, J. Aptamer-Based Biosensors. TrAC Trends Anal. Chem. 2008, 27, 108–117. [Google Scholar] [CrossRef]
- Mir, M.; Vreeke, M.; Katakis, I. Different Strategies to Develop an Electrochemical Thrombin Aptasensor. Electrochem. Commun. 2006, 8, 505–511. [Google Scholar] [CrossRef]
- Polsky, R.; Gill, R.; Kaganovsky, L.; Willner, I. Nucleic Acid-Functionalized Pt Nanoparticles: Catalytic Labels for the Amplified Electrochemical Detection of Biomolecules. Anal. Chem. 2006, 78, 2268–2271. [Google Scholar] [CrossRef] [PubMed]
- Numnuam, A.; Chumbimuni-Torres, K.Y.; Xiang, Y.; Bash, R.; Thavarungkul, P.; Kanatharana, P.; Pretsch, E.; Wang, J.; Bakker, E. Aptamer-Based Potentiometric Measurements of Proteins Using Ion-Selective Microelectrodes. Anal. Chem. 2008, 80, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Ji, J.; Liu, Y.; Kong, J.; Liu, B. An Aptamer-Based Biosensor for Sensitive Thrombin Detection. Electrochem. Commun. 2009, 11, 38–40. [Google Scholar] [CrossRef]
- Peng, K.; Zhao, H.; Wu, X.; Yuan, Y.; Yuan, R. Ultrasensitive Aptasensor Based on Graphene-3,4,9,10-Perylenetetracarboxylic Dianhydride as Platform and Functionalized Hollow PtCo Nanochains as Enhancers. Sens. Actuators B Chem. 2012, 169, 88–95. [Google Scholar] [CrossRef]
- Centi, S.; Messina, G.; Tombelli, S.; Palchetti, I.; Mascini, M. Different Approaches for the Detection of Thrombin by an Electrochemical Aptamer-Based Assay Coupled to Magnetic Beads. Biosens. Bioelectron. 2008, 23, 1602–1609. [Google Scholar] [CrossRef] [PubMed]
- Centi, S.; Tombelli, S.; Minunni, M.; Mascini, M. Aptamer-Based Detection of Plasma Proteins by an Electrochemical Assay Coupled to Magnetic Beads. Anal. Chem. 2007, 79, 1466–1473. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wang, Y.; Wei, H.; Dong, S. Amplified Electrochemical Aptasensor Taking AuNPs Based Sandwich Sensing Platform as a Model. Biosens. Bioelectron. 2008, 23, 965–970. [Google Scholar] [CrossRef] [PubMed]
- Ikebukuro, K.; Kiyohara, C.; Sode, K. Electrochemical Detection of Protein Using a Double Aptamer Sandwich. Anal. Lett. 2004, 37, 2901–2909. [Google Scholar] [CrossRef]
- Ikebukuro, K.; Kiyohara, C.; Sode, K. Novel Electrochemical Sensor System for Protein Using the Aptamers in Sandwich Manner. Biosens. Bioelectron. 2005, 20, 2168–2172. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Feng, K.-J.; Chen, J.-W.; Jiang, J.-H.; Shen, G.-L.; Yu, R.-Q. Electrochemical Detection of Thrombin by Sandwich Approach Using Antibody and Aptamer. Bioelectrochemistry 2008, 73, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Son, J.I.; Won, M.-S.; Shim, Y.-B. Gold Nanoparticles Doped Conducting Polymer Nanorod Electrodes: Ferrocene Catalyzed Aptamer-Based Thrombin Immunosensor. Anal. Chem. 2009, 81, 6604–6611. [Google Scholar] [CrossRef] [PubMed]
- Lubin, A.A.; Plaxco, K.W. Folding-Based Electrochemical Biosensors: The Case for Responsive Nucleic Acid Architectures. Acc. Chem. Res. 2010, 43, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Plaxco, K.W.; Soh, H.T. Switch-Based Biosensors: A New Approach towards Real-Time, in Vivo Molecular Detection. Trends Biotechnol. 2011, 29, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.-Y.; Del Campo, F.J.; Tsai, Y.-C. Sensitive Electrochemical Thrombin Aptasensor Based on Gold Disk Microelectrodearrays. Biosens. Bioelectron. 2013, 42, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Gorgy, K.; Gondran, C.; Le Goff, A.; Spinelli, N.; Lopez, C.; Defrancq, E.; Cosnier, S. Label-Free Impedimetric Thrombin Sensor Based on Poly(pyrrole-Nitrilotriacetic Acid)-Aptamer Film. Biosens. Bioelectron. 2013, 41, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Shen, L.; Zhang, D.; Qi, H.; Gao, Q.; Ma, F.; Zhang, C. Electrochemical Impedance Spectroscopy for Study of Aptamer-Thrombin Interfacial Interactions. Biosens. Bioelectron. 2008, 23, 1624–1630. [Google Scholar] [CrossRef] [PubMed]
- Suprun, E.; Shumyantseva, V.; Bulko, T.; Rachmetova, S.; Rad’ko, S.; Bodoev, N.; Archakov, A. Au-Nanoparticles as an Electrochemical Sensing Platform for Aptamer-Thrombin Interaction. Biosens. Bioelectron. 2008, 24, 831–836. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Chen, Y.; Ren, J.; Qu, X. A Graphene Functionalized Electrochemical Aptasensor for Selective Label-Free Detection of Cancer Cells. Biomaterials 2011, 32, 2930–2937. [Google Scholar] [CrossRef] [PubMed]
- Elahi, M.Y.; Bathaie, S.Z.; Mousavi, M.F.; Hoshyar, R.; Ghasemi, S. A New DNA-Nanobiosensor Based on G-Quadruplex Immobilized on Carbon Nanotubes Modified Glassy Carbon Electrode. Electrochim. Acta 2012, 82, 143–151. [Google Scholar] [CrossRef]
- Xiao, Y.; Lubin, A.A.; Heeger, A.J.; Plaxco, K.W. Label-Free Electronic Detection of Thrombin in Blood Serum by Using an Aptamer-Based Sensor. Angew. Chem. 2005, 117, 5592–5595. [Google Scholar] [CrossRef]
- Radi, A.-E.; Acero Sánchez, J.L.; Baldrich, E.; O’Sullivan, C.K. Reusable Impedimetric Aptasensor. Anal. Chem. 2005, 77, 6320–6323. [Google Scholar] [CrossRef] [PubMed]
- Radi, A.-E.; Acero Sánchez, J.L.; Baldrich, E.; O’Sullivan, C.K. Reagentless, Reusable, Ultrasensitive Electrochemical Molecular Beacon Aptasensor. J. Am. Chem. Soc. 2006, 128, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, J.L.A.; Baldrich, E.; Radi, A.E.-G.; Dondapati, S.; Sánchez, P.L.; Katakis, I.; O’Sullivan, C.K. Electronic “Off-On” Molecular Switch for Rapid Detection of Thrombin. Electroanalysis 2006, 18, 1957–1962. [Google Scholar] [CrossRef]
- Bang, G.S.; Cho, S.; Kim, B.-G. A Novel Electrochemical Detection Method for Aptamer Biosensors. Biosens. Bioelectron. 2005, 21, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Wang, F.; Chen, Z. Aptamer-Based Electrochemical Biosensor for Label-Free Voltammetric Detection of Thrombin and Adenosine. Sens. Actuators B Chem. 2011, 160, 1380–1385. [Google Scholar] [CrossRef]
- Xiao, Y.; Piorek, B.D.; Plaxco, K.W.; Heeger, A.J. A Reagentless Signal-on Architecture for Electronic, Aptamer-Based Sensors via Target-Induced Strand Displacement. J. Am. Chem. Soc. 2005, 127, 17990–17991. [Google Scholar] [CrossRef] [PubMed]
- Meini, N.; Farre, C.; Chaix, C.; Kherrat, R.; Dzyadevych, S.; Jaffrezic-Renault, N. A Sensitive and Selective Thrombin Impedimetric Aptasensor Based on Tailored Aptamers Obtained by Solid-Phase Synthesis. Sens. Actuators B Chem. 2012, 166–167, 715–720. [Google Scholar] [CrossRef]
- Mir, M.; Jenkins, A.T.A.; Katakis, I. Ultrasensitive Detection Based on an Aptamer Beacon Electron Transfer Chain. Electrochem. Commun. 2008, 10, 1533–1536. [Google Scholar] [CrossRef]
- Prabhakar, N.; Matharu, Z.; Malhotra, B.D. Polyaniline Langmuir-Blodgett Film Based Aptasensor for Ochratoxin A Detection. Biosens. Bioelectron. 2011, 26, 4006–4011. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, J.; Li, J.; Yang, H.-H.; Fu, F.; Chen, G. An Ultrasensitive Signal-on Electrochemical Aptasensor via Target-Induced Conjunction of Split Aptamer Fragments. Biosens. Bioelectron. 2010, 25, 996–1000. [Google Scholar] [CrossRef] [PubMed]
- Castillo, G.; Lamberti, I.; Mosiello, L.; Hianik, T. Impedimetric DNA Aptasensor for Sensitive Detection of Ochratoxin A in Food. Electroanalysis 2012, 24, 512–520. [Google Scholar] [CrossRef]
- Hayat, A.; Andreescu, S.; Marty, J.-L. Design of PEG-Aptamer Two Piece Macromolecules as Convenient and Integrated Sensing Platform: Application to the Label Free Detection of Small Size Molecules. Biosens. Bioelectron. 2013, 45, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Jalit, Y.; Gutierrez, F.A.; Dubacheva, G.; Goyer, C.; Coche-Guerente, L.; Defrancq, E.; Labbé, P.; Rivas, G.A.; Rodríguez, M.C. Characterization of a Modified Gold Platform for the Development of a Label-Free Anti-Thrombin Aptasensor. Biosens. Bioelectron. 2013, 41, 424–429. [Google Scholar] [CrossRef] [PubMed]
- De Rache, A.; Kejnovská, I.; Vorlíčková, M.; Buess-Herman, C. Elongated Thrombin Binding Aptamer: A G-Quadruplex Cation-Sensitive Conformational Switch. Chemistry 2012, 18, 4392–4400. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yang, W.; Wang, J.; Yang, C.; Yang, F.; Yang, X. A Sensitive Impedimetric Thrombin Aptasensor Based on Polyamidoamine Dendrimer. Talanta 2009, 78, 1240–1245. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Shen, B.; Zhang, F.; Wu, J.; Xu, Y.; He, P.; Fang, Y. A New Electrochemically Active-Inactive Switching Aptamer Molecular Beacon to Detect Thrombin Directly in Solution. Biosens. Bioelectron. 2010, 25, 2265–2269. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhou, G.; Xu, X.; Cao, L.; Liang, G.; Chen, H.; Liu, B.; Kong, J. Development of an Electrochemical Aptamer-Based Sensor with a Sensitive Fe3O4 Nanopaticle-Redox Tag for Reagentless Protein Detection. Electrochem. Commun. 2011, 13, 928–931. [Google Scholar] [CrossRef]
- Li, Y.; Deng, L.; Deng, C.; Nie, Z.; Yang, M.; Si, S. Simple and Sensitive Aptasensor Based on Quantum Dot-Coated Silica Nanospheres and the Gold Screen-Printed Electrode. Talanta 2012, 99, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Radi, A.-E.; O’Sullivan, C.K. Aptamer Conformational Switch as Sensitive Electrochemical Biosensor for Potassium Ion Recognition. Chem. Commun. (Camb.) 2006, 32, 3432–3434. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.-S.; Chen, C.-R.; Shen, G.-L.; Yu, R.-Q. Reversible Electronic Nanoswitch Based on DNA G-Quadruplex Conformation: A Platform for Single-Step, Reagentless Potassium Detection. Biomaterials 2008, 29, 2689–2696. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wan, Y.; Wang, L.; Song, S.; Li, D.; Fan, C. Switchable Charge Transport Path via a Potassium Ions Promoted Conformational Change of G-Quadruplex Probe Monolayer. Electrochem. Commun. 2008, 10, 1258–1260. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, L.; Ma, H.; Zhou, T.; Li, X. Aptamer Biosensor for Label-Free Impedance Spectroscopy Detection of Potassium Ion Based on DNA G-Quadruplex Conformation. Biosens. Bioelectron. 2013, 48, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, J.; Chen, R.; Chen, G.; Fu, F. An Electrochemical Biosensor for Ultratrace Terbium Based on Tb3+ Promoted Conformational Change of Human Telomeric G-Quadruplex. Biosens. Bioelectron. 2009, 25, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, J.; Zhang, X.; Zeng, Z.; Chen, M.; Wang, S. An Electrochemical Biosensor Based on Hairpin-DNA Aptamer Probe and Restriction Endonuclease for Ochratoxin A Detection. Electrochem. Commun. 2012, 25, 5–7. [Google Scholar] [CrossRef]
- Han, G.; Feng, X.; Chen, Z. Hemin/G-Quadruplex DNAzyme for Designing of Electrochemical Sensors. Int. J. Electrochem. Sci 2015. [Google Scholar]
- Pelossof, G.; Tel-Vered, R.; Elbaz, J.; Willner, I. Amplified Biosensing Using the Horseradish Peroxidase-Mimicking DNAzyme as an Electrocatalyst. Anal. Chem. 2010, 82, 4396–4402. [Google Scholar] [CrossRef] [PubMed]
- Kosman, J.; Juskowiak, B. Peroxidase-Mimicking DNAzymes for Biosensing Applications: A Review. Anal. Chim. Acta 2011, 707, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, N.; Horii, K.; Kato, S.; Akitomi, J.; Waga, I. High-Throughput Quantitative Screening of Peroxidase-Mimicking DNAzymes on a Microarray by Using Electrochemical Detection. Anal. Chem. 2013, 85, 5430–5435. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Xu, S.; Minteer, S.D.; Baum, D.A. Investigation of a Deoxyribozyme as a Biofuel Cell Catalyst. J. Am. Chem. Soc. 2011, 133, 15890–15893. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Lu, J.; Chen, Z.; Yu, Y.; Mo, M. A Repeatable Assembling and Disassembling Electrochemical Aptamer Cytosensor for Ultrasensitive and Highly Selective Detection of Human Liver Cancer Cells. Anal. Chim. Acta 2015, 885, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Lu, J.; Zhong, Y.; Yu, Y.; Wang, Y.; Zhang, B.; Chen, Z. Sensitive Electrochemical Aptamer Cytosensor for Highly Specific Detection of Cancer Cells Based on the Hybrid Nanoelectrocatalysts and Enzyme for Signal Amplification. Biosens. Bioelectron. 2016, 75, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Shlyahovsky, B.; Elbaz, J.; Willner, I. Amplified Analysis of Low-Molecular-Weight Substrates or Proteins by the Self-Assembly of DNAzyme-Aptamer Conjugates. J. Am. Chem. Soc. 2007, 129, 5804–5805. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhu, X.; Wang, J.; Xu, L.; Li, G. Strategy to Fabricate an Electrochemical Aptasensor: Application to the Assay of Adenosine Deaminase Activity. Anal. Chem. 2010, 82, 3207–3211. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Cao, Y.; Han, P.; Zhu, X.; Sun, L.; Li, G. Tools for Investigation of the RNA Endonuclease Activity of Mammalian Argonaute2 Protein. Anal. Chem. 2012, 84, 2492–2497. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liang, Z.; Li, Y. Label Free, Highly Sensitive and Selective Recognition of Small Molecule Using Gold Surface Confined Aptamers. Solid State Sci. 2012, 14, 1060–1063. [Google Scholar] [CrossRef]
- Wang, Z.-H.; Lu, C.-Y.; Liu, J.; Xu, J.-J.; Chen, H.-Y. An Improved G-Quadruplex DNAzyme for Dual-Functional Electrochemical Biosensing of Adenosines and Hydrogen Peroxide from Cancer Cells. Chem. Commun. (Camb.) 2014, 50, 1178–1180. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Nie, Y.; Zhu, X.; Liu, X.; Li, G. Study on the Electrocatalytic Activity of Human Telomere G-Quadruplex–hemin Complex and Its Interaction with Small Molecular Ligands. Electrochim. Acta 2009, 55, 276–280. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, W.; Xiao, H.; Huang, J.; Li, G. Electrochemical Study of a hemin–DNA Complex and Its Activity as a Ligand Binder. Electrochim. Acta 2008, 53, 4407–4413. [Google Scholar] [CrossRef]
- Yang, C.; Lates, V.; Prieto-Simón, B.; Marty, J.-L.; Yang, X. Aptamer-DNAzyme Hairpins for Biosensing of Ochratoxin A. Biosens. Bioelectron. 2012, 32, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Xu, L.; Ma, W.; Chen, W.; Yan, W.; Kuang, H.; Wang, L.; Xu, C. G-Quadruplex DNAzyme-Based Microcystin-LR (Toxin) Determination by a Novel Immunosensor. Biosens. Bioelectron. 2011, 26, 4393–4398. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Liu, X. G-Quadruplex Based Impedimetric 2-Hydroxyfluorene Biosensor Using Hemin as a Peroxidase Enzyme Mimic. Microchim. Acta 2015, 182, 2233–2240. [Google Scholar] [CrossRef]
- Liang, G.; Liu, X.; Li, X. Highly Sensitive Detection of α-Naphthol Based on G-DNA Modified Gold Electrode by Electrochemical Impedance Spectroscopy. Biosens. Bioelectron. 2013, 45, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yin, J.; Wu, Z.; Yu, R. Electrocatalytic Assay of mercury(II) Ions Using a Bifunctional Oligonucleotide Signal Probe. Anal. Chim. Acta 2013, 762, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Pelossof, G.; Tel-Vered, R.; Willner, I. Amplified Surface Plasmon Resonance and Electrochemical Detection of Pb2+ Ions Using the Pb2+-Dependent DNAzyme and hemin/G-Quadruplex as a Label. Anal. Chem. 2012, 84, 3703–3709. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Gou, X.; Yuan, R.; Chai, Y.; Zhuo, Y.; Mao, L.; Gan, X. Electrochemical Aptasensor Based on the Dual-Amplification of G-Quadruplex Horseradish Peroxidase-Mimicking DNAzyme and Blocking Reagent-Horseradish Peroxidase. Biosens. Bioelectron. 2011, 26, 4236–4240. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Wang, M.; Li, C.; Xie, J. Label-Free and Amplified Aptasensor for Thrombin Detection Based on Background Reduction and Direct Electron Transfer of Hemin. Biosens. Bioelectron. 2013, 43, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, C.; Zhang, C.; Wang, Y.; Tang, B. Label-Free and Ultrasensitive Electrochemical Detection of Nucleic Acids Based on Autocatalytic and Exonuclease III-Assisted Target Recycling Strategy. Anal. Chem. 2013, 85, 2282–2288. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Yuan, R.; Chai, Y.; Yuan, Y.; Zhuo, Y.; Mao, L. Bi-Enzyme Functionlized Hollow PtCo Nanochains as Labels for an Electrochemical Aptasensor. Biosens. Bioelectron. 2011, 26, 4331–4336. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Chai, Y.; Yuan, R.; Bai, L.; Yuan, Y.; Wang, Y. A Dual-Amplification Aptasensor for Highly Sensitive Detection of Thrombin Based on the Functionalized Graphene-Pd Nanoparticles Composites and the hemin/G-Quadruplex. Anal. Chim. Acta 2012, 755, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Yuan, R.; Chai, Y.; Zhuo, Y.; Ye, X.; Gan, X.; Bai, L. Hemin/G-Quadruplex Simultaneously Acts as NADH Oxidase and HRP-Mimicking DNAzyme for Simple, Sensitive Pseudobienzyme Electrochemical Detection of Thrombin. Chem. Commun. (Camb.) 2012, 48, 4621–4623. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Liu, G.; Yuan, R.; Chai, Y.; Gan, X.; Bai, L. Dendrimer Functionalized Reduced Graphene Oxide as Nanocarrier for Sensitive Pseudobienzyme Electrochemical Aptasensor. Biosens. Bioelectron. 2013, 42, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Song, Y.; Chai, Y.; Pan, G.; Li, T.; Yuan, Y.; Yuan, R. Electrochemical Immunosensor for Detecting the Spore Wall Protein of Nosema Bombycis Based on the Amplification of hemin/G-Quadruplex DNAzyme Concatamers Functionalized Pt@Pd Nanowires. Biosens. Bioelectron. 2014, 60, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Gao, M.; Liu, G.; Chai, Y.; Wei, S.; Yuan, R. Sensitive Pseudobienzyme Electrocatalytic DNA Biosensor for mercury(II) Ion by Using the Autonomously Assembled hemin/G-Quadruplex DNAzyme Nanowires for Signal Amplification. Anal. Chim. Acta 2014, 811, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Chai, Y.; Yuan, Y.; Yuan, R. A Pseudo Triple-Enzyme Electrochemical Aptasensor Based on the Amplification of Pt-Pd Nanowires and hemin/G-Quadruplex. Anal. Chim. Acta 2014, 834, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Sun, A.; Qi, Q.; Wang, X.; Bie, P. Porous Platinum Nanotubes Labeled with hemin/G-Quadruplex Based Electrochemical Aptasensor for Sensitive Thrombin Analysis via the Cascade Signal Amplification. Biosens. Bioelectron. 2014, 57, 16–21. [Google Scholar] [CrossRef] [PubMed]












© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiorcea-Paquim, A.-M.; Oliveira-Brett, A.M. Guanine Quadruplex Electrochemical Aptasensors. Chemosensors 2016, 4, 13. https://doi.org/10.3390/chemosensors4030013
Chiorcea-Paquim A-M, Oliveira-Brett AM. Guanine Quadruplex Electrochemical Aptasensors. Chemosensors. 2016; 4(3):13. https://doi.org/10.3390/chemosensors4030013
Chicago/Turabian StyleChiorcea-Paquim, Ana-Maria, and Ana Maria Oliveira-Brett. 2016. "Guanine Quadruplex Electrochemical Aptasensors" Chemosensors 4, no. 3: 13. https://doi.org/10.3390/chemosensors4030013
APA StyleChiorcea-Paquim, A. -M., & Oliveira-Brett, A. M. (2016). Guanine Quadruplex Electrochemical Aptasensors. Chemosensors, 4(3), 13. https://doi.org/10.3390/chemosensors4030013