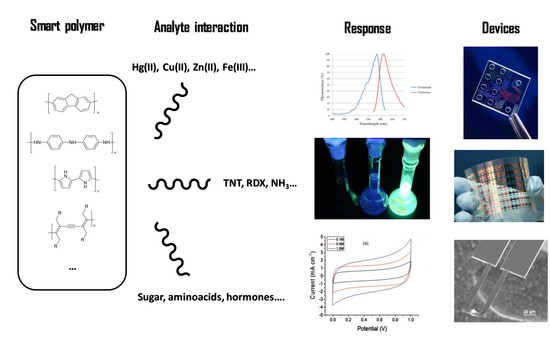
Smart Polymers in Micro and Nano Sensory Devices
Abstract
:1. Introduction
2. Polymers with Sensory Properties
3. Mechanisms of Detection and Target Species
4. New Micro and Nano Sensory Devices Based on Smart Polymers
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Okatno, T. Molecular design of temperature-responsive polymers as intelligent materials. In Advances in Polymer Chemistry, 1st ed.; Dušek, K., Ed.; Springer: Berlin, Germany, 1993; pp. 180–197. ISBN 978-3-540-47836-2. [Google Scholar]
- Thévenot, J.; Oliveira, H.; Sandre, O.; Lecommandoux, S. Magnetic responsive polymer composite materials. Chem. Soc. Rev. 2013, 42, 7099–7116. [Google Scholar] [CrossRef] [PubMed]
- Colson, Y.L.; Grinstaff, M.W. Biologically responsive polymeric nanoparticles for drug delivery. Adv. Mater. 2012, 24, 3878–3886. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Ravi, P.; Tam, K.C. pH-Responsive polymers: Synthesis, properties and applications. Soft Matter 2008, 4, 435–449. [Google Scholar] [CrossRef]
- Hu, J.; Liu, S. Responsive polymers for detection and sensing applications: Current status and future developments. Macromolecules 2010, 43, 8315–8330. [Google Scholar] [CrossRef]
- Chaterji, S.; Kwon, I.K.; Park, K. Smart polymeric gels: Redefining the limits of biomedical devices. Prog. Polym. Sci. 2007, 32, 1083–1122. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, A.K.; Sandeep, K.S.; Bhanu, S.; Kankane, S. Responsive polymers in controlled drug delivery. Prog. Polym. Sci. 2008, 33, 1088–1118. [Google Scholar] [CrossRef]
- García, J.M.; Pablos, J.L.; García, F.C.; Serna, F. Sensory polymers for detecting explosives and chemical warfare agents. In Industrial Applications for Intelligent Polymers and Coatings, 1st ed.; Hosseini, M., Makhlouf, A.S., Eds.; Springer International Publishing: Basel, Switzerland, 2016; pp. 553–576. ISBN 978-3-319-26893-4. [Google Scholar]
- García, J.M.; García, F.C.; Serna, F.; de la Peña, J.L. Fluorogenic and chromogenic polymer chemosensors. Polym. Rev. 2011, 51, 341–390. [Google Scholar] [CrossRef]
- Uzun, L.; Turner, A.P. Molecularly-imprinted polymer sensors: Realizing their potential. Biosens. Bioelectron. 2016, 76, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Zhang, T.; Zhao, H.; Fei, T. Preparation of organic-inorganic hybrid polymers and their humidity sensing properties. Sens. Actuators B Chem. 2017, 242, 1108–1114. [Google Scholar] [CrossRef]
- Balint, R.; Cassidy, N.; Cartmell, S. Conductive polymers: Towards a smart biomaterial for tissue engineering. Acta Biomater. 2014, 10, 2341–2353. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yin, J.; Yoon, J. Recent advances in development of chiral fluorescent and colorimetric sensors. Chem. Rev. 2014, 114, 4918–4959. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zheng, L.; Huang, X.; Cheng, Y.; Zhu, C. Fluorescence sensors based on chiral polymer for highly enantioselective recognition of phenylglycinol. Polymer 2010, 51, 994–997. [Google Scholar] [CrossRef]
- Greene, N.T.; Shimizu, K.D. Colorimetric molecularly imprinted polymer sensor array using dye displacement. J. Am. Chem. Soc. 2005, 127, 5695–5700. [Google Scholar] [CrossRef] [PubMed]
- Woodka, M.D.; Schnee, V.P.; Polcha, M.P. Fluorescent polymer sensor array for detection and discrimination of explosives in water. Anal. Chem. 2010, 82, 9917–9924. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Si, Y.; Wang, J.; Ding, B.; Yu, J.; Al-Deyab, S.S. A facile and highly sensitive colorimetric sensor for the detection of formaldehyde based on electro-spinning/netting nano-fiber/nets. Sens. Actuators B Chem. 2012, 163, 186–193. [Google Scholar] [CrossRef]
- Yoon, J.; Chae, S.; Kim, J.-M. Colorimetric sensors for volatile organic compounds (VOCs) based on conjugated polymer-embedded electrospun fibers. J. Am. Chem. Soc. 2007, 129, 3038–3039. [Google Scholar] [CrossRef] [PubMed]
- Adewuyi, S.; Ondigo, D.; Zugle, R.; Tshentu, Z.; Nyokong, T.; Torto, N. A highly selective and sensitive pyridylazo-2-naphthol-poly(acrylic acid) functionalized electrospun nanofiber fluorescence “turn-off” chemosensory system for Ni2+. Anal. Meth. 2012, 4, 1729–1735. [Google Scholar] [CrossRef]
- Wang, W.; Yang, Q.; Sun, L.; Wang, H.; Zhang, C.; Fei, X.; Li, Y. Preparation of fluorescent nanofibrous film as a sensing material and adsorbent for Cu(II) in aqueous solution via copolymerization and electrospinning. J. Hazard. Mater. 2011, 194, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Chu, F.; Tsiminis, G.; Spooner, N.A.; Monro, T.M. Explosives detection by fluorescence quenching of conjugated polymers in suspended core optical fibers. Sens. Actuators B Chem. 2014, 199, 22–26. [Google Scholar] [CrossRef]
- Geng, L.; Zhao, Y.; Huang, X.; Wang, S.; Zhang, S.; Wu, S. Characterization and gas sensitivity study of polyaniline/SnO2 hybrid material prepared by hydrothermal route. Sens. Actuators B Chem. 2007, 120, 568–572. [Google Scholar] [CrossRef]
- Khot, L.R.; Panigrahi, S.; Lin, D. Development and evaluation of piezoelectric-polymer thin film sensors for low concentration detection of volatile organic compounds related to food safety applications. Sens. Actuators B Chem. 2011, 153, 1–10. [Google Scholar] [CrossRef]
- Chen, A.; Wu, W.; Fegley, M.E.; Pinnock, S.S.; Duffy-Matzner, J.L.; Bernier, W.E.; Jones, W.E., Jr. Pentiptycene-derived fluorescence turn-off polymer chemosensor for copper(II) cation with high selectivity and sensitivity. Polymers 2017, 9, 118. [Google Scholar] [CrossRef]
- Xu, Y.; Meng, J.; Meng, L.; Dong, Y.; Cheng, Y.; Zhu, C. A highly selective fluorescence-based polymer sensor incorporating an (R,R)-Salen moiety for Zn2+ detection. Chem. Eur. J. 2010, 16, 12898–12903. [Google Scholar] [CrossRef] [PubMed]
- Somerset, V.; Leaner, J.; Mason, R.; Iwuoha, E.; Aoife, M. Determination of inorganic mercury using a polyaniline and polyaniline-methylene blue coated screen-printed carbon electrode. Int. J. Environ. Anal. Chem. 2010, 90, 671–685. [Google Scholar] [CrossRef]
- Petzoldt, M.; Eschenbaum, C.; Schwaebel, S.T.; Broedner, K.L.; Hamburger, M.; Bunz, U.H. A biphasic mercury-ion sensor: Exploiting microfluidics to make simple anilines competitive ligands. Chem. Eur. J. 2015, 21, 14297–14300. [Google Scholar] [CrossRef] [PubMed]
- Vallejos, S.; Reglero, J.A.; García, F.C.; García, J.M. Direct visual detection and quantification of mercury in fresh fish meat using facilely prepared polymeric sensory labels. J. Mater. Chem. A 2017, 5, 13710–13716. [Google Scholar] [CrossRef]
- Coronado, E.; Galán-Mascarós, J.R.; Martí-Gastaldo, C.; Palomares, E.; Durrant, J.R.; Vilar, R.; Gratzel, M.; Nazeeruddin, K. Reversible colorimetric probes for mercury sensing. J. Am. Chem. Soc. 2005, 127, 12351–12356. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Song, F.; Wang, L.; Cheng, Y.; Zhu, C. Polymer-based colorimetric and “turn-off” fluorescence sensor incorporating benzo[2,1,3]thiadiazole moiety for Hg2+ detection. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 517–522. [Google Scholar] [CrossRef]
- Liu, B.; Dai, H.; Bao, Y.; Du, F.; Tian, R.; Bai, R. 2,6-Substituted pyridine derivative-containing conjugated polymers: Synthesis, photoluminescence and ion-sensing properties. Polym. Chem. 2011, 2, 1699–1705. [Google Scholar] [CrossRef]
- Liu, B.; Dai, H.; Bao, Y.; Wang, H.; Du, F.; Tian, J.; Li, Q.; Bai, R. An efficient conjugated polymer sensor based on the aggregation-induced fluorescence quenching mechanism for the specific detection of palladium and platinum ions. J. Mater. Chem. 2012, 22, 3555–3561. [Google Scholar] [CrossRef]
- Lin, Q.; Li, Y.; Yang, M. Polyaniline nanofiber humidity sensor prepared by electrospinning. Sens. Actuators B Chem. 2012, 161, 967–972. [Google Scholar] [CrossRef]
- Liu, H.; Kameoka, J.; Czaplewski, D.A.; Craighead, H.G. Polymeric nanowire chemical sensor. Nano Lett. 2004, 4, 671–675. [Google Scholar] [CrossRef]
- Xue, M.; Li, F.; Wang, Y.; Cai, X.; Pan, F.; Chen, J. Ultralow-limit gas detection in nano-dumbbell polymer sensor via electrospinning. Nanoscale 2013, 5, 1803–1805. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, J.E.; Zhu, J.; Bravo-Vasquez, J.P. Cross-reactive, self-encoded polymer film arrays for sensor applications. RSC Adv. 2016, 6, 82616–82624. [Google Scholar] [CrossRef]
- Lu, W.; Xiao, P.; Gu, J.; Zhang, J.; Huang, Y.; Huang, Q.; Chen, T. Aggregation-induced emission of tetraphenylethylene-modified polyethyleneimine for highly selective CO2 detection. Sens. Actuators B Chem. 2016, 228, 551–556. [Google Scholar] [CrossRef]
- Fei, T.; Dai, J.; Jiang, K.; Zhao, H.; Zhang, T. Stable cross-linked amphiphilic polymers from a one-pot reaction for application in humidity sensors. Sens. Actuators B Chem. 2016, 227, 649–654. [Google Scholar] [CrossRef]
- Ghosh, K.R.; Saha, S.K.; Wang, Z.Y. Ultra-sensitive detection of explosives in solution and film as well as the development of thicker film effectiveness by tetraphenylethene moiety in AEI active fluorescent conjugated polymer. Polym. Chem. 2014, 5, 5638–5643. [Google Scholar] [CrossRef]
- Thomas, S.W.; Swager, T.M. Trace hydrazine detection with fluorescent conjugated polymers: A turn-on sensory mechanism. Adv. Mater. 2006, 18, 1047–1050. [Google Scholar] [CrossRef]
- Pablos, J.L.; Trigo-López, M.; Serna, F.; García, F.C.; García, J.M. Solid polymer substrates and smart fibres for the selective visual detection of TNT both in vapor and in aqueous media. RSC Adv. 2014, 4, 25562–25568. [Google Scholar] [CrossRef]
- Pablos, J.L.; Trigo-López, M.; Serna, F.; García, F.; García, J.M. Water-soluble polymers, solid polymer membranes, and coated fibres as smart sensory materials for the naked eye detection and quantification of TNT in aqueous media. Chem. Commun. 2014, 50, 2484–2487. [Google Scholar] [CrossRef] [PubMed]
- Pablos, J.L.; Vallejos, S.; Muñoz, A.; Rojo, M.J.; Serna, F.; García, F.C.; García, J.M. Solid polymer substrates and coated fibers containing 2,4,6-trinitrobenzene motifs as smart labels for the visual detection of biogenic amine vapors. Chem. Eur. J. 2015, 21, 8733–8736. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Swager, T.M. A fluorescent self-amplifying wavelength-responsive sensory polymer for fluoride ions. Angew. Chem. Int. Ed. 2003, 115, 4951–4954. [Google Scholar] [CrossRef]
- Zeng, Q.; Cai, P.; Li, Z.; Qin, J.; Tang, B.Z. An imidazole-functionalized polyacetylene: Convenient synthesis and selective chemosensor for metal ions and cyanide. Chem. Commun. 2008, 9, 1094–1096. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Xu, B.; Tong, H.; Wang, L. Highly selective and sensitive detection of cyanide by a reaction-based conjugated polymer chemosensor. Macromolecules 2011, 44, 4241–4248. [Google Scholar] [CrossRef]
- Karimian, N.; Zavar, M.H.A.; Chamsaz, M.; Turner, A.P.; Tiwari, A. On/off-switchable electrochemical folic acid sensor based on molecularly imprinted polymer electrode. Electrochem. Commun. 2013, 36, 92–95. [Google Scholar] [CrossRef]
- Osman, B.; Uzun, L.; Besirli, N.; Denizli, A. Microcontact imprinted surface plasmon resonance sensor for myoglobin detection. Mater. Sci. Eng. C 2013, 33, 3609–3614. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Piro, B.; Reisberg, S.; Anquetin, G.; de Rocquigny, H.; Jiang, P.; Dong, C.Z. Direct, reagentless electrochemical detection of the BIR3 domain of X-linked inhibitor of apoptosis protein using a peptide-based conducting polymer sensor. Biosens. Bioelectron. 2014, 61, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Cysewska, C.; Karczewski, J.; Jasiński, P. Electrochemical synthesis of 3D nano-/micro-structured porous PPy. Mater. Lett. 2016, 183, 397–400. [Google Scholar] [CrossRef]
- Fuchs, Y.; Soppera, O.; Mayes, A.G.; Haup, K. Holographic molecularly imprinted polymers for label-free chemical sensing. Adv. Mater. 2013, 25, 566–570. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Deng, Q.; Guo, T.; Su, R.; Gu, Y.; Wang, S. High selectivity and sensitivity fluorescence sensing of melamine based on the combination of a fluorescent chemosensor and molecularly imprinted polymers. RSC Adv. 2015, 5, 94084–94090. [Google Scholar] [CrossRef]
- Liu, C.-L.; Lin, C.-H.; Kuo, C.-C.; Lin, S.-T.; Chen, W.-C. Conjugated rod–coil block copolymers: Synthesis, morphology, photophysical properties, and stimuli-responsive applications. Prog. Polym. Sci. 2011, 36, 603–637. [Google Scholar] [CrossRef]
- Li, D.; Li, H.; Liu, M.; Chen, J.; Ding, J.; Huang, X.; Wu, H. A novel D-π-A conjugated polymer chemosensor based on benzo[c][1,2,5]selenadiazole for highly selective and sensitive recognition of mercury(II) ions. Macromol. Chem. Phys. 2014, 215, 82–89. [Google Scholar] [CrossRef]
- El Kaoutit, H.; Estévez, P.; García, F.; Serna, F.; García, J. Sub-ppm quantification of Hg(II) in aqueous media using both the naked eye and digital information from pictures of a colorimetric sensory polymer membrane taken with the digital camera of a conventional mobile. Anal. Meth. 2013, 5, 54–58. [Google Scholar] [CrossRef]
- Geng, T.M.; Wu, D.Y. Water-soluble polymeric chemosensor for selective detection of Hg2+ in aqueous solution using rhodamine-based modified poly(acrylamide-acrylic acid). Luminescence 2015, 30, 1263–1268. [Google Scholar] [CrossRef] [PubMed]
- Geng, T.M.; Guo, C.; Dong, Y.J.; Chen, M.; Wang, Y. Turn-on fluorogenic and chromogenic detection of cations in complete water media with poly(N-vinyl pyrrolidone) bearing rhodamine B derivatives as polymeric chemosensor. Polym Adv Technol. 2016, 27, 90–97. [Google Scholar] [CrossRef]
- Geng, T.M.; Zhang, W.Y.; Li, D.K.; Xia, H.Y.; Wang, Y.; Wang, Z.Q.; Zheng, Q. The chromogenic and fluorescent sensing properties for a water soluble polymeric chemosensor bearing rhodamine ethanediamine moieties with oxethyl (OCH2CH2) as a spacer. J. Environ. Chem. Eng. 2017, 5, 906–914. [Google Scholar] [CrossRef]
- Vallejos, S.; Estévez, P.; Ibeas, S.; Muñoz, A.; García, F.C.; Serna, F.; García, J.M. A selective and highly sensitive fluorescent probe of Hg2+ in organic and aqueous media: The role of a polymer network in extending the sensing phenomena to water environments. Sens. Actuators B Chem. 2011, 157, 686–690. [Google Scholar] [CrossRef]
- Pablos, J.L.; Ibeas, S.; Muñoz, A.; Serna, F.; García, F.C.; García, J.M. Solid polymer and metallogel networks based on a fluorene derivative as fluorescent and colourimetric chemosensors for Hg(II). React. Funct. Polym. 2014, 79, 14–23. [Google Scholar] [CrossRef]
- Abbas, K.; Znad, H.; Awual, Md. R. A ligand anchored conjugate adsorbent for effective mercury(II) detection and removal from aqueous media. Chem. Eng. J. 2018, 334, 432–443. [Google Scholar] [CrossRef]
- Li, J.; Wu, Y.; Song, F.; Wei, G.; Cheng, Y.; Zhu, C. A highly selective and sensitive polymer-based OFF-ON fluorescent sensor for Hg2+ detection incorporating salen and perylenyl moieties. J. Mater. Chem. 2012, 22, 478–482. [Google Scholar] [CrossRef]
- Jiao, J.; Li, F.; Zhang, S.; Quan, Y.; Zhen, W.; Cheng, Y.; Zhu, C. Hg2+-Induced in situ generated radical cation of (S)-BINOL-based polymer for highly enantioselective recognition of phenylalaninol. Macromol. Rapid Commun. 2014, 35, 1443–1449. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Kanhere, E.; Kottapalli, A.G.P.; Miao, J.; Triantayllou, M.S. Flexible liquid crystal polymer-based electrochemical sensor for in-situ detection of zinc(II) in seawater. Microchimica Acta 2017, 184, 3007–3015. [Google Scholar] [CrossRef]
- Sakthivel, S.; Punniyamurthy, T. Fluorescent OFF-ON polymer chemosensor bonded alternatively with 1,4-dioctyloxybenzene and (R,R)-salen for cascade Zn2+ and chiral recognition. Tetrahedron Asymmetry 2012, 23, 570–576. [Google Scholar] [CrossRef]
- Song, F.; Ma, X.; Hou, J.; Huang, X.; Cheng, Y.; Zhu, C. (R,R)-salen/salan-based polymer fluorescence sensors for Zn2+ detection. Polymers 2011, 52, 6029–6036. [Google Scholar] [CrossRef]
- Sakthivel, S.; Jammi, S.; Punniyamurthy, T. Fluorescent non-linear chiral polymer chemosensor bonded alternatively with 1,4-diethynyl-2,5-dioctyloxybenzene and (R,R)-salen for Zn2+ recognition. Tetrahedron Asymmetry 2012, 23, 101–107. [Google Scholar] [CrossRef]
- Dong, Y.; Wu, Y.; Jiang, X.; Huang, X.; Cheng, Y.; Zhu, C. A polymer based fluorescent sensor for Zn2+ detection and its application for constructing logic gates. Polymer 2011, 52, 5811–5816. [Google Scholar] [CrossRef]
- Liu, T.; Liu, S. Responsive polymers-based dual fluorescent chemosensors for Zn2+ ions and temperatures working in purely aqueous media. Anal. Chem. 2011, 83, 2775–2785. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Tao, F.; Wang, H.; Wang, L.; Zhang, J.; Ge, P.; Sung, S. A novel reversible colorimetric chemosensor for the detection of Cu2+ based on a water-soluble polymer containing rhodamine receptor pendants. RSC Adv. 2015, 5, 18983–18989. [Google Scholar] [CrossRef]
- Sergeev, A.A.; Mironenko, A.Y.; Leonov, A.A.; Nazirov, A.E.; Voznesenskiy, S.S.; Bratskaya, S.Y.; Kulchin, Y.N. Special features of copper(II) detection in aqueous solutions. Phys. Procedia 2017, 86, 152–154. [Google Scholar] [CrossRef]
- Trigo-López, M.; Muñoz, A.; Ibeas, S.; Serna, F.; García, F.C.; García, J.M. Colorimetric detection and determination of Fe(III), Co(II), Cu(II), Sn(II) in aqueous media by acrylic polymers with pendant terpyridine motifs. Sens. Actuators B Chem. 2016, 8, 118–126. [Google Scholar] [CrossRef]
- Barrio-Manso, J.L.; Calvo, P.; García, F.C.; Pablos, J.L.; Torroba, T.; García, J.M. Functional fluorescent aramids: Aromatic polyamides containing a dipicolinic acid derivative as luminescent converters and sensory materials for the fluorescence detection and quantification of Cr(VI), Fe(III), Cu(II). Polym. Chem. 2013, 4, 4256–4264. [Google Scholar] [CrossRef]
- Chakraborty, C.; Singh, P.; Maji, S.K.; Malik, S. Conjugated polyfluorene-based reversible fluorescent sensor for Cu(II) and cyanide ions in aqueous medium. Chem. Lett. 2013, 42, 1355–1357. [Google Scholar] [CrossRef]
- El Kaoutit, H.; Estévez, P.; Ibeas, S.; García, F.C.; Serna, F.; Benabdelouahab, F.B.; García, J.M. Chromogenic and fluorogenic detection of cations in aqueous media by means of an acrylic polymer chemosensor with pendant Rhodamine-based dyes. Dyes Pigm. 2013, 96, 414–423. [Google Scholar] [CrossRef]
- Vallejos, S.; Muñoz, A.; Ibeas, S.; Serna, F.; García, F. Solid sensory polymer substrates for the quantification of iron in blood, wine and water by a scalable RGB technique. J. Mater. Chem. A 2013, 1, 15435–15441. [Google Scholar] [CrossRef]
- Ghosh, S.; Dey, C.K.; Manna, R. Epoxy-based polymer bearing 1-napthylamine units: Highly selective fluorescent chemosensor for ferric ion. Tetrahedron Lett. 2010, 51, 3177–3180. [Google Scholar] [CrossRef]
- Wang, L.; Li, F.; Liu, X.; Wei, G.; Cheng, Y.; Zhu, C. A helical chiral polymer-based chromo-fluorescence and CD response sensor for selective detection of trivalent cations. J. Polym. Sci. Part A: Polym. Chem. 2013, 51, 4070–4075. [Google Scholar] [CrossRef]
- Li, Y.; Ashizawa, M.; Uchida, S.; Michinobu, T. A novel polymeric chemosensor: Dual colorimetric detection of metal ions through click synthesis. Macromol. Rapid Commun. 2011, 32, 1804–1808. [Google Scholar] [CrossRef] [PubMed]
- Vallejos, S.; Muñoz, A.; Ibeas, S.; Serna, F.; García, F.C.; García, J.M. Selective and sensitive detection of aluminium ions in water via fluorescence “turn-on” with both solid and water soluble sensory polymer substrates. J. Hazard. Mater. 2014, 276, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Vallejos, S.; Muñoz, S.; Ibeas, S.; Serna, F.; García, F.; García, J.M. Forced solid-state interactions for the selective “Turn-On” fluorescence sensing of aluminum ions in water using a sensory polymer substrate. ACS Appl. Mater. Interfaces 2015, 7, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wei, G.; Sheng, Y.; Quan, Y.; Cheng, Y.; Zhu, C. (S)-Binapthalene-based fluorescence polymer sensors for direct and visual F− detection. Polymer 2014, 55, 5689–5694. [Google Scholar] [CrossRef]
- Alaei, P.; Rouhani, S.; Gharanjig, K.; Ghasemi, J. A new polymerizable fluorescent PET chemosensor of fluoride (F−) based on naphthalimide-thiourea dye. Spectrochim. Acta Part A 2012, 90, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Trigo-López, M.; Muñoz, A.; Mendía, A.; Ibeas, S.; Serna, F.; García, F.; García, J.M. Palladium-containing polymers as hybrid sensory materials (water-soluble polymers, films and smart textiles) for the colorimetric detection of cyanide in aqueous and gas phases. Sens. Actuators B Chem. 2017, 255, 2750–2755. [Google Scholar] [CrossRef]
- Vallejos, S.; Estévez, P.; García, F.C.; Serna, F.; De la Peña, J.; García, J.M. Putting to work organic sensing molecules in aqueous media: Fluorene derivative-containing polymers as sensory materials for the colorimetric sensing of cyanide in water. Chem. Commun. 2010, 46, 7951–7953. [Google Scholar] [CrossRef] [PubMed]
- Vallejos, S.; El Kaoutit, H.; Estévez, P.; García, F.C.; De la Peña, J.L.; Serna, F.; García, J.M. Working with water insoluble organic molecules in aqueous media: Fluorene derivative-containing polymers as sensory materials for the colorimetric sensing of cyanide in water. Polym. Chem. 2011, 2, 1129–1138. [Google Scholar] [CrossRef]
- Son, J.H.; Jang, G.; Lee, T.S. Synthesis of water-soluble, fluorescent, conjugated polybenzodiazaborole for detection of cyanide anion in water. Polymer 2013, 54, 3542–3547. [Google Scholar] [CrossRef]
- Lin, Q.; Zhong, K.-P.; Zhu, J.-H.; Ding, L.; Su, J.-X.; Yao, H.; Wei, T.-B.; Zhang, Y.-M. Iodine controlled pillar[5]arene-based multiresponsive supramolecular polymer for fluorescence detection of cyanide, mercury and cysteine. Macromolecules 2017, 50, 7863–7871. [Google Scholar] [CrossRef]
- Xu, H.; Wu, W.; Chen, Y.; Qiu, T.; Fan, L. Construction of response patterns for metal cations by using a fluorescent conjugated polymer sensor array from parallel combinatorial synthesis. Appl. Mater. Interfaces 2014, 6, 5041–5049. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, J.; Ying, Z.; Meng, L.; Fu, C. Highly selective molecularly imprinted polymer sensor for indium detection based on recognition of In-Alizarin complexes. Electroanalysis 2015, 27, 1758–1765. [Google Scholar] [CrossRef]
- Song, M.; Xu, J. Preparation of polyethylenimine-functionalized graphene oxide composite and its application in electrochemical ammonia sensors. Electroanalysis 2013, 25, 523–530. [Google Scholar] [CrossRef]
- Hong, L.; Li, Y.; Yang, M. Fabrication and ammonia gas sensing of palladium/PPy nanocomposite. Sens. Actuators B Chem. 2010, 145, 25–31. [Google Scholar] [CrossRef]
- Zhang, D.; Jian, C.; Sun, Y. Room-temperature high-performance ammonia gas sensor based on layer-by-layer self-assembled molybdenum disulfide/zinc oxide nanocomposite film. J. Alloys Compd. 2017, 698, 476–483. [Google Scholar] [CrossRef]
- Ahmad, Z.; Choudhary, M.A.; Mehmood, A.; Wakeeh, R.; Akhtar, T.; Rafiq, M.A. Synthesis of PPy nano/microspheres using cobalt(III) as an oxidizing agent and its ammonia sensing behavior. Macromol. Res. 2016, 24, 596–601. [Google Scholar] [CrossRef]
- Xu, Y.; Sui, X.; Guan, S.; Zhai, J.; Gao, L. Olfactory sensory neuron-mimetic CO2 activated nanofluidic diode with fast response rate. Adv. Mater. 2015, 27, 1851–1855. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lim, L.-T. Colorimetric array indicator for NH3 and CO2 detection. Sens. Actuators B Chem. 2018, 255, 3216–3226. [Google Scholar] [CrossRef]
- Li, Y.; Yang, M.J.; She, Y. Humidity sensors using in situ synthesized sodium polystyrenesulfonate/ZnO nanocomposites. Talanta 2004, 62, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Suri, K.; Annapoorni, S.; Sarkar, A.K.; Tandon, R.P. Gas and humidity sensors based on iron oxide-PPy nanocomposites. Sens. Actuators B Chem. 2002, 81, 277–282. [Google Scholar] [CrossRef]
- Najjar, R.; Nematdoust, S. A resistive-type humidity sensor based on PPy and ZnO nanoparticles: Hybrid polymers vis-a-vis nanocomposites. RSC Adv. 2016, 6, 112129–112139. [Google Scholar] [CrossRef]
- Su, P.G.; Huang, L.N. Humidity sensors based on TiO2 nanoparticles/PPy composite thin films. Sens. Actuators B Chem. 2007, 123, 501–507. [Google Scholar] [CrossRef]
- Hosono, K.; Matsubara, I.; Murayama, N.; Woosuck, S.; Izu, N. Synthesis of PPy/MoO3 hybrid thin films and their volatile organic compound gas-sensing properties. Chem. Mater. 2005, 17, 349–354. [Google Scholar] [CrossRef]
- Savage, N.O. Gas sensing composites of metal oxides with vapor-deposited PPy. Sens. Actuators B Chem. 2009, 143, 6–11. [Google Scholar] [CrossRef]
- Kwan, P.H.; MacLachlan, M.J.; Swager, T.M. Rotaxaned conjugated sensory polymers. J. Am. Chem. Soc. 2004, 126, 8638–8639. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Jung, S.H.; Han, W.S.; Moon, J.H.; Kang, S.; Lee, J.Y.; Shinkai, S. A chromo-fluorogenic tetrazole-based CoBr2 coordination polymer gel as a highly sensitive and selective chemosensor for volatile gases containing chloride. Chem. Eur. J. 2011, 17, 2823–2827. [Google Scholar] [CrossRef] [PubMed]
- Patil, J.; Duragkar, N.; Rao, V.R. An ultra-sensitive piezoresistive polymer nano-composite microcantilever sensor electronic nose platform for explosive vapor detection. Sens. Actuators B Chem. 2014, 192, 444–451. [Google Scholar] [CrossRef]
- Calvo-Gredilla, P.; García-Calvo, J.; Cuevas, J.; Torroba, T.; Pablos, J.-L.; García, F.; García, J.M.; Fernández-Lázaro, F. Solvent-free off–on detection of the improvised explosive triacetone triperoxide (TATP) with fluorogenic materials. Chem. Eur. J. 2017, 23, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pangeni, D.; Nesterov, E.E. “Higher energy gap” control in fluorescent conjugated polymers: Turn-on amplified detection of organophosphorous agents. Macromolecules 2013, 46, 7266–7273. [Google Scholar] [CrossRef]
- Yang, Z.; Sheng, Q.; Zhang, S.; Zheng, X.; Zheng, J. One-pot synthesis of Fe3O4/polypyrrole/graphene oxide nanocomposites for electrochemical sensing of hydrazine. Microchimica Acta 2017, 184, 2219–2226. [Google Scholar] [CrossRef]
- Roales, J.; Moscoso, F.G.; Gámez, F.; Lopes-Costa, T.; Sousaraei, A.; Casado, S.; Castro-Smirnov, J.R.; Cabanillas-Gonzalez, J.; Almeida, J.; Queirós, C.; Cunha-Silva, L.; Silva, A.M.G.; Pedrosa, J.M. Preparation of luminescent metal-organic framework films by soft-imprinting for 2,4-dinitrotoluene sensing. Materials 2017, 10, 992. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Sun, D.; Li, H.; Sun, X.; Liu, J.; Ren, Z.; Yan, S. High efficiency organosilicon-containing polymer sensors for the detection of trinitrotoluene and dinitrotoluene. J. Mater. Chem. C 2016, 4, 6756–6760. [Google Scholar] [CrossRef]
- Tokranova, N.A.; Novak, S.W.; Castracane, J.; Levitsky, I.A. Deep infiltration of emissive polymers into mesoporous silicon microcavities: Nanoscale confinement and advanced vapor sensing. J. Phys. Chem. C 2013, 117, 22667–22676. [Google Scholar] [CrossRef]
- Zhang, H.; Feng, L.; Liu, B.; Tong, C.; Lü, C. Conjugation of PPV functionalized mesoporous silica nanoparticles with graphene oxide for facile and sensitive fluorescence detection of TNT in water through FRET. Dyes Pigm. 2014, 101, 122–129. [Google Scholar] [CrossRef]
- Hussein, L.A.; El-Kosasy, A.M.; Trabik, Y.A. Comparative study of normal, micro- and nano-sized iron oxide effect in potentiometric determination of fluconazole in biological fluids. RSC Adv. 2015, 5, 37957–37963. [Google Scholar] [CrossRef]
- Yu, G.; Qiang, L. Preparation and characterization of PTFE coating in new polymer quartz piezoelectric crystal sensor for testing liquor products. Chin. Phys. B 2015, 24, 078106. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, L.; Luo, Z.; Tang, H. Fabrication of molecular imprinted polymer sensor for chlortetracycline based on controlled electrochemical reduction of graphene oxide. Sens. Actuators B Chem. 2013, 185, 438–444. [Google Scholar] [CrossRef]
- Li, J.; Jiang, F.; Li, Y.; Chen, Z. Fabrication of an oxytetracycline molecular-imprinted sensor based on the competition reaction via a GOD-enzymatic amplifier. Biosens. Bioelectron 2011, 26, 2097–2101. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-Q.; Lin, F.-Y.; Yu, L.-P. A molecularly imprinted photonic polymer sensor with high selectivity for tetracyclines analysis in food. Analyst 2012, 137, 3502–3509. [Google Scholar] [CrossRef] [PubMed]
- Prasad, B.; Mahavir, P.; Rashmi, M.; Sharma, P. Enantioselective quantitative separation of d- and l-thyroxine by molecularly imprinted micro-solid phase extraction silver fiber coupled with complementary molecularly imprinted polymer-sensor. J. Chromatogr. A 2010, 1217, 4255–4266. [Google Scholar] [CrossRef] [PubMed]
- Prasad, B.B.; Srivastava, A.; Prasad, A.; Tiwari, M.P. Molecularly imprinted micro solid-phase extraction technique coupled with complementary molecularly imprinted polymer-sensor for ultra-trace analysis of epinephrine in real samples. Colloids Surf. B 2014, 113, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Wang, Y.; Thakur, G.; Kotsuchibashi, Y.; Naicker, S.; Narain, R.; Thundat, T. Rapid and highly sensitive detection of dopamine using conjugated oxaborole-based polymer and glycopolymer systems. ACS Appl. Mater. Interfaces 2017, 9, 15225–15231. [Google Scholar] [CrossRef] [PubMed]
- Pablos, J.L.; Vallejos, S.; Ibeas, S.; Muñoz, A.; Serna, F.; García, F.C.; García, J.M. Acrylic polymers with pendant phenylboronic acid moieties as “turn-off” and “ turn-on” fluorescence solid sensors for detection of dopamine, glucose and, fructose in water. ACS Macro Letters 2015, 4, 979–983. [Google Scholar] [CrossRef]
- Oh, S.; Uh, K.; Jeon, S.; Kim, J.M. A free-standing self-assembled tubular conjugated polymer sensor. Macromolecules 2016, 49, 5841–5848. [Google Scholar] [CrossRef]
- Shirsat, M.D.; Too, C.O.; Wallace, G.G. Amperometric glucose biosensor on layer by layer assembled carbon nanotube and PPy multilayer film. Electroanalysis 2008, 20, 150–156. [Google Scholar] [CrossRef]
- Barone, P.W.; Yoon, H.; Ortiz-García, R.; Zhang, J.; Ahan, J.H.; Kim, J.H.; Strano, M.S. Modulation of single-walled carbon nanotube photoluminescence by hydrogel swelling. ACS Nano 2009, 3, 3869–3877. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xia, J.; Qiang, X.; Xia, Y.; Shi, G.; Zhang, F.; Tang, J. Polymer-assisted in situ growth of copper nanoparticles on graphene surface for non-enzymatic electrochemical sensing of glucose. Int. J. Electrochem. Sci. 2013, 8, 6941–6950. [Google Scholar]
- Ivanov, A.; Thammakhet, C.; Kuzimenkova, M.; Thavarungkul, P.; Kanatharana, P.; Mikhalovska, L.; Mattiasson, B. Thin semitransparent gels containing phenylboronic acid: Porosity, optical response and permeability for sugars. J. Mol. Recognit. 2008, 21, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Hajizadeh, S.; Ivanov, A.E.; Jahanshahi, M.; Sanati, M.H.; Zhuravleva, N.V.; Mikhalovska, L.I.; Galaev, I.Y. Glucose sensors with increased sensitivity based on composite gels containing immobilized boronic acid. React. Funct. Polym. 2008, 68, 1625–1635. [Google Scholar] [CrossRef]
- Thammakhet, C.; Thavarungkul, P.; Kanatharana, P. Development of an on-column affinity smart polymer gel glucose. Anal. Chim. Acta 2011, 695, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Ouiganon, S.; Thammakhet, C.; Thavarungkul, P. An application of optical coherence tomography and a smart polymer gel to construct enzyme-free sugar sensor. Appl. Phys. B: Lasers Opt. 2016, 122, 1–8. [Google Scholar] [CrossRef]
- Dabrowski, M.; Sharma, P.S.; Iskierko, Z.; Noworyta, K.; Cieplak, M.; Lisowski, W.; Kutner, W. Early diagnosis of fungal infections using piezomicrogravimetric and electric chemosensors based on polymers molecularly imprinted with d-arabitol. Biosens. Bioelectron 2016, 79, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Prasad, B.; Srivastava, A.; Pandey, I.; Tiwari, M. Electrochemically grown imprinted polybenzidine nanofilm on multiwalled carbon nanotubes anchored pencil graphite fibers for enantioselective micro-solid phase extraction coupled with ultratrace sensing of d- and l-methionine. J. Chromatogr. B 2013, 912, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Iskierko, Z.; Checinska, A.; Sharma, P.S.; Golebiewska, K.; Noworyta, K.; Borowicz, P.; Kutner, W. Molecularly imprinted polymer based extended-gate field-effect transistor chemosensors for phenylalanine enantioselective sensing. J. Mater. Chem. C 2017, 5, 969–977. [Google Scholar] [CrossRef]
- Hou, J.; Song, F.; Wang, L.; Wei, G.; Cheng, Y.; Zhu, C. In situ generated 1:1 Zn(II)-containing polymer complex sensor for highly enantioselective recognition of N-Boc-protected alanine. Macromolecules 2012, 45, 7835–7842. [Google Scholar] [CrossRef]
- Wei, G.; Zhan, S.; Dai, C.; Quan, Y.; Cheng, Y.; Zhu, C. A new chiral binapthalene-based fluorescence polymer sensor for the highly enantioselective recognition of phenylalaninol. Chem. Eur. J. 2013, 19, 16066–16071. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wei, G.; Wang, Z.; Ma, J.; Zhu, C.; Cheng, Y. Highly enantioselective recognition of alaninol via the chiral BINAM-based fluorescence polymer sensor. Polymer 2016, 101, 93–97. [Google Scholar] [CrossRef]
- Song, F.; Weir, G.; Wang, L.; Jiao, J.; Chen, Y.; Zhu, C. Salen-based chiral fluorescence polymer sensor for enantioselective recognition of α-hydroxyl carboxylic acids. J. Org. Chem. 2012, 77, 4759–4764. [Google Scholar] [CrossRef] [PubMed]
- Prasad, B.B.; Pandey, I. Molecularly imprinted polymer-based piezoelectric sensor for enantio-selective analysis of malic acid isomers. Sens. Actuators B Chem. 2013, 181, 596–604. [Google Scholar] [CrossRef]
- Yadav, S.; Kumar, A.; Pundir, C.S. Amperometric creatinine biosensor based on covalently coimmobilized enzymes onto carboxylated multiwalled carbon nanotubes/polyaniline composite film. Anal. Biochem. 2011, 419, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Chow, C.F. Supramolecular polymeric chemosensor for biomedical applications: Design and synthesis of a luminescent zinc metallopolymer as a chemosensor for adenine detection. J. Fluoresc. 2012, 22, 1539–1546. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Y.; Ding, B. Electrospun nanofiber-based sensors. In Electrospun Nanofibers for Energy and Environmental Applications, 1st ed.; Ding, B., Yu, J., Eds.; Springer: Berlin, Germany, 2014; pp. 267–297. ISBN 978-3-642-54160-5. [Google Scholar]
- Ye, L.; Mosbach, L. Molecular imprinting: Synthetic materials as substitutes for biological antibodies and receptors. Chem. Mater. 2008, 20, 859–868. [Google Scholar] [CrossRef]
- Creran, B.; Bunz, U.; Rotello, V. Polymer-nanoparticle assemblies for array based sensing. Curr. Org. Chem. 2015, 109, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Arshak, K.; Moore, E.; Lyons, G.; Harris, J.; Clifford, S. A review of gas sensors employed in electronic nose applications. Sens. Rev. 2004, 24, 181–198. [Google Scholar] [CrossRef]
- Rodríguez-Méndez, M.-L.; De Saja, J.A.; González-Antón, R.; García-Hernández, C.; Medina-Plaza, C.; García-Cabezón, C.; Martín-Pedrosa, F. Electronic noses and tongues in wine industry. Front. Bioeng. Biotechnol. 2016, 4, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Nanto, H.; Douguchi, Y.; Yokoi, Y. Smart electronic nose using polymer-film coated quartz resonator gas sensor for identification of harmful gases. Proc. Soc. Photo Opt. Instrum. Eng. 1999, 3856, 317–327. [Google Scholar] [CrossRef]
- Sartore, L.; Barbaglio, M.; Borgese, L.; Bontempi, E. Polymer-grafted QCM chemical sensor and application to heavy metal ions real time detection. Sens. Actuators B Chem. 2011, 155, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Guo, J.; Fan, X.; Xu, J.; Fan, Z.; Du, B. Detection of heavy metal ions in aqueous solution by Poly(MBTVBC-co-VIM)-coated QCM sensor. Sens. Actuators B 2017, 157, 34–41. [Google Scholar] [CrossRef]
- Fan, Y.-Q.; Gao, F.; Wang, M.; Zhuang, J.; Tang, G.; Zhang, Y.-J. Recent development of wearable microfluidics applied in body fluid testing and drug delivery. Chin. J. Anal. Chem. 2017, 45, 455–463. [Google Scholar] [CrossRef]
- Qi, J.; Li, B.; Wang, X.; Zhang, Z.; Wang, Z.; Han, J.; Chen, L. Three-dimensional paper-based microfluidic chip device for multiplexed fluorescence detection of Cu2+ and Hg2+ ions based on ion imprinting technology. Sens. Actuators B Chem. 2017, 251, 224–233. [Google Scholar] [CrossRef]
- Kudr, J.; Ondrej, Z.; Klimanek, M.; Vrba, R.; Adam, V. Microfluidic electrochemical devices for pollution analysis—A review. Sens. Actuators B Chem. 2017, 246, 578–590. [Google Scholar] [CrossRef]
- Naveen, M.; Gurudatt, N.; Shim, Y.-B. Applications of conducting polymer composites to electrochemical sensors: A review. Appl. Mater. Today 2017, 9, 419–433. [Google Scholar] [CrossRef]
- Bai, H.; Wang, S.; Liu, P.; Xiong, C.; Zhang, K.; Cao, Q. Electrochemical sensor based on in situ polymerized ion-imprinted membranes at graphene modified electrode for palladium determination. J. Electroanal. Chem. 2016, 771, 29–36. [Google Scholar] [CrossRef]
- Caliò, A.; Dardano, P.; Di Palma, V.; Bevilacqua, M.; Di Matteo, A.; Iuele, H.; De Stefano, L. Polymeric microneedles based enzymatic electrodes for electrochemical biosensing of glucose and lactic acid. Sens. Actuators B Chem. 2016, 236, 343–349. [Google Scholar] [CrossRef]
- Liu, Y.; Meiting, W.; Yue, H.; Zhu, L.; Du, Y. An electrochemical sensor based on a molecularly imprinted polymer for determination of anticancer drug mitoxantrone. Sens. Actuators B Chem. 2018, 255, 544–551. [Google Scholar] [CrossRef]
- Yu, C.H.; Huang, X.Y.; Lei, F.H.; Tan, X.C.; Wei, Y.C.; Li, H. Molecularly imprinted electrochemical sensor based on nickel nanoparticle-modified electrodes for phenobarbital determination. Electrochim. Acta 2014, 141, 45–50. [Google Scholar] [CrossRef]
- Shrivastava, S.; Jadon, N.; Jain, R. Next-generation polymer nanocomposite-based electrochemical sensors and biosensors: A review. Trends Anal. Chem. 2016, 82, 55–67. [Google Scholar] [CrossRef]
- Lv, A.; Wang, M.; Wang, Y.; Bo, Z.; Chi, L. Investigation into the sensing process of high-performance H2S sensors based on polymer transistors. Chemistry 2016, 22, 3654–3659. [Google Scholar] [CrossRef] [PubMed]
- Liqiang, L.; Baumgarten, M.; Müllen, K.; Nan, L.; Fuchs, H.; Lifeng, C. High performance field-effect ammonia sensors based on a structured ultrathin organic semiconductor film. Adv. Mater. 2013, 25, 3419–3425. [Google Scholar] [CrossRef]
- Lv, A.; Pan, Y.; Chi, L. Gas sensors based on polymer field-effect transistors. Sensors 2017, 17, 213. [Google Scholar] [CrossRef] [PubMed]
- Chuan-Guang, Q.; Cai-Xi, L.; Gao-Wei, O.; Ke, Q.; Feng, Z.; Hai-Tong, S.; Xiao-Hui, W. Progress of azobenzene-based photoswitchable molecular probes and sensory chips for chemical and biological analysis. Chin. J. Anal. Chem. 2015, 43, 433–443. [Google Scholar] [CrossRef]
- Song, S.; Choi, H.; Hong, J.; Kim, B.; Sim, S.; Yoon, H. Selective antigen–antibody recognition on SPR sensor based on the heat-sensitive conformational change of poly(N-isopropylacrylamide). Colloids Surf. A Physicochem. Eng. Asp. 2008, 313, 504–508. [Google Scholar] [CrossRef]
- Wilhelmina de Groot, G.; Demarche, S.; Santonicola, M.; Tiefenauer, L.; Vancso, G. Smart polymer brush nanostructures guide the self-assembly of pore-spanning lipid bilayers with integrated membrane proteins. Nanoscale 2014, 6, 2228–2237. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Sun, H.; Nagarajan, R.; Kumar, J.; Gu, Z.; Cho, J.; Kurup, P. Dynamic chemical vapor sensing with nanofibrous film based surface acoustic wave sensors. Sens. Actuators A Phys. 2011, 167, 8–13. [Google Scholar] [CrossRef]
- Qianqian, L.; Yang, L.; Mujie, Y. Highly sensitive and ultrafast response surface acoustic wave humidity sensor based on electrospun polyaniline/poly(vinyl butyral) nanofibers. Anal. Chim. Acta 2012, 748, 73–80. [Google Scholar] [CrossRef]
- Thiha, A.; Ibrahim, F.; Muniandy, S.; Dinshaw, I.J.; Teh, S.J.; Thong, K.L.; Leo, B.F.; Madou, M. All-carbon suspended nanowire sensors as rapid highly sensitive label-free chemiresistive biosensing platform. Biosens. Bioelectron. 2018, 107, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Scampicchio, M.; Arecchi, A.; Lawrence, N.; Mannino, S. Nylon nanofibrous membrane for mediated glucose biosensing. Sens. Actuators B Chem. 2010, 145, 394–397. [Google Scholar] [CrossRef]
- Manesh, K.; Santhosh, P.; Gopalan, A.; Lee, K. Electrospun poly(vinylidene fluoride)/poly(aminophenylboronic acid) composite nanofibrous membrane as a novel glucose sensor. Anal. Biochem. 2007, 360, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Trigo-López, M.; Pablos, J.L.; Muñoz, A.; Ibeas, S.; Serna, F.; García, F.C.; García, J.M. Aromatic polyamides and acrylic polymers as solid sensory materials and smart coated fibres for high acidity colorimetric sensing. Polym. Chem. 2015, 6, 3110–3120. [Google Scholar] [CrossRef]
- Ghosh, K.R.; Saha, S.K.; Gao, J.P.; Wang, Z.Y. Direct detection of ultralow trace amounts of isocyanates in air using a fluorescent conjugated polymer. Chem. Commun. 2014, 50, 716–718. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.; Anandakathir, R.; Kumar, A.; Kumar, J.; Kurup, P.U. Sensitive and fast recognition of explosives using fluorescent polymer sensors and pattern recognition analysis. Sens. Actuators B Chem. 2011, 160, 1237–1243. [Google Scholar] [CrossRef]
- Dong, L.; Deng, C.; He, C.; Shi, L.; Fu, Y.; Zhu, D.; Chen, J. Highly sensitive vapor detection of amines with fluorescent conjugated polymer: A novel lasing turn-on sensory mechanism. Sens. Actuators B Chem. 2013, 180, 28–43. [Google Scholar] [CrossRef]
- Dong, W.; Fei, T.; Scherf, U. Conjugated polymers containing tetraphenylethylene in the backbones and side-chains for highly sensitive TNT detection. RSC Adv. 2018, 8, 5760–5767. [Google Scholar] [CrossRef]
- Trigo-López, M.; Muñoz, A.; Ibeas, S.; García, F.C.; Serna, F.; García, J.M. Solid sensory polymer kit for the easy and rapid determination of the concentration of water in organic solvents and ambient humidity. Sens. Actuators B Chem. 2014, 191, 233–238. [Google Scholar] [CrossRef]
- Toncelli, C.; Arzhakova, O.; Dolgova, A.; Volynskii, A.; Kerry, J.; Papkovsky, D. Phosphorescent oxygen sensors produced by spotcrazing of polyphenylenesulfide films. J. Mater. Chem. C 2014, 2, 8035–8041. [Google Scholar] [CrossRef]
- Waskitoaji, W.; Tsuyoshi, H.; Watanabe, M.; Nishide, H. Pt-porpholactone- and -porphyrin-based luminescent sensory polymer coating for visualization of oxygen pressure distribution on biplanar surface. React. Funct. Polym. 2010, 70, 669–673. [Google Scholar] [CrossRef]
- Hyakutake, T.; Ishigami, Y.; Kato, J.; Inukai, J.; Miyatake, K.; Nishide, H.; Watanabe, M. Luminescent sensory polymer coating composed of platinumporphyrin and poly(trimethylsilylpropyne) for real-time poly(trimethylsilylpropyne) oxygen visualization in operating PEFCs. Macromol. Chem. Phys. 2011, 212, 42–47. [Google Scholar] [CrossRef]
- Rezaei, B.; Boroujeni, M.K.; Ensafi, A.A. Fabrication of DNA, o-phenylenediamine, and gold nanoparticle bioimprinted polymer electrochemical sensor for the determination of dopamine. Biosens. Bioelectron. 2015, 66, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Wang, E.; Wang, J. Gas-breathing polymer film for constructing switchable ionic diodes. RSC Adv. 2015, 5, 35622–35630. [Google Scholar] [CrossRef]
- Kolodziejczyk, B.; Winther-Jensen, O.; Pereira, B.; Nair, S.; Winther-Jensen, B. Patterning of conducting layers on breathable substrates using laser engraving for gas sensors. J. Appl. Polym. Sci. 2015, 132, 42359–42361. [Google Scholar] [CrossRef]
- Huang, W.; Bender, M.; Seehafer, K.; Wacker, I.; Schröder, R.R.; Bunz, U.H.F. A tetraphenylethene-based polymer array discriminates nitroarenes. Macromolecules. 2018, 51, 1345–1350. [Google Scholar] [CrossRef]
- Lorwongtragool, P.; Sowade, E.; Watthanawisuth, N.; Baumann, R.; Kerdcharoen, T. A novel wearable electronic nose for healthcare based on flexible printed chemical sensor array. Sensors 2014, 14, 19700–19712. [Google Scholar] [CrossRef] [PubMed]
- Long, Z.; Lu, Y.; Zhang, M.; Qiu, H. Selective recognition and discrimination of water-soluble azo dyes by a seven-channel molecularly imprinted polymer sensor array. J. Sep. Sci. 2014, 37, 2764–2770. [Google Scholar] [CrossRef] [PubMed]
- Benz, M.; Patel, S.V. Freestanding chemiresistive polymer composite ribbons as high-flux sensors. J. Appl. Polym. Sci. 2012, 125, 3986–3995. [Google Scholar] [CrossRef]
- Stella, J.; Barisci, J.; Serra, G.; Wallace, G.; De Rossi, D. Characterization of olive oil by an electronic nose based on conducting polymers. Sens. Actuators B Chem. 2000, 63, 1–9. [Google Scholar] [CrossRef]
- Hossain, M.; Aminur Rahman, G.; Freund, M.; Jayas, D.; White, N.D.; Shafai, C.; Thomson, D.J. Fabrication and optimization of a conducting polymer sensor array using stored grain model volatiles. J. Agric. Food Chem. 2012, 60, 2863–2873. [Google Scholar] [CrossRef] [PubMed]
- Mirmohseni, A.; Hassanzadeh, V. Application of polymer-coated quartz crystal microbalance (QCM) as a sensor for BTEX compounds vapors. J. Appl. Polym. Sci. 2001, 79, 1062–1066. [Google Scholar] [CrossRef]
- Temel, F.; Tabakci, M. Calix[4]arene coated QCM sensors for detection of VOC emissions: Methylene chloride sensing studies. Talanta 2016, 153, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Pasupathy, P.; Trivedi, T.; Leonhardt, B.; Zhang, S.; Ekerdt, J.; Neikirk, D. Miniature passive wireless resonant platform for chemical memory-based threshold sensing. IEEE Sens. J. 2017, 17, 1209–1210. [Google Scholar] [CrossRef]
- Seyama, M.; Sugimoto, I.; Nakamura, M. Aroma sensing and indoor air monitoring by quartz crystal resonators with sensory films prepared by sputtering of biomaterials and sintered polymers. Biosens. Bioelectron. 2004, 20, 814–824. [Google Scholar] [CrossRef] [PubMed]
- Royal, M.; Jokerst, N.; Fair, R. Integrated sample preparation and sensing: Polymer microresonator sensors embedded in digital electrowetting microfluidic systems. IEEE Photonics J. 2012, 4, 2126–2135. [Google Scholar] [CrossRef]
- Matsumoto, K.; Tiu, K.; Kawamura, A.; Advincula, R.; Miyata, T. QCM sensing of bisphenol A using molecularly imprinted hydrogel/conducting polymer matrix. Polymer 2016, 48, 525–532. [Google Scholar] [CrossRef]
- Tokuyama, H.; Kitamura, E.; Seida, Y. Detection of Au(III) ions using a poly(N,N-dimethylacrylamide)-coated QCM sensor. Talanta 2016, 146, 507–509. [Google Scholar] [CrossRef] [PubMed]
- Eo, S.H.; Song, S.; Yoon, B.; Kim, J.M. A microfluidic conjugated-polymer sensor chip. Adv. Mater. 2008, 20, 1690–1694. [Google Scholar] [CrossRef]
- Funfak, A.; Hartung, R.; Cao, J.; Martin, K.; Wiesmüller, K.-H.; Wolfbeis, O.; Köhler, J. Highly resolved dose–response functions for drug-modulated bacteria cultivation obtained by fluorometric and photometric flow-through sensing in microsegmented flow. Sens. Actuators B Chem. 2009, 142, 66–72. [Google Scholar] [CrossRef]
- Chou, J.; Du, N.; Ou, T.; Floriano, P.; Christodoulides, N.; McDevitt, J. Hot embossed polyethylene through-hole chips for bead-based microfluidic devices. Biosens. Bioelectron. 2013, 42, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Coelho, M.; Giarola, J.; Da Silva, A.; Tarley, C.; Borges, K.; Pereira, A. Development and application of electrochemical sensor based on molecularly imprinted polymer and carbon nanotubes for the determination of carvedilol. Chemosensors 2016, 4, 22. [Google Scholar] [CrossRef]
- Zhiani, R.; Ghanei-Motlag, M.; Razavipanah, I. Selective voltammetric sensor for nanomolar detection of silver ions using carbon paste electrode modified with novel nanosized Ag(I)-imprinted polymer. J. Mol. Liq. 2016, 219, 554–560. [Google Scholar] [CrossRef]
- Khadem, M.; Faridbod, F.; Norouzi, P.; Foroushani, A.R.; Ganjali, M.R.; Shahtaheri, S.J.; Yarahmadi, R. Modification of carbon paste electrode based on molecularly imprinted polymer for electrochemical determination of diazinon in biological and environmental samples. Electroanalysis 2017, 29, 708–715. [Google Scholar] [CrossRef]
- Karimian, N.; Gholivand, M.; Malekzadeh, G. Cefixime detection by a novel electrochemical sensor based on glassy carbon electrode modified with surface imprinted polymer/multiwall carbon nanotubes. J. Electroanal. Chem. 2016, 771, 64–72. [Google Scholar] [CrossRef]
- Xu, M.; Song, Y.; Ye, Y.; Gong, C.; Shen, Y.; Wang, L. A novel flexible electrochemical glucose sensor based on gold nanoparticles/polyaniline arrays/carbon cloth electrode. Sens. Actuators B Chem. 2017, 252, 1187–1193. [Google Scholar] [CrossRef]
- Aziz, S.; Bum, K.; Yang, J.; Yang, B.-S.; Kang, C.; Doh, Y.; Kim, Y. Fabrication of ZnSnO3 based humidity sensor onto arbitrary substrates by micro-nano scale transfer printing. Sens. Actuators A Phys. 2016, 246, 1–8. [Google Scholar] [CrossRef]
- Klink, M.; Iwuoh, E.; Ebenso, E. The electro-catalytic and redox-mediator effects of nanostructured PDMA-PSA modified-electrodes as phenol derivative sensors. Int. J. Electrochem. Sci. 2011, 6, 2429–2442. [Google Scholar]
- Ong, P.-L.; Levitsky, I. Fluorescent gas sensors based on nanoporous optical resonators (microcavities) infiltrated with sensory emissive polymers. IEEE Sens. J. 2010, 11, 2947–2951. [Google Scholar] [CrossRef]
- Che, Y.; Gross, D.; Huang, H.; Yang, D.; Yang, X.; Discekici, E.; Zang, L. Diffusion-controlled detection of trinitrotoluene: Interior nanoporous structure and low highest occupied molecular orbital level of building blocks enhance selectivity and sensitivity. J. Am. Chem. Soc. 2012, 134, 4978–4982. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, H.; Ren, X. Ratiometric fluorescence and mesoporous structured imprinting nanoparticles for rapid and sensitive detection 2,4,6-trinitrophenol. Biosens. Bioelectron. 2017, 89, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, H. Selective dye adsorption and metal ion detection using multifunctional silsesquioxane-based tetraphenylethene-linked nanoporous polymers. J. Mater. Chem. A 2017, 5, 9156–9162. [Google Scholar] [CrossRef]
- Stofik, M.; Semerádtová, A.; Malý, J.; Kolska, Z.; Nedela, Z.; Wrobel, D.; Slepicka, P. Direct immobilization of biotin on the micro-patterned PEN foil treated by excimer laser. Colloids Surf. B 2015, 128, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Semerádtová, A.; Stofik, M.; Nedela, O.; Stanek, O.; Slepicka, P.; Kolska, Z.; Malý, J. A simple approach for fabrication of optical affinity-based bioanalytical microsystem on polymeric PEN foils. Colloids Surf. B 2018, 165, 28–36. [Google Scholar] [CrossRef] [PubMed]


| Type of Analyte | Target Species | References |
|---|---|---|
| Cations and anions | Hg2+ | [26,27,28,29,30,54,55,56,57,58,59,60,61,62,63] |
| Zn2+ | [64,65,66,67,68,69] | |
| Cu2+ | [20,24,70,71,72,73,74] | |
| Fe2+,Fe3+ | [57,73,75,76,77,78,79] | |
| Al3+ | [58,78,80,81] | |
| Cr3+,Cr6+ | [27,58,73,78] | |
| F− | [44,82,83] | |
| CN− | [45,46,74,84,85,86,87,88] | |
| Other cations | [19,31,32,72,79,89,90] | |
| Gases and Volatile Organic Compounds | NH3 | [35,91,92,93,94] |
| CO2 | [37,95,96] | |
| H2O | [38,97,98,99,100] | |
| VOCs | [17,18,22,23,36,101,102,103,104] | |
| Explosives and harmful substances | PETN 1/RDX 2 | [105] |
| TATP 3 | [106] | |
| DCP 4 | [107] | |
| Hydrazine | [40,108] | |
| DNB 5, DNT 6 | [21,109,110] | |
| TNT 7 | [41,42,43,105,110,111,112] | |
| Biomolecules | Drugs | [113,114,115,116,117] |
| Hormones | [51,118,119] | |
| Neurotransmitters | [120,121,122] | |
| Sugars/Saccharides | [50,122,123,124,125,126,127,128,129,130] | |
| Amino acids and proteins | [48,131,132,133,134,135] | |
| Metabolites | [47,136,137] | |
| Other biomolecules | [52,62,63,138,139] |
| Group of Sensory Devices | Type of Response | References |
|---|---|---|
| Polymer nanofibers | SAW | [163,164] |
| Electrical | [34,35,118,165,166,167] | |
| Optical | [17,18,19,168] | |
| Polymer films and coatings | Optical | [39,48,169,170,171,172,173,174,175,176] |
| Electrochemical | [115,177,178,179] | |
| Electrical | [93] | |
| Sensory polymeric arrays | Optical | [89,180,181,182] |
| Electrical | [183,184,185] | |
| Quartz Crystal Microbalances | Piezoelectric | [14,114,130,146,186,187,188,189,190,191,192] |
| Microfluidic devices | Optical | [27,128,150,193,194,195] |
| Electrical | [148,190] | |
| Modified electrodes | Electrochemical | [26,99,152,153,154,155,196,197,198,199,200,201,202] |
| Sensory chips | Optical | [161,193,194,195] |
| Micro and nanoporous materials | Optical | [111,203,204,205] |
| Electrochemical | [162,206] | |
| LIPSS and SERS effect | Optical | [207,208] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reglero Ruiz, J.A.; Sanjuán, A.M.; Vallejos, S.; García, F.C.; García, J.M. Smart Polymers in Micro and Nano Sensory Devices. Chemosensors 2018, 6, 12. https://doi.org/10.3390/chemosensors6020012
Reglero Ruiz JA, Sanjuán AM, Vallejos S, García FC, García JM. Smart Polymers in Micro and Nano Sensory Devices. Chemosensors. 2018; 6(2):12. https://doi.org/10.3390/chemosensors6020012
Chicago/Turabian StyleReglero Ruiz, José Antonio, Ana María Sanjuán, Saúl Vallejos, Félix Clemente García, and José Miguel García. 2018. "Smart Polymers in Micro and Nano Sensory Devices" Chemosensors 6, no. 2: 12. https://doi.org/10.3390/chemosensors6020012
APA StyleReglero Ruiz, J. A., Sanjuán, A. M., Vallejos, S., García, F. C., & García, J. M. (2018). Smart Polymers in Micro and Nano Sensory Devices. Chemosensors, 6(2), 12. https://doi.org/10.3390/chemosensors6020012