Film Carbon Veil-Based Electrode Modified with Triton X-100 for Nitrite Determination
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Instruments
2.3. Procedures
2.3.1. Phytosynthesis of Gold Nanoparticles (Phyto-Au)
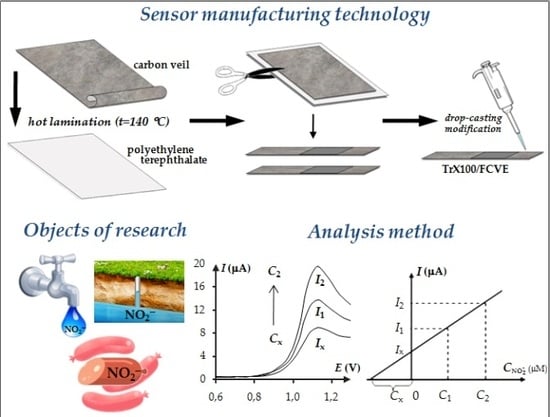
2.3.2. Manufacturing of the Sensor
2.3.3. Electrochemical Measurements
2.3.4. Sample Preparation
2.4. Statistical Analysis and Data Treatment
3. Results
3.1. Electrochemical Behavior of NO2− on FCVE and Modified FCVE
3.2. Choice of Surfactant for FCVE Modification
3.3. Characterization of the FCVE and the TrX100/FCVE
3.4. Effect of pH
3.5. Effect of Potential Scan Rate
3.6. Analytic Characteristics of TrX100/FCVE
3.7. Determination of Nitrite in Real Samples
3.7.1. Analysis of Sausage Products
3.7.2. Analysis of Water Samples
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Genualdi, S.; Matos, M.; Mangrum, J.; de Jager, L.S. Investigation into the concentration and sources of nitrates and nitrites in milk and plant-based powders. J. Agric. Food Chem. 2020, 68, 1725–1730. [Google Scholar] [CrossRef]
- Song, P.; Wu, L.; Guan, W. Dietary nitrates, nitrites, and nitrosamines intake and the risk of 400 gastric cancer: A meta-analysis. Nutrients 2015, 7, 9872–9895. [Google Scholar] [CrossRef]
- Barry, K.H.; Jones, R.R.; Cantor, K.P. Ingested nitrate and nitrite and bladder cancer in northern new England. Epidemiology 2020, 31, 136–144. [Google Scholar] [CrossRef]
- WHO. Nitrate and nitrite in drinking-water. In Background Document for Preparation of WHO Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- State Standard 33673-2015. Cooked sausages. In General Specifications; Izdatelstvo Standartinform: Moscow, Russian, 2016. [Google Scholar]
- Murray, E.; Roche, P.; Briet, M.; Moore, B.; Morrin, A.; Diamond, D.; Paull, B. Fully automated, low-cost ion chromatography system for in-situ analysis of nitrite and nitrate in natural waters. Talanta 2020, 216, 120955. [Google Scholar] [CrossRef]
- Lenghartova, K.; Lauko, L.; Cacho, F.; Beinrohr, E. Determination of nitrites in water by in-electrode coulometric titration in reticulated vitreous carbon electrode. Acta Chim. Slov. 2015, 62, 152–158. [Google Scholar] [CrossRef] [Green Version]
- He, Z.K.; Fuhrmann, B.; Spohn, U. Precise and sensitive determination of nitrite by coulometric back titration under flow conditions. Fresenius J. Anal. Chem. 2000, 367, 264–269. [Google Scholar] [CrossRef]
- Li, Y.-S.; Zhao, C.-L.; Li, B.-L.; Gao, X.-F. Evaluating nitrite content changes in some Chinese home cooking a newely-developed CDs diazotization spectrophotometry. Food Chem. 2020, 330, 127151. [Google Scholar] [CrossRef]
- Abou-Melha, K.S. Analytical chemistry optical chemosensor for spectrophotometric determination of nitrite in wastewater. Chem. Select. 2020, 5, 6216–6223. [Google Scholar] [CrossRef]
- Nithyayini, K.N.; Harish, M.N.K.; Nagashree, K.L. Electrochemical detection of nitrite at NiFe2O4 nanoparticles synthesized by solvent deficient method. Electrochim. Acta 2019, 317, 701–710. [Google Scholar] [CrossRef]
- Peng, Z.W.; Yuan, D.; Jiang, Z.W.; Li, Y.F. Novel metal-organic gels of bis(benzimidazole)-based ligands with copper(II) for electrochemical selectively sensing of nitrite. Electrochim. Acta 2017, 238, 1–8. [Google Scholar] [CrossRef]
- Yenil, N.; Yemiş, F. Nitrite in nature: Determination with polymeric materials. Pak. J. Anal. Environ. Chem. 2018, 19, 104–114. [Google Scholar] [CrossRef]
- Stozhko, N.Y.; Malakhova, N.A.; Byzov, I.V.; Brainina, K.Z. Electrodes in stripping voltammetry: From a macro- to a micro- and nano-structured surface. J. Anal. Chem. 2009, 64, 1148–1157. [Google Scholar] [CrossRef]
- Brainina, K.; Stozhko, N.; Bukharinova, M.; Vikulova, E. Nanomaterials: Electrochemical properties and application in sensors. Phys. Sci. Rev. 2019, 3, 8050. [Google Scholar]
- Su, C.-H.; Sun, C.-L.; Liao, Y.-C. Printed combinatorial sensors for simultaneous detection of ascorbic acid, uric acid, dopamine, and nitrite. ACS Omega 2017, 2, 4245–4252. [Google Scholar] [CrossRef] [Green Version]
- Jian, J.-M.; Fu, L.; Ji, J.; Lin, L.; Guo, X.; Ren, T.-L. Electrochemically reduced graphene oxide/gold nanoparticles composite modified screen-printed carbon electrode for effective electrocatalytic analysis of nitrite in foods. Sens. Actuators B 2018, 262, 125–136. [Google Scholar] [CrossRef]
- Talbi, M.; Al-Hamry, A.; Teixeira, P.R.; Bouhamed, A.; Azzouzi, S.; Paterno, L.G.; Kanoun, O. Graphite screen printed electrodes functionalized with AuNPs-PEI for nitrite detection. In Proceedings of the 16th International Multi-Conference on Systems, Signals & Devices (SSD), Istanbul, Turkey, 21–24 March 2019; pp. 607–610. [Google Scholar]
- Lo, N.-C.; Sun, I.-W.; Chen, P.-Y. CuAg nanoparticles formed in situ on electrochemically pre-anodized screen-printed carbon electrodes for the detection of nitrate and nitrite anions. J. Chin. Chem. Soc. 2018, 65, 928–988. [Google Scholar] [CrossRef]
- Jaiswal, N.; Tiwari, I.; Foster, C.W.; Banks, C.E. Highly sensitive amperometric sensing of nitrite utilizing bulk-modified MnO2 decorated graphene oxide nanocomposite screen-printed electrodes. Electrochim. Acta 2017, 227, 255–266. [Google Scholar] [CrossRef]
- Palanisamy, S.; Thirumalraj, B.; Chen, S.-M. A novel amperometric nitrite sensor based on screen printed carbon electrode modified with graphite/β-cyclodextrin composite. J. Electroanal. Chem. 2016, 760, 97–104. [Google Scholar] [CrossRef]
- Promsuwan, K.; Thavarungkul, P.; Kanatharana, P.; Limbut, W. Flow injection amperometric nitrite sensor based on silver microcubics-poly (acrylic acid)/poly (vinyl alcohol) modified screen printed carbon electrode. Electrochim. Acta 2017, 232, 357–369. [Google Scholar] [CrossRef]
- Zhe, T.; Sun, X.; Wang, Q.; Liu, Y.; Li, R.; Li, F.; Wang, L. A screen printed carbon electrode modified with a lamellar nanocomposite containing dendritic silver nanostructures, reduced graphene oxide, and β-cyclodextrin for voltammetric sensing of nitrite. Microchim. Acta 2019, 186, 319. [Google Scholar] [CrossRef]
- Kuntolaksono, S.; Matsuura, H. Coulometric analysis of nitrite using electrochemically activated carbon felt electrode. Sens. Mater. 2019, 31, 1215–1224. [Google Scholar] [CrossRef]
- Wei, W.; Wu, S.-G. Study of electrooxidation behavior of nitrite on gold nanoparticles/graphitizing carbon felt electrode and its analytical application. Chin. J. Anal. Chem. 2019, 47, e19014–e19020. [Google Scholar] [CrossRef]
- Zhu, W.; Zhang, Y.; Gong, J.; Ma, Y.; Sun, J.; Li, T.; Wang, J. Surface engineering of carbon fiber paper toward exceptionally high-performance and stable electrochemical nitrite sensing. ACS Sens. 2019, 4, 2980–2987. [Google Scholar] [CrossRef] [PubMed]
- Muthumariappan, A.; Govindasamy, M.; Chen, S.-M.; Sakthivel, K.; Mani, V. Screen-printed electrode modified with a composite prepared from graphene oxide nanosheets and Mn3O4microcubes for ultrasensitive determination of nitrite. Microchim. Acta 2017, 184, 3625–3634. [Google Scholar] [CrossRef]
- Wang, L.; Tricard, S.; Cao, L.; Liang, Y.; Zhao, J.; Fang, J.; Shen, W. Prussian blue/1-butyl-3-methylimidazolium tetrafluoroborate–graphite felt electrodes for efficient electrocatalytic determination of nitrite. Sens. Actuators B 2015, 214, 70–75. [Google Scholar] [CrossRef]
- Kunpatee, K.; Traipop, S.; Chailapakul, O.; Chuanuwatanakul, S. Simultaneous determination of ascorbic acid, dopamine and uric acid using grapheme quantum dots/ionic liquid modified screen-printed carbon electrode. Sens. Actuators B 2020, 314, 128059. [Google Scholar] [CrossRef]
- Pérez-Fernández, B.; Costa-García, A.; De La Escosura-Muñiz, A. Electrochemical (bio)sensors for pesticides detection using screen-printed electrodes. Biosensors 2020, 10, 32. [Google Scholar]
- Brainina, K.; Bukharinova, M.A.; Stozhko, N.Y.; Sokolkov, S.V.; Tarasov, A.V.; Vidrevich, M.B. Electrochemical sensor based on a carbon veil modified by phytosynthesized gold nanoparticles for determination of ascorbic acid. Sensors 2020, 20, 1800. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhu, W.; Wang, Y.; Ma, Y.; Sun, J.; Li, T.; Ji, Y. High-performance electrochemical nitrite sensing enabled by the commercial carbon fiber cloth. Inorg. Chem. Front. 2019, 6, 1501–1506. [Google Scholar] [CrossRef]
- Yatsunami, T.; Takase, S.; Shimizu, Y. Amperometric nitrite-ion sensor based on electrodeposited Sm-based perovskite-type oxide thick-film electrode. Sens. Mater. 2016, 28, 777–784. [Google Scholar]
- Brainina, K.; Stozhko, N.; Bukharinova, M.; Khamzina, E.; Vidrevich, M. Potentiometric method of plant microsuspensions antioxidant activity determination. Food. Chem. 2019, 278, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Stozhko, N.Y.; Bukharinova, M.A.; Khamzina, E.I.; Tarasov, A.V.; Vidrevich, M.B.; Brainina, K.Z. The effect of the antioxidant activity of plant extracts on the properties of gold nanoparticles. Nanomaterials 2019, 9, 1655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balasubramanian, P.; Settu, R.; Chen, S.-M.; Chen, T.-W.; Sharmila, G. A new electrochemical sensor for highly sensitive and selective detection of nitrite in food samples based on sonochemical synthesized Calcium Ferrite (CaFe2O4) clusters modified screen printed carbon electrode. J. Colloid Interface Sci. 2018, 524, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, G.; Oztekin, N.; Orbay, A.; Senkal, F. Voltammetric determination of nitrite in meat products using polyvinylimidazole modified carbone paste electrode. Food Chem. 2014, 152, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Burns, D.T.; Danzer, K.; Townshend, A. Use of the terms “recovery” and “apparent recovery” in analytical procedures (IUPAC Recommendations 2002). Pure Appl. Chem. 2003, 74, 2201–2205. [Google Scholar] [CrossRef]
- Chen, H.; Yang, T.; Liu, F.; Li, W. Electrodeposition of gold nanoparticles on Cu-based metal-organic framework for the electrochemical detection of nitrite. Sens. Actuators B 2018, 286, 401–407. [Google Scholar] [CrossRef]
- Pan, F.; Chen, D.; Zhuang, X.; Wu, X.; Luan, F.; Zhang, S.; Li, X. Fabrication of gold nanoparticles/l -cysteine functionalized graphene oxide nanocomposites and application for nitrite detection. J. Alloys Compd. 2018, 744, 51–56. [Google Scholar] [CrossRef]
- Brainina, K.Z.; Galperin, L.G.; Bukharinova, M.A.; Stozhko, N.Y. Mathematical modeling and experimental study of electrode processes. J. Solid State Electrochem. 2014, 19, 599–606. [Google Scholar] [CrossRef]
- Fotouhi, L.; Fatollahzadeh, M.; Heravi, M.M. Electrochemical behavior and voltammetric determination of sulfaguanidine at a glassy carbon electrode modified with a multi-walled carbon nanotube. Int. J. Electrochem. Sci. 2012, 7, 3919–3928. [Google Scholar]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; John Wiley & Sons Inc: Hoboken, NJ, USA, 2001. [Google Scholar]
- Afkhami, A.; Soltani-Felehgari, F.; Madrakian, T.; Ghaedi, H. Surface decoration of multi-walled carbone nanotubes modified carbone paste electrode with gold nanoparticles for electrooxidation and sensitive determination of nitrite. Biosens. Bioelectron. 2014, 51, 379–385. [Google Scholar] [CrossRef]
- Huang, X.; Li, Y.; Chen, Y.; Wang, L. Electrochemical determination of nitrite and iodate by use of gold nanoparticles/poly(3-methylthiophene) composites coated glassy carbon electrode. Sens. Actuators B 2008, 134, 780–786. [Google Scholar] [CrossRef]
- Pham, X.-H.; Li, C.A.; Han, K.N.; Huynh-Nguyen, B.-C.; Le, T.-H.; Ko, E.; Kim, J.H.; Seong, G.H. Electrochemical detection of nitrite using urchin-like palladium nanostructures on carbon nanotube thin film electrodes. Sens. Actuators B 2014, 193, 815–822. [Google Scholar] [CrossRef]
- Fu, L.; Yu, S.; Thompson, L.; Yu, A. Development of a novel nitrite electrochemical sensor by stepwise in situ formation of palladium and reduced graphene oxide nanocomposites. RSC Adv. 2015, 5, 40111–40116. [Google Scholar] [CrossRef]
- Gowda, J.I.; Nandibewoor, S.T. Electrochemical behavior of paclitaxel and its determination at glassy carbon electrode. Asian J. Pharm. Sci. 2014, 9, 42–49. [Google Scholar] [CrossRef] [Green Version]
- Heli, H.; Eskandari, I.; Sattarahmady, N.; Moosavi-Movahedi, A.A. Cobalt nanoflowers: Synthesis, characterization and derivatization to cobalt hexacyanoferrate—Electrocatalytic oxidation and determination of sulfite and nitrite. Electrochim. Acta 2012, 77, 294–301. [Google Scholar] [CrossRef]
- Zhang, S.; Li, B.Q.; Zheng, J.B. An electrochemical sensor for the sensitive determination of nitrites based on Pt-PANI-graphenenanocomposites. Anal. Methods 2015, 7, 8366–8372. [Google Scholar] [CrossRef]
- Balasubramanian, P.; Velmurugan, M.; Chen, S.-M.; Chen, T.-W.; Ye, Y.-T. A single-step electrochemical preparation of cadmium sulfide anchored ERGO/β-CD modified screen-printed carbon electrode for sensitive and selective detection of nitrite. J. Electrochem. Soc. 2019, 166, B690–B696. [Google Scholar] [CrossRef]
- Caetano, L.P.; Lima, A.P.; Tormin, T.F.; Richter, E.M.; Espindola, F.S.; Botelho, F.V.; Munoz, R.A.A. Carbon-nanotube modified screen-printed electrode for the simultaneous determination of nitrite and uric acid in biological fluids using batch-injection amperometric detection. Electroanalysis 2018, 30, 1862–1871. [Google Scholar] [CrossRef]
- Koyun, O.; Sahin, Y. Voltammetric determination of nitrite with gold nanoparticles/poly(methylene blue)-modified pencil graphite electrode: Application in food and water samples. Ionics 2018, 24, 3187–3197. [Google Scholar] [CrossRef]
- Monteiro, T.; Rodrigues, P.R.; Gonçalves, A.L.; Moura, J.J.G.; Jubete, E.; Añorga, L.; Almeida, M.G. Construction of effective disposable biosensors for point of care testing of nitrite. Talanta 2015, 142, 246–251. [Google Scholar] [CrossRef] [Green Version]
- State Standard 29299-92. Meat and meat products. In Determination of Nitrite Content; Izdatelstvo Standartov: Moscow, Russia, 2003. [Google Scholar]
- State Standard 33045-2014. Water. In Methods for Determination of Nitrogen-Containing Matters; Izdatelstvo Standartinform: Moscow, Russia, 2019. [Google Scholar]











| Interfering Substance | Concentration of Interfering Substance, μM | Response Change, % |
|---|---|---|
| Glucose | 1000 | 0.0 |
| Cl− | 1000 | 0.0 |
| SO42− | 1000 | 0.0 |
| Fe(III) | 1000 | 0.0 |
| CH3COO− | 500 | 0.0 |
| Co(II) | 425 | −3.1 |
| Ni(II) | 150 | −4.5 |
| Cu(II) | 150 | −3.3 |
| BrO3− | 110 | −2.3 |
| Citric acid | 50 | −2.9 |
| Ascorbic acid | 50 | −0.5 |
| Sensor * | Limit of Detection, μM | Linear Range, μM | Technique ** | Sample | Ref. |
|---|---|---|---|---|---|
| GO/PEDOT:PSS/SPCE | 0.018 | 0.05–16.55 | Am | background solution | [16] |
| rGO/AuNPs/SPCE | 0.13 | 1–6000 | DPV, CV | purified water, packaged mineral water, dried shrimps, cured/salted fish, sausage | [17] |
| AuNPs-PEI/GSPE | 1.0 | 1–10 | DPV | background solution | [18] |
| CuAgNPs/SPCEanodized | 11.1/15.6 | 20–370 | CV | tap water, river water deionized water | [19] |
| MnO2/GO-SPCE | 0.09 | 0.1–1 1–1000 | Am | tap water, packaged water | [20] |
| GR+ β-CD/SPCE | 0.26 | 0.7–2150 | Am | drinking water tap water | [21] |
| AgMCs-PAA/PVA/SPCE | 4.45 | 2–800 | FI-Am | ham, bacon, fermented pork, and sausage | [22] |
| Ag/rGO/β-CD/SPCE | 0.24 | 1–2000 | LSV | spiked pickles | [23] |
| EACFE | 1.0 | 1.0–1000 | Cm | background solution | [24] |
| AuNPs/GCFE | 0.95 | 1.0–3350 | CV | mustard | [25] |
| ACFPE | 0.07 | 0.1–3838.5 | Am | mineral water, sausage | [26] |
| PB/[Bmim][BF4]-GFE | 0.013 | 1.0–8.0 | CV | tap water | [28] |
| CFE | 0.03 | 0.25–3838.5 | Am | mineral water, sausage | [32] |
| SmFeO3/CFE | 50 | 50–1000 | Am | background solution | [33] |
| rGO/β-CD/CdS/SPCE | 0.021 | 0.05–447 | Am | tap water and river water | [51] |
| MWCNT/SPE | 0.02 | 1–500 | DPV, Am | saliva, urine, and blood samples | [52] |
| AuNPs/PMB/PGE | 0.314 | 5–5000 | DPV | sausage, mineral water | [53] |
| ccNiR + carbon ink/SPE | 1.2 | 0.7–370 | CV | drinking water, tap water, milk, urine, plasma | [54] |
| TrX100/FCVE | 0.01 | 0.1–0.9 0.9–100 | LSV | sausage products, water | this paper |
| Sample | Found in Extract, μM | Added, μM | Found in Extract with Additive, μM | Found Additive, μM | R, % |
|---|---|---|---|---|---|
| Sausages | 24 ± 2 | 20 | 43 ± 1 | 19 ± 1 | 93 |
| “Doctorskaya” sausage | 88 ± 1 | 80 | 163 ± 1 | 76 ± 5 | 95 |
| “Molochnaya” sausage | 228 ± 6 | 300 | 512 ± 19 | 284 ± 17 | 95 |
| Sample | Voltammetry with TrX100/FCVE, μM | RSD, % | Spectrophotometry, μM | RSD, % | F-Test | t-Test |
|---|---|---|---|---|---|---|
| Sausages | 24 ± 2 | 2.6 | 23 ± 1 | 1.5 | 3.21 | 2.29 |
| “Doctorskaya” sausage | 88 ± 1 | 0.5 | 87 ± 2 | 0.8 | 3.45 | 0.08 |
| “Molochnaya” sausage | 228 ± 6 | 1.2 | 239 ± 11 | 1.9 | 3.02 | 2.43 |
| Water Sample | Added, μM | Found, μM | RSD,% | R,% |
|---|---|---|---|---|
| Sample 1 | 0.99 | 1.02 ± 0.06 | 2.3 | 103 |
| Sample 2 | 4.95 | 4.87 ± 0.31 | 1.9 | 98 |
| Sample 3 | 10.0 | 9.8 ± 0.2 | 1.1 | 99 |
| Water Sample | Voltammetry with TrX100/FCVE, μM | RSD,% | Spectrophotometry, μM | RSD,% | F-test | t-test |
|---|---|---|---|---|---|---|
| Sample 1 | 1.02 ± 0.06 | 2.3 | 0.96 ± 0.12 | 4.9 | 4.19 | 1.32 |
| Sample 2 | 4.87 ± 0.31 | 1.9 | 5.15 ± 0.34 | 2.7 | 2.18 | 1.90 |
| Sample 3 | 9.8 ± 0.2 | 1.1 | 9.3 ± 0.5 | 2.2 | 4.33 | 2.67 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stozhko, N.Y.; Bukharinova, M.A.; Khamzina, E.I.; Tarasov, A.V.; Sokolkov, S.V. Film Carbon Veil-Based Electrode Modified with Triton X-100 for Nitrite Determination. Chemosensors 2020, 8, 78. https://doi.org/10.3390/chemosensors8030078
Stozhko NY, Bukharinova MA, Khamzina EI, Tarasov AV, Sokolkov SV. Film Carbon Veil-Based Electrode Modified with Triton X-100 for Nitrite Determination. Chemosensors. 2020; 8(3):78. https://doi.org/10.3390/chemosensors8030078
Chicago/Turabian StyleStozhko, Natalia Yu., Maria A. Bukharinova, Ekaterina I. Khamzina, Aleksey V. Tarasov, and Sergey V. Sokolkov. 2020. "Film Carbon Veil-Based Electrode Modified with Triton X-100 for Nitrite Determination" Chemosensors 8, no. 3: 78. https://doi.org/10.3390/chemosensors8030078
APA StyleStozhko, N. Y., Bukharinova, M. A., Khamzina, E. I., Tarasov, A. V., & Sokolkov, S. V. (2020). Film Carbon Veil-Based Electrode Modified with Triton X-100 for Nitrite Determination. Chemosensors, 8(3), 78. https://doi.org/10.3390/chemosensors8030078