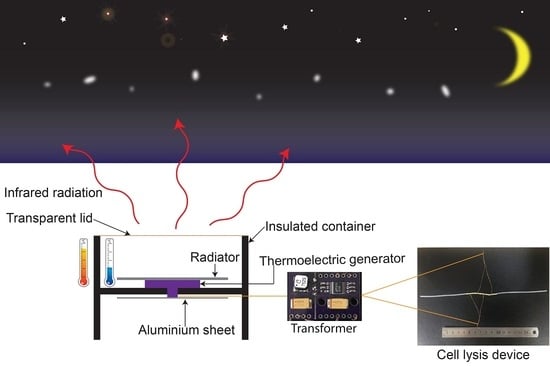
Off-Grid Electrical Cell Lysis Microfluidic Device Utilizing Thermoelectricity and Thermal Radiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Cell Culture and Harvesting
2.2.2. Fabrication of Cell Electrical Lysis Device
2.2.3. Fabrication of Radiator
2.2.4. Fourier-Transform Infrared Spectroscopy (FT-IR) Analysis
2.2.5. Setup and Measurement of Thermoelectric Generation
2.2.6. Electrical Cell Lysis
3. Results and Discussion
3.1. IR Emissivity of Cellulose-Based Radiator
3.2. Electrical Cell Lysis Efficiency of Microfluidic Device
3.2.1. Indoor Conditions
3.2.2. Outdoor Conditions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Lindström, S.; Andersson-Svahn, H. Overview of single-cell analyses: Microdevices and applications. Lab Chip 2010, 10, 3363–3372. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhao, Z.; Rahim, N.A.A.; van Noort, D.; Yu, H. Towards a human-on-chip: Culturing multiple cell types on a chip with compartmentalized microenvironments. Lab Chip 2009, 9, 3185–3192. [Google Scholar] [CrossRef] [PubMed]
- Sontheimer-Phelps, A.; Hassell, B.A.; Ingber, D.E. Modelling cancer in microfluidic human organs-on-chips. Nat. Rev. Cancer 2019, 19, 65–81. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zheng, J.; Liu, L.; Xu, L. Application of Microfluidics in Wearable Devices. Small Methods 2019, 3, 1900688. [Google Scholar] [CrossRef]
- Lu, K.-Y.; Wo, A.M.; Lo, Y.-J.; Chen, K.-C.; Lin, C.-M.; Yang, C.-R. Three dimensional electrode array for cell lysis via electroporation. Biosens. Bioelectron. 2006, 22, 568–574. [Google Scholar] [CrossRef]
- Mohammed, M.I.; Haswell, S.; Gibson, I. Lab-on-a-chip or Chip-in-a-lab: Challenges of Commercialization Lost in Translation. Procedia Technol. 2015, 20, 54–59. [Google Scholar] [CrossRef] [Green Version]
- Shen, W.; Chen, X.; Qiu, J.; Hayward, J.A.; Sayeef, S.; Osman, P.; Meng, K.; Dong, Z.Y. A comprehensive review of variable renewable energy levelized cost of electricity. Renew. Sustain. Energy Rev. 2020, 133, 110301. [Google Scholar] [CrossRef]
- Jaziri, N.; Boughamoura, A.; Müller, J.; Mezghani, B.; Tounsi, F.; Ismail, M. A comprehensive review of Thermoelectric Generators: Technologies and common applications. Energy Rep. 2020, 6, 264–287. [Google Scholar] [CrossRef]
- Chen, X.; Cui, D.; Cai, H.; Li, H.; Sun, J.; Zhang, L. MEMS-based microdevice for cell lysis and DNA extraction. Microelectromechanical Systems Devices. 2012, pp. 23–39. Available online: https://www.researchgate.net/publication/221920990_MEMS-Based_Microdevice_for_Cell_Lysis_and_DNA_Extraction (accessed on 14 September 2021).
- He, W.; Zhang, G.; Zhang, X.; Ji, J.; Li, G.; Zhao, X. Recent development and application of thermoelectric generator and cooler. Appl. Energy 2015, 143, 1–25. [Google Scholar] [CrossRef]
- Kim, S.J.; We, J.H.; Cho, B.J. A wearable thermoelectric generator fabricated on a glass fabric. Energy Environ. Sci. 2014, 7, 1959–1965. [Google Scholar] [CrossRef]
- Kim, M.-K.; Kim, M.-S.; Lee, S.; Kim, C.; Kim, Y.-J. Wearable thermoelectric generator for harvesting human body heat energy. Smart Mater. Struct. 2014, 23, 105002. [Google Scholar] [CrossRef]
- Kim, S.K.; Kim, J.H.; Kim, K.P.; Chung, T.D. Continuous low-voltage dc electroporation on a microfluidic chip with polyelectrolytic salt bridges. Anal. Chem. 2007, 79, 7761–7766. [Google Scholar] [CrossRef]
- Ren, W.; Sun, Y.; Zhao, D.; Aili, A.; Zhang, S.; Shi, C.; Zhang, J.; Geng, H.; Zhang, J.; Zhang, L. High-performance wearable thermoelectric generator with self-healing, recycling, and Lego-like reconfiguring capabilities. Sci. Adv. 2021, 7, eabe0586. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Hu, J. A novel design for a wearable thermoelectric generator based on 3D fabric structure. Smart Mater. Struct. 2017, 26, 045037. [Google Scholar] [CrossRef]
- Yan, J.; Liao, X.; Yan, D.; Chen, Y. Review of Micro Thermoelectric Generator. J. Microelectromechanical Syst. 2018, 27, 1–18. [Google Scholar] [CrossRef]
- Stordeur, M.; Stark, I. Low power thermoelectric generator-self-sufficient energy supply for micro systems. In Proceedings of theXVI ICT '97. Proceedings ICT'97. 16th International Conference on Thermoelectrics (Cat. No.97TH8291), Dresden, Germany, 26–29 August 1997; pp. 575–577. [Google Scholar]
- Lu, X.; Xu, P.; Wang, H.; Yang, T.; Hou, J. Cooling potential and applications prospects of passive radiative cooling in buildings: The current state-of-the-art. Renew. Sustain. Energy Rev. 2016, 65, 1079–1097. [Google Scholar] [CrossRef]
- Perrakis, G.; Tasolamprou, A.C.; Kenanakis, G.; Economou, E.N.; Tzortzakis, S.; Kafesaki, M. Passive radiative cooling and other photonic approaches for the temperature control of photovoltaics: A comparative study for crystalline silicon-based architectures. Opt. Express 2020, 28, 18548–18565. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, J.; Fu, B.; Song, C.; Shang, W.; Tao, P.; Deng, T. All-Day Freshwater Harvesting through Combined Solar-Driven Interfacial Desalination and Passive Radiative Cooling. ACS Appl. Mater. Interfaces 2020, 12, 47612–47622. [Google Scholar] [CrossRef]
- Zhao, B.; Hu, M.; Ao, X.; Chen, N.; Pei, G. Radiative cooling: A review of fundamentals, materials, applications, and prospects. Appl. Energy 2019, 236, 489–513. [Google Scholar] [CrossRef]
- Xu, D.; Zheng, G.; Xian, F.; Wang, S.; Hua, X. Ultra-broadband and angle-insensitive perfect absorber in the mid-infrared atmospheric window. Optik 2021, 245, 167633. [Google Scholar] [CrossRef]
- Raman, A.P.; Anoma, M.A.; Zhu, L.; Rephaeli, E.; Fan, S. Passive radiative cooling below ambient air temperature under direct sunlight. Nature 2014, 515, 540–544. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, Z.; Zhang, J.; Feng, W.; Zuo, J. Advances and challenges in commercializing radiative cooling. Mater. Today Phys. 2019, 11, 100161. [Google Scholar] [CrossRef]
- Zhu, L.; Raman, A.P.; Fan, S. Radiative cooling of solar absorbers using a visibly transparent photonic crystal thermal blackbody. Proc. Natl. Acad. Sci. USA 2015, 112, 12282–12287. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Zhu, L.; Raman, A.; Fan, S. Radiative cooling to deep sub-freezing temperatures through a 24-h day–night cycle. Nat. Commun. 2016, 7, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Tso, C.Y.; Chan, K.C.; Chao, C.Y.H. A field investigation of passive radiative cooling under Hong Kong’s climate. Renew. Energy 2017, 106, 52–61. [Google Scholar] [CrossRef]
- Orel, B.; Gunde, M.K.; Krainer, A. Radiative cooling efficiency of white pigmented paints. Sol. Energy 1993, 50, 477–482. [Google Scholar] [CrossRef]
- Granqvist, C.; Hjortsberg, A.; Eriksson, T. Radiative cooling to low temperatures with selectivity IR-emitting surfaces. Thin Solid Film. 1982, 90, 187–190. [Google Scholar] [CrossRef]
- Hossain, M.M.; Jia, B.; Gu, M. A metamaterial emitter for highly efficient radiative cooling. Adv. Opt. Mater. 2015, 3, 1047–1051. [Google Scholar] [CrossRef]
- Bathgate, S.N.; Bosi, S.G. A robust convection cover material for selective radiative cooling applications. Sol. Energy Mater. Sol. Cells 2011, 95, 2778–2785. [Google Scholar] [CrossRef]
- Li, Y.; Su, L.; Xu, X.; Zhang, C.; Wang, B. Energy conversion within infrared plasmonic absorption metamaterials for multi-band resonance. Opt. Commun. 2015, 342, 247–252. [Google Scholar] [CrossRef]
- El-Kady, I.; Farfan, G.; Rammohan, R.; Taha, M.R. Photonic crystal high-efficiency multispectral thermal emitters. Appl. Phys. Lett. 2008, 93, 153501. [Google Scholar] [CrossRef]
- Cui, Y.; He, Y.; Jin, Y.; Ding, F.; Yang, L.; Ye, Y.; Zhong, S.; Lin, Y.; He, S. Plasmonic and metamaterial structures as electromagnetic absorbers. Laser Photonics Rev. 2014, 8, 495–520. [Google Scholar] [CrossRef] [Green Version]
- Hervé, A.; Drévillon, J.; Ezzahri, Y.; Joulain, K. Radiative cooling by tailoring surfaces with microstructures: Association of a grating and a multi-layer structure. J. Quant. Spectrosc. Radiat. Transf. 2018, 221, 155–163. [Google Scholar] [CrossRef]
- Bao, H.; Yan, C.; Wang, B.; Fang, X.; Zhao, C.; Ruan, X. Double-layer nanoparticle-based coatings for efficient terrestrial radiative cooling. Sol. Energy Mater. Sol. Cells 2017, 168, 78–84. [Google Scholar] [CrossRef]
- Kecebas, M.A.; Menguc, M.P.; Kosar, A.; Sendur, K. Passive radiative cooling design with broadband optical thin-film filters. J. Quant. Spectrosc. Radiat. Transf. 2017, 198, 179–186. [Google Scholar] [CrossRef]
- Xiang, B.; Zhang, R.; Luo, Y.; Zhang, S.; Xu, L.; Min, H.; Tang, S.; Meng, X. 3D porous polymer film with designed pore architecture and auto-deposited SiO2 for highly efficient passive radiative cooling. Nano Energy 2021, 81, 105600. [Google Scholar] [CrossRef]
- McNerney, R. Diagnostics for Developing Countries. Diagnostics 2015, 5, 200–209. [Google Scholar] [CrossRef]
- Hanna, S.E.; Connor, C.J.; Wang, H.H. Real-time Polymerase Chain Reaction for the Food Microbiologist: Technologies, Applications, and Limitations. J. Food Sci. 2005, 70, R49–R53. [Google Scholar] [CrossRef]
- Shehadul Islam, M.; Aryasomayajula, A.; Selvaganapathy, P.R. A review on macroscale and microscale cell lysis methods. Micromachines 2017, 8, 83. [Google Scholar] [CrossRef]
- Won, E.-J.; Thai, D.A.; Duong, D.D.; Lee, N.Y.; Song, Y.-J. Microfluidic electrical cell lysis for high-throughput and continuous production of cell-free varicella-zoster virus. J. Biotechnol. 2021, 335, 19–26. [Google Scholar] [CrossRef]
- Nan, L.; Jiang, Z.; Wei, X. Emerging microfluidic devices for cell lysis: A review. Lab A Chip 2014, 14, 1060–1073. [Google Scholar] [CrossRef] [PubMed]
- Tsong, T.Y. Electroporation of Cell Membranes. In Electroporation and Electrofusion in Cell Biology; Neumann, E., Sowers, A.E., Jordan, C.A., Eds.; Springer US: Boston, MA, USA, 1989; pp. 149–163. [Google Scholar]
- Weaver, J.C. Electroporation theory. In Plant Cell Electroporation and Electrofusion Protocols; Springer: Berlin/Heidelberg, Germany, 1995; pp. 3–28. [Google Scholar]
- Jordan, C.A.; Neumann, E.; Sowers, A.E. Electroporation and Electrofusion in Cell Biology; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Ho, S.; Mittal, G.S. Electroporation of cell membranes: A review. Crit. Rev. Biotechnol. 1996, 16, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Olofsson, J.; Levin, M.; Stromberg, A.; Weber, S.G.; Ryttsen, F.; Orwar, O. Generation of focused electric field patterns at dielectric surfaces. Anal. Chem. 2005, 77, 4667–4672. [Google Scholar] [CrossRef] [Green Version]
- Shin, Y.S.; Cho, K.; Kim, J.K.; Lim, S.H.; Park, C.H.; Lee, K.B.; Park, Y.; Chung, C.; Han, D.-C.; Chang, J.K. Electrotransfection of Mammalian Cells Using Microchannel-Type Electroporation Chip. Anal. Chem. 2004, 76, 7045–7052. [Google Scholar] [CrossRef]
- Geng, T.; Zhan, Y.; Wang, H.-Y.; Witting, S.R.; Cornetta, K.G.; Lu, C. Flow-through electroporation based on constant voltage for large-volume transfection of cells. J. Control. Release 2010, 144, 91–100. [Google Scholar] [CrossRef]
- Hossain, M.M.; Gu, M. Radiative Cooling: Principles, Progress, and Potentials. Adv. Sci. 2016, 3, 1500360. [Google Scholar] [CrossRef]
- Chen, Y.; Dang, B.; Fu, J.; Wang, C.; Li, C.; Sun, Q.; Li, H. Cellulose-Based Hybrid Structural Material for Radiative Cooling. Nano Lett. 2021, 21, 397–404. [Google Scholar] [CrossRef]
- Li, T.; Zhai, Y.; He, S.; Gan, W.; Wei, Z.; Heidarinejad, M.; Dalgo, D.; Mi, R.; Zhao, X.; Song, J.; et al. A radiative cooling structural material. Science 2019, 364, 760–763. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Xu, J.-J.; Liu, Y.; Chen, H.-Y. In-situ synthesis of poly (dimethylsiloxane)–gold nanoparticles composite films and its application in microfluidic systems. Lab Chip 2008, 8, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Feifel, S.C.; Lisdat, F. Silica nanoparticles for the layer-by-layer assembly of fully electro-active cytochrome c multilayers. J. Nanobiotechnology 2011, 9, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, D.; Liu, C.; Xu, Z.; Liu, Y.; Yu, Z.; Yu, L.; Chen, L.; Li, R.; Ma, R.; Ye, H. The design of ultra-broadband selective near-perfect absorber based on photonic structures to achieve near-ideal daytime radiative cooling. Mater. Des. 2018, 139. [Google Scholar] [CrossRef]
- Jeon, S.; Son, S.; Lee, S.Y.; Chae, D.; Bae, J.H.; Lee, H.; Oh, S.J. Multifunctional Daytime Radiative Cooling Devices with Simultaneous Light-Emitting and Radiative Cooling Functional Layers. ACS Appl. Mater. Interfaces 2020, 12, 54763–54772. [Google Scholar] [CrossRef] [PubMed]
- Ko, B.; Lee, D.; Badloe, T.; Rho, J. Metamaterial-Based Radiative Cooling: Towards Energy-Free All-Day Cooling. Energies 2019, 12, 89. [Google Scholar] [CrossRef] [Green Version]
- Vall, S.; Castell, A. Radiative cooling as low-grade energy source: A literature review. Renew. Sustain. Energy Rev. 2017, 77, 803–820. [Google Scholar] [CrossRef] [Green Version]
- Zhao, B.; Ao, X.; Chen, N.; Xuan, Q.; Hu, M.; Pei, G. General strategy of passive sub-ambient daytime radiative cooling. Sol. Energy Mater. Sol. Cells 2019, 199, 108–113. [Google Scholar] [CrossRef]
- Di Carlo, D.; Jeong, K.H.; Lee, L.P. Reagentless mechanical cell lysis by nanoscale barbs in microchannels for sample preparation. Lab Chip 2003, 3, 287–291. [Google Scholar] [CrossRef]
- Lo, Y.-J.; Lei, U. A Continuous Flow-through Microfluidic Device for Electrical Lysis of Cells. Micromachines 2019, 10, 247. [Google Scholar] [CrossRef] [PubMed] [Green Version]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duong, D.-D.; Lee, N.-Y. Off-Grid Electrical Cell Lysis Microfluidic Device Utilizing Thermoelectricity and Thermal Radiation. Chemosensors 2021, 9, 292. https://doi.org/10.3390/chemosensors9100292
Duong D-D, Lee N-Y. Off-Grid Electrical Cell Lysis Microfluidic Device Utilizing Thermoelectricity and Thermal Radiation. Chemosensors. 2021; 9(10):292. https://doi.org/10.3390/chemosensors9100292
Chicago/Turabian StyleDuong, Duong-Duy, and Nae-Yoon Lee. 2021. "Off-Grid Electrical Cell Lysis Microfluidic Device Utilizing Thermoelectricity and Thermal Radiation" Chemosensors 9, no. 10: 292. https://doi.org/10.3390/chemosensors9100292
APA StyleDuong, D. -D., & Lee, N. -Y. (2021). Off-Grid Electrical Cell Lysis Microfluidic Device Utilizing Thermoelectricity and Thermal Radiation. Chemosensors, 9(10), 292. https://doi.org/10.3390/chemosensors9100292