A Paper-Based Ultrasensitive Optical Sensor for the Selective Detection of H2S Vapors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis
2.2.1. Preparation of 7-Nitrobenzo[c][1,2,5]Oxadiazole-4-Thiol (NBD-SH)
2.2.2. Preparation of (bis(7-Nitrobenzo[c][1,2,5]Oxadiazol-4-yl)Sulfane (NBD)2S
2.2.3. Sensor Preparation
2.3. Sensing Experiments and Instrumentation
2.3.1. Mass Spectrometry
2.3.2. Spectroscopy
2.3.3. Exposure of (NBD)2S in Solution to Gaseous H2S
2.3.4. Exposure of the Paper Sensor to Gaseous H2S
2.3.5. Camera-Based Color Recognition
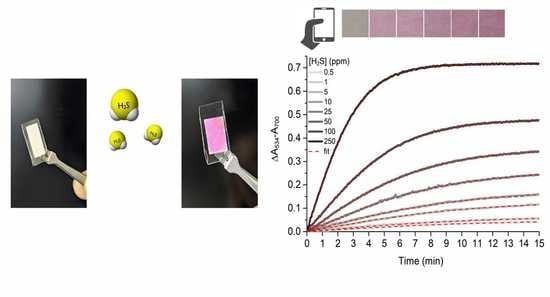
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Dorman, D.C.; Brenneman, K.A.; Struve, M.F.; Miller, K.L.; James, R.A.; Marshall, M.W.; Foster, P.M.D. Fertility and developmental neurotoxicity effects of inhaled hydrogen sulfide in Sprague-Dawley rats. Neurotoxicol. Teratol. 2000, 22, 71–84. [Google Scholar] [CrossRef]
- Struve, M.F.; Brisbois, J.N.; Arden James, R.; Marshall, M.W.; Dorman, D.C. Neurotoxicological effects associated with short-term exposure of Sprague—Dawley rats to hydrogen sulfide. Neurotoxicology 2001, 22, 375–385. [Google Scholar] [CrossRef]
- Lindenmann, J.; Matzi, V.; Neuboeck, N.; Ratzenhofer-Komenda, B.; Maier, A.; Smolle-Juettner, F.-M. Severe hydrogen sulphide poisoning treated with 4-dimethylaminophenol and hyperbaric oxygen. Diving Hyperb. Med. 2010, 40, 213–217. [Google Scholar] [PubMed]
- Beauchamp, R.O.; Bus, J.S.; Popp, J.A.; Boreiko, C.J.; Andjelkovich, D.A.; Leber, P. A critical review of the literature on hydrogen sulfide toxicity. Crit. Rev. Toxicol. 1984, 13, 25–97. [Google Scholar] [CrossRef]
- OSHA Hydrogen Sulfide Hazards. Available online: https://www.osha.gov/SLTC/hydrogensulfide/hazards.html (accessed on 3 July 2020).
- Powers, W. The Science of Smell Part 1: Odor Perception and Physiological Response; Iowa State University Extension: Ames, IA, USA, 2004. [Google Scholar]
- Wang, R. Physiological implications of hydrogen sulfide: A whiff exploration that blossomed. Physiol. Rev. 2012, 92, 791–896. [Google Scholar] [CrossRef] [Green Version]
- Montoya, L.A.; Shen, X.; McDermott, J.J.; Kevil, C.G.; Pluth, M.D. Mechanistic investigations reveal that dibromobimane extrudes sulfur from biological sulfhydryl sources other than hydrogen sulfide. Chem. Sci. 2015, 6, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H.; Shibuya, N.; Kimura, Y. Hydrogen sulfide is a signaling molecule and a cytoprotectant. Antioxid. Redox Signal. 2012, 17, 45–57. [Google Scholar] [CrossRef] [Green Version]
- Olson, K.R. A practical look at the chemistry and biology of hydrogen sulfide. Antioxid. Redox Signal. 2012, 17, 32–44. [Google Scholar] [CrossRef] [Green Version]
- Khalid, T.Y.; Saad, S.; Greenman, J.; De Lacy Costello, B.; Probert, C.S.J.; Ratcliffe, N.M. Volatiles from oral anaerobes confounding breath biomarker discovery. J. Breath Res. 2013, 7. [Google Scholar] [CrossRef] [Green Version]
- Jiménez, D.; Martínez-Máñez, R.; Sancenón, F.; Ros-Lis, J.V.; Benito, A.; Soto, J. A new chromo-chemodosimeter selective for sulfide anion. J. Am. Chem. Soc. 2003, 125, 9000–9001. [Google Scholar] [CrossRef] [PubMed]
- Lei, W.; Dasgupta, P.K. Determination of sulfide and mercaptans in caustic scrubbing liquor. Anal. Chim. Acta 1989, 226, 165–170. [Google Scholar] [CrossRef]
- Searcy, D.G.; Peterson, M.A. Hydrogen sulfide consumption measured at low steady state concentrations using a sulfidostat. Anal. Biochem. 2004, 324, 269–275. [Google Scholar] [CrossRef]
- Lawrence, N.S.; Davis, J.; Jiang, L.; Jones, T.G.J.; Davies, S.N.; Compton, R.G. The electrochemical analog of the methylene blue reaction: A novel amperometric approach to the detection of hydrogen sulfide. Electroanalysis 2000, 12, 1453–1460. [Google Scholar] [CrossRef]
- Radford-Knoery, J.; Cutter, G.A. Determination of Carbonyl Sulfide and Hydrogen Sulfide Species in Natural Waters Using Specialized Collection Procedures and Gas Chromatography with Flame Photometric Detection. Anal. Chem. 1993, 65, 976–982. [Google Scholar] [CrossRef]
- Pandey, S.K.; Kim, K.-H.; Tang, K.-T. A review of sensor-based methods for monitoring hydrogen sulfide. TrAC Trends Anal. Chem. 2012, 32, 87–99. [Google Scholar] [CrossRef]
- Wang, C.; Chu, X.; Wu, M. Detection of H2S down to ppb levels at room temperature using sensors based on ZnO nanorods. Sens. Actuators B 2006, 113, 320–323. [Google Scholar] [CrossRef]
- Advani, G.N.; Nanis, L. Effects of humidity on hydrogen sulfide detection by SnO2 solid state gas sensors. Sens. Actuators 1981, 2, 201–206. [Google Scholar] [CrossRef]
- Liu, J.; Huang, X.; Ye, G.; Liu, W.; Jiao, Z.; Chao, W.; Zhou, Z.; Yu, Z. H2S detection sensing characteristic of CuO/SnO2 sensor. Sensors 2003, 3, 110. [Google Scholar] [CrossRef] [Green Version]
- Sarfraz, J.; Ihalainen, P.; Määttänen, A.; Gulin, T.; Koskela, J.; Wilén, C.-E.; Kilpelä, A.; Peltonen, J. A printed H2S sensor with electro-optical response. Sens. Actuators B 2014, 191, 821–827. [Google Scholar] [CrossRef]
- Virji, S.; Kaner, R.B.; Weiller, B.H. Direct electrical measurement of the conversion of metal acetates to metal sulfides by hydrogen sulfide. Inorg. Chem. 2006, 45, 10467–10471. [Google Scholar] [CrossRef]
- Rosolina, S.M.; Carpenter, T.S.; Xue, Z.-L. Bismuth-Based, Disposable Sensor for the Detection of Hydrogen Sulfide Gas. Anal. Chem. 2016, 88, 1553–1558. [Google Scholar] [CrossRef]
- Sarfraz, J.; Tobjork, D.; Osterbacka, R.; Linden, M. Low-cost hydrogen sulfide gas sensor on paper substrates: Fabrication and demonstration. IEEE Sens. J. 2012, 12, 1973–1978. [Google Scholar] [CrossRef]
- Fang, G.; Liu, Z.; Liu, C.; Yao, K.-L. Room temperature H2S sensing properties and mechanism of CeO2-SnO2 sol-gel thin films. Sens. Actuators B 2000, 66, 46–48. [Google Scholar] [CrossRef]
- Chowdhuri, A.; Gupta, V.; Sreenivas, K. Fast response H2S gas sensing characteristics with ultra-thin CuO islands on sputtered SnO2. Sens. Actuators B 2003, 93, 572–579. [Google Scholar] [CrossRef]
- Patil, L.A.; Patil, D.R. Heterocontact type CuO-modified SnO2 sensor for the detection of a ppm level H2S gas at room temperature. Sens. Actuators B 2006, 120, 316–323. [Google Scholar] [CrossRef]
- Vaishampayan, M.V.; Deshmukh, R.G.; Walke, P.; Mulla, I.S. Fe-doped SnO2 nanomaterial: A low temperature hydrogen sulfide gas sensor. Mater. Chem. Phys. 2008, 109, 230–234. [Google Scholar] [CrossRef]
- Gao, B.; Cui, L.; Pan, Y.; Xue, M.; Zhu, B.; Zhang, G.; Zhang, C.; Shuang, S.; Dong, C. A highly selective fluorescent probe based on Michael addition for fast detection of hydrogen sulfide. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 173, 457–461. [Google Scholar] [CrossRef]
- Pla-Tolós, J.; Moliner-Martínez, Y.; Verdú-Andrés, J.; Casanova-Chafer, J.; Molins-Legua, C.; Campíns-Falcó, P. New optical paper sensor for in situ measurement of hydrogen sulphide in waters and atmospheres. Talanta 2016, 156–157, 79–86. [Google Scholar] [CrossRef]
- Petruci, J.F.D.S.; Cardoso, A.A. Sensitive luminescent paper-based sensor for the determination of gaseous hydrogen sulfide. Anal. Methods 2015, 7, 2687–2692. [Google Scholar] [CrossRef]
- Choi, M.M.F.; Hawkins, P. Development of an optical hydrogen sulphide sensor. Sens. Actuators B 2003, 90, 211–215. [Google Scholar] [CrossRef]
- Toda, K.; Ohira, S.-I.; Tanaka, T.; Nishimura, T.; Dasgupta, P.K. Field Instrument for Simultaneous Large Dynamic Range Measurement of Atmospheric Hydrogen Sulfide, Methanethiol, and Sulfur Dioxide. Environ. Sci. Technol. 2004, 38, 1529–1536. [Google Scholar] [CrossRef] [PubMed]
- Toda, K.; Dasgupta, P.K.; Li, J.; Tarver, G.A.; Zarus, G.M. Fluorometric field instrument for continuous measurement of atmospheric hydrogen sulfide. Anal. Chem. 2001, 73, 5716–5724. [Google Scholar] [CrossRef] [PubMed]
- Petruci, J.F.D.S.; Cardoso, A.A. Portable and Disposable Paper-Based Fluorescent Sensor for in Situ Gaseous Hydrogen Sulfide Determination in Near Real-Time. Anal. Chem. 2016, 88, 11714–11719. [Google Scholar] [CrossRef]
- Kubaáň, V.; Dasgupta, P.K.; Marx, J.N. Nitroprusside and Methylene Blue Methods for Silicone Membrane Differentiated Flow Injection Determination of Sulfide in Water and Wastewater. Anal. Chem. 1992, 64, 36–43. [Google Scholar] [CrossRef]
- Fischer, E. Bildung von Methylenblau als Reaktion auf Schwefelwasserstoff. Berichte Dtsch. Chem. Gesellschaft 1883, 16, 2234–2236. [Google Scholar] [CrossRef]
- Sen, A.; Albarella, J.D.; Carey, J.R.; Kim, P.; McNamara, W.B., III. Low-cost colorimetric sensor for the quantitative detection of gaseous hydrogen sulfide. Sens. Actuators B 2008, 134, 234–237. [Google Scholar] [CrossRef]
- Wallace, K.J.; Cordero, S.R.; Tan, C.P.; Lynch, V.M.; Anslyn, E.V. A colorimetric response to hydrogen sulfide. Sens. Actuators B 2007, 120, 362–367. [Google Scholar] [CrossRef]
- Tanaka, Y.; Nakamoto, T.; Moriizumi, T. Study of highly sensitive smell sensing system using gas detector tube combined with optical sensor. Sens. Actuators B 2006, 119, 84–88. [Google Scholar] [CrossRef]
- Ninh, H.P.; Tanaka, Y.; Nakamoto, T.; Hamada, K. A bad-smell sensing network using gas detector tubes and mobile phone cameras. Sens. Actuators B 2007, 125, 138–143. [Google Scholar] [CrossRef]
- Jarosz, A.P.; Yep, T.; Mutus, B. Microplate-based colorimetric detection of free hydrogen sulfide. Anal. Chem. 2013, 85, 3638–3643. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Lu, F.; Peng, M.; Wang, C.-W.; Tseng, Y.-T.; Du, Y.; Cai, N.; Lien, C.-W.; Chang, H.-T.; He, Y.; et al. Selective Colorimetric Detection of Hydrogen Sulfide Based on Primary Amine-Active Ester Cross-Linking of Gold Nanoparticles. Anal. Chem. 2015, 87, 7267–7273. [Google Scholar] [CrossRef]
- Cha, J.-H.; Kim, D.-H.; Choi, S.-J.; Koo, W.-T.; Kim, I.-D. Sub-Parts-per-Million Hydrogen Sulfide Colorimetric Sensor: Lead Acetate Anchored Nanofibers toward Halitosis Diagnosis. Anal. Chem. 2018, 90, 8769–8775. [Google Scholar] [CrossRef]
- Liu, Y.; Feng, G. A visible light excitable colorimetric and fluorescent ESIPT probe for rapid and selective detection of hydrogen sulfide. Org. Biomol. Chem. 2014, 12, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Jin, W. Highly selective and sensitive colorimetric probe for hydrogen sulfide by a copper (II) complex of azo-dye based on chemosensing ensemble approach. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 90, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Adarsh, N.; Krishnan, M.S.; Ramaiah, D. Sensitive naked eye detection of hydrogen sulfide and nitric oxide by aza-BODIPY dyes in aqueous medium. Anal. Chem. 2014, 86, 9335–9342. [Google Scholar] [CrossRef] [PubMed]
- Engel, L.; Tarantik, K.R.; Pannek, C.; Wöllenstein, J. Colorimetric Detection of Hydrogen Sulfide in Ambient Air. Proceedings 2018, 2, 804. [Google Scholar] [CrossRef] [Green Version]
- Fu, H.; Duan, X. Highly sensitive and colorimetric detection of hydrogen sulphide by in situ formation of Ag2S@Ag nanoparticles in polyelectrolyte multilayer film. RSC Adv. 2015, 5, 3508–3511. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Z.; Wang, S.; Qu, C.; Chen, L. On-site visual detection of hydrogen sulfide in air based on enhancing the stability of gold nanoparticles. ACS Appl. Mater. Interfaces 2014, 6, 6300–6307. [Google Scholar] [CrossRef]
- Deng, H.-H.; Weng, S.-H.; Huang, S.-L.; Zhang, L.-N.; Liu, A.-L.; Lin, X.-H.; Chen, W. Colorimetric detection of sulfide based on target-induced shielding against the peroxidase-like activity of gold nanoparticles. Anal. Chim. Acta 2014, 852, 218–222. [Google Scholar] [CrossRef]
- El Sayed, S.; Milani, M.; Licchelli, M.; Martínez-Máñez, R.; Sancenón, F. Hexametaphosphate-capped silica mesoporous nanoparticles containing CuII complexes for the selective and sensitive optical detection of hydrogen sulfide in water. Chem. Eur. J. 2015, 21, 7002–7006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, D.; Gao, Z.; Tang, D.; Niessner, R.; Knopp, D. Target-Induced Nanocatalyst Deactivation Facilitated by Core at Shell Nanostructures for Signal-Amplified Headspace-Colorimetric Assay of Dissolved Hydrogen Sulfide. Anal. Chem. 2015, 87, 10153–10160. [Google Scholar] [CrossRef]
- Puacz, W.; Szahun, W.; Linke, K. Catalytic determination of sulfide in blood. Analyst 1995, 120, 939–941. [Google Scholar] [CrossRef]
- Xu, H.; Wu, J.; Chen, C.-H.; Zhang, L.; Yang, K.-L. Detecting hydrogen sulfide by using transparent polymer with embedded CdSe/CdS quantum dots. Sens. Actuators B 2010, 143, 535–538. [Google Scholar] [CrossRef]
- Bhadra, N.; Hussain, S.; Das, S.; Bhunia, R.; Bhar, R.; Pal, A.K. H2S Gas Sensor Based on Nanocrystalline Copper/DLC Composite Films. Plasmonics 2015, 10, 503–509. [Google Scholar] [CrossRef]
- Ravi, P.V.; Thangadurai, D.T.; Nataraj, D.; Senthilkumar, K.; Manonmani, G.; Kalarikkal, N.; Thomas, S.; Govindh, P. Graphene Nanobuds: A New Second-Generation Phosgene Sensor with Ultralow Detection Limit in Aqueous Solution. ACS Appl. Mater. Interfaces 2019, 11, 19339–19349. [Google Scholar] [CrossRef]
- Yu, F.; Li, P.; Song, P.; Wang, B.; Zhao, J.; Han, K. An ICT-based strategy to a colorimetric and ratiometric fluorescence probe for hydrogen sulfide in living cells. Chem. Commun. 2012, 48, 2852–2854. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, X.; Kan, H.; Wang, W.; Zhu, B.; Du, B.; Zhang, X. A highly selective colorimetric chemodosimeter for fast and quantitative detection of hydrogen sulfide. Analyst 2012, 137, 5576–5580. [Google Scholar] [CrossRef]
- Wu, M.-Y.; Li, K.; Hou, J.-T.; Huang, Z.; Yu, X.-Q. A selective colorimetric and ratiometric fluorescent probe for hydrogen sulfide. Org. Biomol. Chem. 2012, 10, 8342–8347. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Liu, C.; Zhu, Y.-C.; Zhu, Y.-Z. Development of a boron-dipyrromethene-Cu2+ ensemble based colorimetric probe toward hydrogen sulfide in aqueous media. Tetrahedron Lett. 2011, 52, 5000–5003. [Google Scholar] [CrossRef]
- Liu, J.; Sun, Y.-Q.; Zhang, J.; Yang, T.; Cao, J.; Zhang, L.; Guo, W. A ratiometric fluorescent probe for biological signaling molecule H 2S: Fast response and high selectivity. Chem. Eur. J. 2013, 19, 4717–4722. [Google Scholar] [CrossRef] [PubMed]
- Narayanaswamy, R.; Sevilla, F., III. Umist Flow cell studies with immobilised reagents for the development of an optical fibre sulphide sensor. Analyst 1986, 3, 1085–1088. [Google Scholar] [CrossRef]
- Maiti, S.; Mandal, B.; Sharma, M.; Mukherjee, S.; Das, A.K. A covalent organic polymer as an efficient chemosensor for highly selective H2S detection through proton conduction. Chem. Commun. 2020. [Google Scholar] [CrossRef]
- Sensidyne Hydrogen Sulphide 1–60 ppm Gas Detector Tube. Available online: https://www.sensidyne.com/colorimetric-gas-detector-tubes/detector-tubes/120sd-hydrogen-sulphide.php (accessed on 3 July 2020).
- Gastec Corporation Hydrogen Sulphide Detector Tube 4HT. Available online: https://www.gastec.co.jp/en/product/detail/id=1799 (accessed on 3 July 2020).
- Huang, L.; Jiang, P.; Wang, D.; Luo, Y.; Li, M.; Lee, H.; Gerhardt, R.A. A novel paper-based flexible ammonia gas sensor via silver and SWNT-PABS inkjet printing. Sens. Actuators B 2014, 197, 308–313. [Google Scholar] [CrossRef]
- Cuartero, M.; Crespo, G.A.; Bakker, E. Paper-based thin-layer coulometric sensor for halide determination. Anal. Chem. 2015, 87, 1981–1990. [Google Scholar] [CrossRef]
- Chaiyo, S.; Siangproh, W.; Apilux, A.; Chailapakul, O. Highly selective and sensitive paper-based colorimetric sensor using thiosulfate catalytic etching of silver nanoplates for trace determination of copper ions. Anal. Chim. Acta 2015, 866, 75–83. [Google Scholar] [CrossRef]
- Su, Y.; Ma, S.; Jiang, K.; Han, X. CdTe-paper-based visual sensor for detecting methyl viologen. Chin. J. Chem. 2015, 33, 446–450. [Google Scholar] [CrossRef]
- Ferreira, D.C.M.; Giordano, G.F.; Soares, C.C.D.S.P.; De Oliveira, J.F.A.; Mendes, R.K.; Piazzetta, M.H.; Gobbi, A.L.; Cardoso, M.B. Optical paper-based sensor for ascorbic acid quantification using silver nanoparticles. Talanta 2015, 141, 188–194. [Google Scholar] [CrossRef]
- Firdaus, M.L.; Alwi, W.; Trinoveldi, F.; Rahayu, I.; Rahmidar, L.; Warsito, K. Determination of Chromium and Iron Using Digital Image-based Colorimetry. Procedia Environ. Sci. 2014, 20, 298–304. [Google Scholar] [CrossRef] [Green Version]
- Masawat, P.; Harfield, A.; Srihirun, N.; Namwong, A. Green Determination of Total Iron in Water by Digital Image Colorimetry. Anal. Lett. 2017, 50, 173–185. [Google Scholar] [CrossRef]
- Puchum, S.; Meelapsom, R.; Muniandy, S.S.; Lee, H.L.; Pencharee, S.; Amatatongchai, M.; Suttisintong, K.; Jarujamrus, P. Use of unmodified silver nanoparticles (AgNPs) as colorimetric Hg(II) sensor: A new approach to sensitive and high sample throughput determination of Hg(II) under high influence of ionic suppression. Int. J. Environ. Anal. Chem. 2019, 99, 139–156. [Google Scholar] [CrossRef]
- Salcedo, A.R.M.; Sevilla, F.B. Colorimetric determination of mercury vapor using smartphone camera-based imaging. Instrum. Sci. Technol. 2018, 46, 450–462. [Google Scholar] [CrossRef]
- Choodum, A.; Boonsamran, P.; NicDaeid, N.; Wongniramaikul, W. On-site semi-quantitative analysis for ammonium nitrate detection using digital image colourimetry. Sci. Justice 2015, 55, 437–445. [Google Scholar] [CrossRef]
- David, T.; Grandivoriana, N.A.; Fidelis, N. Digital-based image detection system in simple silver nanoparticles-based cyanide assays. Res. J. Chem. Environ. 2018, 22, 10–14. [Google Scholar]
- Choodum, A.; Parabun, K.; Klawach, N.; Daeid, N.N.; Kanatharana, P.; Wongniramaikul, W. Real time quantitative colourimetric test for methamphetamine detection using digital and mobile phone technology. Forensic Sci. Int. 2014, 235, 8–13. [Google Scholar] [CrossRef]
- Wongniramaikul, W.; Limsakul, W.; Choodum, A. A biodegradable colorimetric film for rapid low-cost field determination of formaldehyde contamination by digital image colorimetry. Food Chem. 2018, 249, 154–161. [Google Scholar] [CrossRef]
- Tambaru, D.; Rupilu, R.H.; Nitti, F.; Gauru, I. Suwari Development of paper-based sensor coupled with smartphone detector for simple creatinine determination. In Proceedings of the AIP Conference Proceedings, Bikaner, India, 24–25 November 2017; American Institute of Physics Inc.: Kupang, Indonesia, 2017; Volume 1823. [Google Scholar]
- Priye, A.; Ball, C.S.; Meagher, R.J. Colorimetric-Luminance Readout for Quantitative Analysis of Fluorescence Signals with a Smartphone CMOS Sensor. Anal. Chem. 2018, 90, 12385–12389. [Google Scholar] [CrossRef] [PubMed]
- Fatoni, A.; Numnuam, A.; Kanatharana, P.; Limbut, W.; Thammakhet, C.; Thavarungkul, P. A highly stable oxygen-independent glucose biosensor based on a chitosan-albumin cryogel incorporated with carbon nanotubes and ferrocene. Sens. Actuators B 2013, 185, 725–734. [Google Scholar] [CrossRef]
- Shen, L.; Hagen, J.A.; Papautsky, I. Point-of-care colorimetric detection with a smartphone. Lab Chip 2012, 12, 4240–4243. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.I.; Chang, B.-Y. Development of the smartphone-based colorimetry for multi-analyte sensing arrays. Lab Chip 2014, 14, 1725–1732. [Google Scholar] [CrossRef]
- Montoya, L.A.; Pearce, T.F.; Hansen, R.J.; Zakharov, L.N.; Pluth, M.D. Development of selective colorimetric probes for hydrogen sulfide based on nucleophilic aromatic substitution. J. Org. Chem. 2013, 78, 6550–6557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montoya, L.A.; Pluth, M.D. Hydrogen sulfide deactivates common nitrobenzofurazan-based fluorescent thiol labeling reagents. Anal. Chem. 2014, 86, 6032–6039. [Google Scholar] [CrossRef] [PubMed]
- Ismail, I.; Chen, Z.; Sun, L.; Ji, X.; Ye, H.; Kang, X.; Huang, H.; Song, H.; Bolton, S.G.; Xi, Z.; et al. Highly efficient H2S scavengers: Via thiolysis of positively-charged NBD amines. Chem. Sci. 2020, 11, 7823–7828. [Google Scholar] [CrossRef]
- Quddious, A.; Yang, S.; Khan, M.M.; Tahir, F.A.; Shamim, A.; Salama, K.N.; Cheema, H.M. Disposable, paper-based, inkjet-printed humidity and H2S gas sensor for passive sensing applications. Sensors 2016, 16, 2073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rae Systems Colorimetric Gas Detection Tubes. Available online: https://www.raesystems.com/products/colorimetric-gas-detection-tubes-and-pump (accessed on 3 July 2020).
- Sanderson, H.P.; Thomas, R.; Katz, M. Limitations of the lead acetate impregnated paper tape method for hydrogen sulfide. J. Air Pollut. Control Assoc. 1966, 16, 328–330. [Google Scholar] [CrossRef] [PubMed]
- Innov Analysis Systems Hydrogen Sulphide Gas Analyser Novasulf Hg 300 Series. Available online: https://www.innov-analysis.com/images/pdf/notice_novasulfhg_en_300_rev0.pdf (accessed on 2 October 2020).
- Nasr-El-Din, H.A.; Al-Humaidan, A.Y. Iron Sulfide Scale: Formation, Removal and Prevention; International Symposium on Oilfield Scale; Society of Petroleum Engineers: Aberdeen, UK, 2001. [Google Scholar] [CrossRef]
- Bro, R.; Rinnan, Å.; Faber, N.M. Standard error of prediction for multilinear PLS 2. Practical implementation in fluorescence spectroscopy. Chemom. Intell. Lab. Syst. 2005, 75, 69–76. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vargas, A.P.; Gámez, F.; Roales, J.; Lopes-Costa, T.; Pedrosa, J.M. A Paper-Based Ultrasensitive Optical Sensor for the Selective Detection of H2S Vapors. Chemosensors 2021, 9, 40. https://doi.org/10.3390/chemosensors9020040
Vargas AP, Gámez F, Roales J, Lopes-Costa T, Pedrosa JM. A Paper-Based Ultrasensitive Optical Sensor for the Selective Detection of H2S Vapors. Chemosensors. 2021; 9(2):40. https://doi.org/10.3390/chemosensors9020040
Chicago/Turabian StyleVargas, Alejandro P., Francisco Gámez, Javier Roales, Tânia Lopes-Costa, and José M. Pedrosa. 2021. "A Paper-Based Ultrasensitive Optical Sensor for the Selective Detection of H2S Vapors" Chemosensors 9, no. 2: 40. https://doi.org/10.3390/chemosensors9020040
APA StyleVargas, A. P., Gámez, F., Roales, J., Lopes-Costa, T., & Pedrosa, J. M. (2021). A Paper-Based Ultrasensitive Optical Sensor for the Selective Detection of H2S Vapors. Chemosensors, 9(2), 40. https://doi.org/10.3390/chemosensors9020040