Influence of Nitrogen-Doped Carbon Dot and Silver Nanoparticle Modified Carbon Paste Electrodes on the Potentiometric Determination of Tobramycin Sulfate: A Comparative Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Instrumentation and Characterization
2.2. Materials
2.2.1. Samples
Pure Sample
Pharmaceutical Formulation
2.2.2. Reagents
2.3. Standard Solutions
2.3.1. Standard Stock Solution
2.3.2. Working Standard Solutions
2.4. Procedures
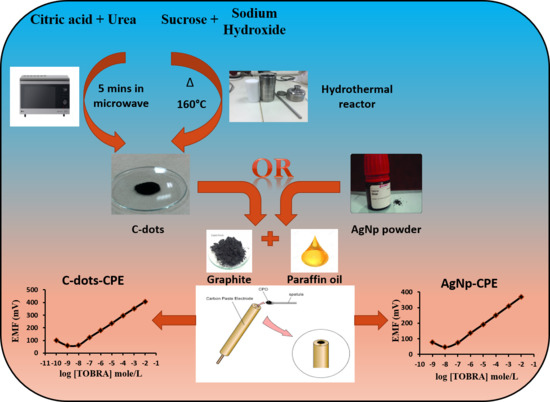
2.4.1. Preparation of C-Dots by Microwave-Assisted Method
2.4.2. Preparation of C-Dots by Hydrothermal Method
2.4.3. Preparation of the Ion-Pair
2.4.4. Sensors Fabrication
Bare Carbon Paste Electrode (Bare-CPE)
Silver Nanoparticles Modified Carbon Paste Electrode (AgNp-CPE)
C-Dots Modified Carbon Paste Electrode (C-Dots-CPE)
2.4.5. Potentiometric Cell—Assembly
2.4.6. Electrodes Calibration
2.4.7. Application to Pharmaceutical Formulation
2.4.8. Application to Spiked Human Plasma
3. Results and Discussion
3.1. Characterization of the Synthesized C-Dots
3.2. Electrodes Fabrication
Optimization of the Modified CPE Composition
3.3. Optimization of the Working pH
3.4. Performance Characteristics of the BARE-CPE, AgNp-CPE, and C-Dots-CPE
3.5. Selectivity of the Proposed Electrodes
3.6. Water-Layer Test
3.7. Application of the Proposed Electrodes for Analysis of Lotepred® T Ophthalmic Solution and Spiked Human Plasma
3.8. Statistical Comparison
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dienstag, J.; Neu, H.C. In Vitro Studies of Tobramycin, an Aminoglycoside Antibiotic. Antimicrob. Agents Chemother. 1972, 1, 41–45. [Google Scholar] [CrossRef] [Green Version]
- Nori, P.; Cowman, K.; Chen, V.; Bartash, R.; Szymczak, W.; Madaline, T.; Punjabi Katiyar, C.; Jain, R.; Aldrich, M.; Weston, G.; et al. Bacterial and fungal coinfections in COVID-19 patients hospitalized during the New York City pandemic surge. Infect. Control Hosp. Epidemiol. 2021, 42, 84–88. [Google Scholar] [CrossRef]
- Chen, L.; Deng, C.; Chen, X.; Zhang, X.; Chen, B.; Yu, H.; Qin, Y.; Xiao, K.; Zhang, H.; Sun, X. Ocular manifestations and clinical characteristics of 535 cases of COVID-19 in Wuhan, China: A cross-sectional study. Acta Ophthalmol. 2020, 98, e951–e959. [Google Scholar] [CrossRef] [PubMed]
- Wilhelmus, K.R.; Gilbert, M.L.; Osato, M.S. Tobramycin in ophthalmology. Surv. Ophthalmol. 1987, 32, 111–122. [Google Scholar] [CrossRef]
- Vaajanen, A.; Vapaatalo, H. A Single Drop in the Eye-Effects on the Whole Body? Open Ophthalmol. J. 2017, 11, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Lai, F.; Sheehan, T. Enhancement of detection sensitivity and cleanup selectivity for tobramycin through pre-column derivatization. J. Chromatogr. A 1992, 609, 173–179. [Google Scholar] [CrossRef]
- Mannan, A.; Asif, S.; Usmanghani, K. A Novel Sensitive Method for Quantitative Determination of Tobramycin by Spectrophotometer using Diphenylamine. RADS J. Pharm. Pharm. Sci 2017, 5, 37–42. [Google Scholar]
- Hassan, A.K.; Ameen, S.T.; Saad, B. Tetracaine-selective electrodes with polymer membranes and their application in pharmaceutical formulation control. Arab. J. Chem. 2017, 10, S1484–S1491. [Google Scholar] [CrossRef]
- Stanić, Z.; Girousi, S. Carbon Paste Electrodes in Potentiometry: The State of the Art and Applications in Modern Electroanalysis (A Review). In Sensing in Electroanalysis; University Press Centre: Pardubice, Czech Republic, 2011; Volume 6, ISBN 9788073954345. [Google Scholar]
- Gupta, V.K.; Yola, M.L.; Özaltin, N.; Atar, N.; Üstündaǧ, Z.; Uzun, L. Molecular imprinted polypyrrole modified glassy carbon electrode for the determination of tobramycin. Electrochim. Acta 2013, 112, 37–43. [Google Scholar] [CrossRef]
- Onac, C.; Kaya, A.; Yola, M.L.; Alpoguz, H.K. Determination of Tobramycin by Square Wave Voltammetry from Milk Sample through the Modified Polymer Inclusion Membrane with Reduced Graphene Oxide. ECS J. Solid State Sci. Technol. 2017, 6, M152–M155. [Google Scholar] [CrossRef]
- Hadi, M.; Mollaei, T. Reduced graphene oxide/graphene oxide hybrid-modified electrode for electrochemical sensing of tobramycin. Chem. Pap. 2019, 73, 291–299. [Google Scholar] [CrossRef]
- El-Kosasy, A.M. Potentiometric assessment of Gram-negative bacterial permeabilization of tobramycin. J. Pharm. Biomed. Anal. 2006, 42, 389–394. [Google Scholar] [CrossRef]
- Kalcher, K. Chemically modified carbon paste electrodes in voltammetric analysis. Electroanalysis 1990, 2, 419–433. [Google Scholar] [CrossRef]
- Afkhami, A.; Soltani-Felehgari, F.; Madrakian, T. Gold nanoparticles modified carbon paste electrode as an efficient electrochemical sensor for rapid and sensitive determination of cefixime in urine and pharmaceutical samples. Electrochim. Acta 2013, 103, 125–133. [Google Scholar] [CrossRef]
- Da Silveira, J.P.; Piovesan, J.V.; Spinelli, A. Carbon paste electrode modified with ferrimagnetic nanoparticles for voltammetric detection of the hormone estriol. Microchem. J. 2017, 133, 22–30. [Google Scholar] [CrossRef]
- Ghapanvari, M.; Madrakian, T.; Afkhami, A.; Ghoorchian, A. A modified carbon paste electrode based on Fe3O4@multi-walled carbon nanotubes@polyacrylonitrile nanofibers for determination of imatinib anticancer drug. J. Appl. Electrochem. 2020, 50, 281–294. [Google Scholar] [CrossRef]
- Fekry, A.M. A new simple electrochemical Moxifloxacin Hydrochloride sensor built on carbon paste modified with silver nanoparticles. Biosens. Bioelectron. 2017, 87, 1065–1070. [Google Scholar] [CrossRef]
- Lima, D.; Calaça, G.N.; Viana, A.G.; Pessôa, C.A. Porphyran-capped gold nanoparticles modified carbon paste electrode: A simple and efficient electrochemical sensor for the sensitive determination of 5-fluorouracil. Appl. Surf. Sci. 2018, 427, 742–753. [Google Scholar] [CrossRef]
- Zhang, Z.; Shen, W.; Xue, J.; Liu, Y.; Liu, Y.; Yan, P.; Liu, J.; Tang, J. Recent advances in synthetic methods and applications of silver nanostructures. Nanoscale Res. Lett. 2018, 13, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maduraiveeran, G.; Jin, W. Nanomaterials based electrochemical sensor and biosensor platforms for environmental applications. Trends Environ. Anal. Chem. 2017, 13, 10–23. [Google Scholar] [CrossRef]
- Zdrachek, E.; Bakker, E. Potentiometric Sensing. Anal. Chem. 2019, 91, 2–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, L.; Wang, A.; Lai, G.; Lin, C.-T.; Yu, J.; Yu, A.; Liu, Z.; Xie, K.; Su, W. A glassy carbon electrode modified with N-doped carbon dots for improved detection of hydrogen peroxide and paracetamol. Microchim. Acta 2018, 185, 87. [Google Scholar] [CrossRef]
- Bonet-San-Emeterio, M.; Algarra, M.; Petković, M.; Del Valle, M. Modification of electrodes with N-and S-doped carbon dots. Evaluation of the electrochemical response. Talanta 2020, 212, 120806. [Google Scholar] [CrossRef] [PubMed]
- Sha, R.; Jones, S.S.; Vishnu, N.; Soundiraraju, B.; Badhulika, S. A Novel Biomass Derived Carbon Quantum Dots for Highly Sensitive and Selective Detection of Hydrazine. Electroanalysis 2018, 30, 2228–2232. [Google Scholar] [CrossRef]
- Spanu, D.; Binda, G.; Dossi, C.; Monticelli, D. Biochar as an alternative sustainable platform for sensing applications: A review. Microchem. J. 2020, 159, 105506. [Google Scholar] [CrossRef]
- Sun, X.; Lei, Y. Fluorescent carbon dots and their sensing applications. TrAC Trends Anal. Chem. 2017, 89, 163–180. [Google Scholar] [CrossRef]
- Tajik, S.; Dourandish, Z.; Zhang, K.; Beitollahi, H.; Van Le, Q.; Jang, H.W.; Shokouhimehr, M. Carbon and graphene quantum dots: A review on syntheses, characterization, biological and sensing applications for neurotransmitter determination. RSC Adv. 2020, 10, 15406–15429. [Google Scholar] [CrossRef] [Green Version]
- Ji, H.; Zhou, F.; Gu, J.; Shu, C.; Xi, K.; Jia, X. Nitrogen-doped carbon dots as a new substrate for sensitive glucose determination. Sensors 2016, 16, 630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palakollu, V.N.; Karpoormath, R.; Wang, L.; Tang, J.N.; Liu, C. A versatile and ultrasensitive electrochemical sensing platform for detection of chlorpromazine based on nitrogen-doped carbon dots/cuprous oxide composite. Nanomaterials 2020, 10, 1513. [Google Scholar] [CrossRef]
- Mongay, C.; Cerdra, V. A Britton-Robinson buffer of known ionic strangth. Ann. Chim. 1974, 64, 409–412. [Google Scholar]
- Qu, S.; Wang, X.; Lu, Q.; Liu, X.; Wang, L. A biocompatible fluorescent ink based on water-soluble luminescent carbon nanodots. Angew. Chemie 2012, 51, 12215–12218. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Li, H.; Liu, Y.; Huang, H.; Kang, Z.; Lee, S.-T. Water soluble carbon nanoparticles: Hydrothermal synthesis and excellent photoluminescence properties. Colloids Surfaces B Biointerfaces 2011, 87, 326–332. [Google Scholar] [CrossRef]
- De Medeiros, T.V.; Manioudakis, J.; Noun, F.; Macairan, J.-R.; Victoria, F.; Naccache, R. Microwave-assisted synthesis of carbon dots and their applications. J. Mater. Chem. C 2019, 7, 7175–7195. [Google Scholar] [CrossRef]
- Jahanbakhshi, M.; Habibi, B. A novel and facile synthesis of carbon quantum dots via salep hydrothermal treatment as the silver nanoparticles support: Application to electroanalytical determination of H2O2 in fetal bovine serum. Biosens. Bioelectron. 2016, 81, 143–150. [Google Scholar] [CrossRef]
- Newman Monday, Y.; Abdullah, J.; Yusof, N.A.; Abdul Rashid, S.; Shueb, R.H. Facile Hydrothermal and Solvothermal Synthesis and Characterization of Nitrogen-Doped Carbon Dots from Palm Kernel Shell Precursor. Appl. Sci. 2021, 11, 1630. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, L.; Cao, F.; Leng, Y. Thermal treatment of hair for the synthesis of sustainable carbon quantum dots and the applications for sensing Hg2+. Sci. Rep. 2016, 6, 35795. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.B.; Borenstein, A.; Markovsky, B.; Aurbach, D.; Gedanken, A.; Talianker, M.; Porat, Z. Activated Carbon Modified with Carbon Nanodots as Novel Electrode Material for Supercapacitors. J. Phys. Chem. C 2016, 120, 13406–13413. [Google Scholar] [CrossRef]
- Peterson, L.N. Inhibition of tobramycin reabsorption in nephron segments by metabolic alkalosis. Kidney Int. 1990, 37, 1492–1499. [Google Scholar] [CrossRef] [Green Version]
- Jaworska, E.; Lewandowski, W.; Mieczkowski, J.; Maksymiuk, K.; Michalska, A. Simple and disposable potentiometric sensors based on graphene or multi-walled carbon nanotubes-Carbon-plastic potentiometric sensors. Analyst 2013, 138, 2363–2371. [Google Scholar] [CrossRef] [PubMed]
- Hussein, L.A.; Magdy, N.; Yamani, H.Z. Stable glycopyrronium bromide solid contact ion selective potentiometric sensors using multi-walled carbon nanotubes, polyaniline nanoparticles and polyaniline microparticles as ion-to-electron transducers: A comparative study. Sens. Actuators B Chem. 2017, 247, 436–444. [Google Scholar] [CrossRef]
- Shabani, R.; Rizi, Z.L.; Moosavi, R. Selective potentiometric sensor for isoniazid ultra-trace determination based on Fe3O4 nanoparticles modified carbon paste electrode (Fe3O4/CPE). Int. J. Nanosci. Nanotechnol. 2018, 14, 241–249. [Google Scholar]
- Buck, R.P.; Lindner, E. Recomendations for nomenclature of ion-selective electrodes (IUPAC recommendations 1994). Pure Appl. Chem. 1994, 66, 2527–2536. [Google Scholar] [CrossRef]
- Qi, L.; Jiang, T.; Liang, R.; Qin, W. Polymeric membrane ion-selective electrodes with anti-biofouling properties by surface modification of silver nanoparticles. Sens. Actuators B Chem. 2021, 328, 129014. [Google Scholar] [CrossRef]
- Zhao, D.L.; Chung, T.S. Applications of carbon quantum dots (CQDs) in membrane technologies: A review. Water Res. 2018, 147, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Koulivand, H.; Shahbazi, A.; Vatanpour, V.; Rahmandoost, M. Novel antifouling and antibacterial polyethersulfone membrane prepared by embedding nitrogen-doped carbon dots for efficient salt and dye rejection. Mater. Sci. Eng. C 2020, 111, 110787. [Google Scholar] [CrossRef]
- Nie, J.; Yuan, L.; Jin, K.; Han, X.; Tian, Y.; Zhou, N. Electrochemical detection of tobramycin based on enzymes-assisted dual signal amplification by using a novel truncated aptamer with high affinity. Biosens. Bioelectron. 2018, 122, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Tohda, K.; Dragoe, D.; Shibata, M.; Umezawa, Y. Studies on the matched potential method for determining the selectivity coefficients of ion-selective electrodes based on neutral ionophores: Experimental and theoretical verification. Anal. Sci. 2001, 17, 733–743. [Google Scholar] [CrossRef] [Green Version]
- Fibbioli, M.; Morf, W.E.; Badertscher, M.; De Rooij, N.F.; Pretsch, E. Potential drifts of solid-contacted ion-selective electrodes due to zero-current ion fluxes through the sensor membrane. Electroanalysis 2000, 12, 1286–1292. [Google Scholar] [CrossRef]




| Parameters | Bare-CPE | Ag-Np-CPE | C-dots-CPE |
|---|---|---|---|
| Slope (mV/decade) | 52.60 | 58.34 | 57.32 |
| Intercept | 605.00 | 484.20 | 522.46 |
| LOD a (M) | 1.8 × 10−7 | 1.8 × 10−8 | 3.2 × 10−9 |
| Response time (s) | 30 | 10 | 10 |
| Working pH range | 5–7.5 | 3.5–8 | 5.5–7.5 |
| Concentration range (M) | 10−6–10−2 | 10−7–10−2 | 10−8–10−2 |
| Stability/Lifetime (days) | 21 days | 28 days | 36 days |
| Accuracy b (mean recovery ± SD) | 100.35 ± 1.002 | 99.77 ± 0.947 | 99.88 ± 1.239 |
| R | 0.9999 | 0.9999 | 0.9999 |
| Intra-day precision (RSD%) c | 0.756 | 0.601 | 0.544 |
| Inter-day precision (RSD%) c | 0.955 | 0.893 | 0.672 |
| Parameters | Bare-CPE | Ag-Np-CPE | C-Dots-CPE | Aptamer-Based Gold Sensor [47] | β-Cyclodextrin-Based Sensor [13] |
|---|---|---|---|---|---|
| Technique | Potentiometry | Differential pulse voltammetry | Potentiometry | ||
| Intercept | 605.00 | 484.20 | 522.46 | 0.1334 | 389.3 |
| LOD [M] a | 1.8 × 10−7 a | 1.8 × 10−8 a | 3.2 × 10−9 a | 5.13 × 10−9 b | 1 × 10−6 a |
| Working pH range | 5–7.5 | 3.5–8 | 5.5–7.5 | 7–7.5 | 5.5–7.5 |
| Concentration range | 10−6–10−2 M | 10−7–10−2 M | 10−8–10−2 M | 10–200 nM | 10−5–10−2 M |
| Stability/Lifetime (days) | 21 days | 28 days | 36 days | 14 days | 21 days |
| R | 0.9999 | 0.9999 | 0.9999 | 0.9969 | 0.998 |
| Intra-day precision (RSD%) | 0.756 | 0.601 | 0.544 | 4.3 | 2.74 |
| Interferents | Bare-CPE | AgNp-CPE | C-Dots-CPE |
|---|---|---|---|
| NaCl | 1.24 × 10−2 | 9.00 × 10−3 | 6.68 × 10−3 |
| CaCl2 | 1.75 × 10−1 | 5.08 × 10−3 | 9.00 × 10−3 |
| Benzalkonium chloride | 1.575 × 10−2 | 3.79 × 10−3 | 9.00 × 10−3 |
| Glucose | 9.90 × 10−2 | 5.51 × 10−3 | 2.986 × 10−3 |
| Loteprednol Etabonate | 1.60 × 10−2 | 9.00 × 10−3 | 4.669 × 10−3 |
| Gentamicin | 1.72 × 10−1 | 3.028 × 10−2 | 9.441 × 10−3 |
| Amikacin | 3.15 × 10−1 | 2.65 × 10−2 | 8.66 × 10−3 |
| In Lotepred® T Ophthalmic Solution | |||
|---|---|---|---|
| Taken TOBRA Conc (M) | Bare-CPE | AgNp-CPE | C-Dots-CPE |
| Recovery% | Recovery% | Recovery% | |
| 1 × 10−5 | 100.00% | 101.00% | 100.56% |
| 1 × 10−4 | 101.70% | 100.00% | 99.31% |
| 1 × 10−3 | 99.31% | 99.20% | 98.01% |
| Mean ±SD | 100.34 ± 1.230 | 100.07 ± 0.902 | 99.29 ± 1.275 |
| In Spiked Human Plasma | |||
| Taken TOBRA Conc (M) | Bare-CPE | AgNp-CPE | C-Dots-CPE |
| Recovery% | Recovery% | Recovery% | |
| 1 × 10−6 | 100.80% | 97.72% | 99.44% |
| 1 × 10−5 | 97.94% | 100.23% | 97.46% |
| 1 × 10−4 | 99.31% | 99.71% | 97.72% |
| Mean ±SD | 99.35 ± 1.430 | 99.22 ± 1.325 | 98.21 ± 1.076 |
| Parameter | Bare-CPE | AgNp-CPE | C-Dots-CPE | Reference Method [7] a |
|---|---|---|---|---|
| Mean | 100.35 | 99.77 | 99.88 | 100.74 |
| SD | 1.002 | 0.947 | 1.239 | 0.505 |
| N | 5 | 6 | 7 | 4 |
| Variance | 1.004 | 0.897 | 1.535 | 0.255 |
| t-test | 0.703 (2.365) * | 1.855 (2.306) * | 1.303 (2.262) * | |
| F | 3.937 (9.117) * | (9.013) * | 6.02 (8.941) * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fares, N.V.; Medhat, P.M.; El Maraghy, C.M.; Okeil, S.; Ayad, M.F. Influence of Nitrogen-Doped Carbon Dot and Silver Nanoparticle Modified Carbon Paste Electrodes on the Potentiometric Determination of Tobramycin Sulfate: A Comparative Study. Chemosensors 2021, 9, 52. https://doi.org/10.3390/chemosensors9030052
Fares NV, Medhat PM, El Maraghy CM, Okeil S, Ayad MF. Influence of Nitrogen-Doped Carbon Dot and Silver Nanoparticle Modified Carbon Paste Electrodes on the Potentiometric Determination of Tobramycin Sulfate: A Comparative Study. Chemosensors. 2021; 9(3):52. https://doi.org/10.3390/chemosensors9030052
Chicago/Turabian StyleFares, Nermine V., Passant M. Medhat, Christine M. El Maraghy, Sherif Okeil, and Miriam F. Ayad. 2021. "Influence of Nitrogen-Doped Carbon Dot and Silver Nanoparticle Modified Carbon Paste Electrodes on the Potentiometric Determination of Tobramycin Sulfate: A Comparative Study" Chemosensors 9, no. 3: 52. https://doi.org/10.3390/chemosensors9030052
APA StyleFares, N. V., Medhat, P. M., El Maraghy, C. M., Okeil, S., & Ayad, M. F. (2021). Influence of Nitrogen-Doped Carbon Dot and Silver Nanoparticle Modified Carbon Paste Electrodes on the Potentiometric Determination of Tobramycin Sulfate: A Comparative Study. Chemosensors, 9(3), 52. https://doi.org/10.3390/chemosensors9030052