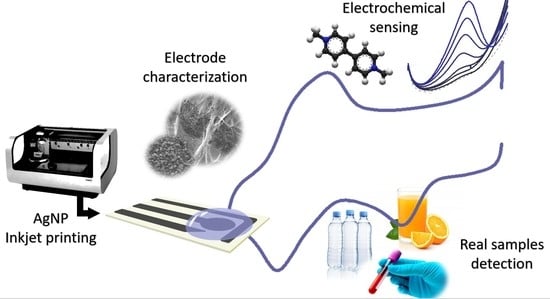
Silver Inkjet-Printed Electrode on Paper for Electrochemical Sensing of Paraquat
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Standards
2.2. Apparatus
2.3. Paper-Based Inkjet Sensor Printing
2.4. Electrochemical Measurements
3. Results
3.1. Morphological and Electrochemical Characterization of Inkjet-Printed Sensors
3.2. Electrochemical Behavior of Paraquat Pesticide


3.3. Determination of Paraquat Using SWV
3.4. Repeatability, Selectivity, and Sample Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Markets and Market. Available online: https://www.marketsandmarkets.com/Market-Reports/printed-electronics-market-197.html?gclid=Cj190KCQiA199P__BRC190ARIsAEZ196irgSR191SFZmKMO570OrbsHSYjrrqCmd105FyCtX-YJnXp192dGcQm193xJ198sIaAv191sEALw_wcB (accessed on 15 January 2021).
- Hayat, A.; Marty, J.L.J.S. Disposable screen printed electrochemical sensors: Tools for environmental monitoring. Sensors 2014, 14, 10432–10453. [Google Scholar] [CrossRef] [PubMed]
- Honeychurch, K.C.; Hart, J.P. Screen-printed electrochemical sensors for monitoring metal pollutants. TrAC Trends Anal. Chem. 2003, 22, 456–469. [Google Scholar] [CrossRef]
- Li, M.; Li, D.-W.; Xiu, G.; Long, Y.-T. Applications of screen-printed electrodes in current environmental analysis. Curr. Opin. Electrochem. 2017, 3, 137–143. [Google Scholar] [CrossRef]
- Thiyagarajan, N.; Chang, J.-L.; Senthilkumar, K.; Zen, J.-M. Disposable electrochemical sensors: A mini review. Electrochem. Commun. 2014, 38, 86–90. [Google Scholar] [CrossRef]
- Hartwig, M.; Zichner, R.; Joseph, Y.J.C. Inkjet-printed wireless chemiresistive sensors—A review. Chemosensors 2018, 6, 66. [Google Scholar] [CrossRef]
- Tortorich, R.P.; Shamkhalichenar, H.; Choi, J.-W.J.A.S. Inkjet-printed and paper-based electrochemical sensors. Appl. Sci. 2018, 8, 288. [Google Scholar] [CrossRef]
- Paschoalino, W.J.; Kogikoski, S.K., Jr.; Barragan, J.T.C.; Giarola, J.F.; Cantelli, L.; Rabelo, T.M.; Pessanha, T.M.; Kubota, L.T. Emerging considerations for the future development of electrochemical paper-based analytical devices. ChemElectroChem 2019, 6, 10–30. [Google Scholar] [CrossRef]
- Lee, V.B.C.; Mohd-Naim, N.F.; Tamiya, E.; Ahmed, M.U. Trends in paper-based electrochemical biosensors: From design to application. Anal. Sci. 2018, 34, 7–18. [Google Scholar] [CrossRef]
- Deroco, P.B.; Giarola, J.D.F.; Wachholz Junior, D.; Lorga, G.A.; Kubota, L.T. Paper-based electrochemical sensing devices. Compr. Anal. Chem. 2020, 89, 91–137. [Google Scholar] [CrossRef]
- Noviana, E.; McCord, C.P.; Clark, K.M.; Jang, I.; Henry, C.S. Electrochemical paper-based devices: Sensing approaches and progress toward practical applications. Lab a Chip 2020, 20, 9–34. [Google Scholar] [CrossRef]
- Gonzalez-Macia, L.; Morrin, A.; Smyth, M.R.; Killard, A.J. Advanced printing and deposition methodologies for the fabrication of biosensors and biodevices. Analist 2010, 135, 845–867. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Rossignol, F.; Macdonald, J. Inkjet printing for biosensor fabrication: Combining chemistry and technology for advanced manufacturing. Lab a Chip 2015, 15, 2538–2558. [Google Scholar] [CrossRef] [PubMed]
- Liana, D.D.; Raguse, B.; Gooding, J.J.; Chow, E. Recent advances in paper-based sensors. Sensors 2012, 12, 11505–11526. [Google Scholar] [CrossRef]
- Singh, A.T.; Lantigua, D.; Meka, A.; Taing, S.; Pandher, M.; Camci-Unal, G. Paper-based sensors: Emerging themes and applications. Sensors 2018, 18, 2838. [Google Scholar] [CrossRef] [PubMed]
- Setti, L.; Fraleoni-Morgera, A.; Ballarin, B.; Filippini, A.; Frascaro, D.; Piana, C. An amperometric glucose biosensor prototype fabricated by thermal inkjet printing. Biosens. Bioelectron. 2005, 20, 2019–2026. [Google Scholar] [CrossRef] [PubMed]
- Moya, A.; Sowade, E.; del Campo, F.J.; Mitra, K.Y.; Ramon, E.; Villa, R.; Baumann, R.R.; Gabriel, G. All-inkjet-printed dissolved oxygen sensors on flexible plastic substrates. Org. Electron. 2016, 39, 168–176. [Google Scholar] [CrossRef]
- Cinti, S.; Arduini, F.; Moscone, D.; Palleschi, G.; Gonzalez-Macia, L.; Killard, A.J. Cholesterol biosensor based on inkjet-printed Prussian blue nanoparticle-modified screen-printed electrodes. Sens. Actuators B Chem. 2015, 221, 187–190. [Google Scholar] [CrossRef]
- Sandström, J.; Broyer, A.; Zoia, D.; Schilt, C.; Greggio, C.; Fournier, M.; Do, K.Q.; Monnet-Tschudi, F. Potential mechanisms of development-dependent adverse effects of the herbicide paraquat in 3D rat brain cell cultures. NeuroToxicology 2017, 60, 116–124. [Google Scholar] [CrossRef]
- Wesseling, C.; Joode, B.V.W.D.; Ruepert, C.; León, C.; Monge, P.; Hermosillo, H.; Partanen, L.J. Paraquat in developing countries. Int. J. Occup. Environ. Health 2001, 7, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Tomková, H.; Sokolová, R.; Opletal, T.; Kučerová, P.; Kučera, L.; Součková, J.; Skopalová, J.; Barták, P. Electrochemical sensor based on phospholipid modified glassy carbon electrode—Determination of paraquat. J. Electroanal. Chem. 2018, 821, 33–39. [Google Scholar] [CrossRef]
- Tcheumi, H.L.; Tassontio, V.N.; Tonle, I.K.; Ngameni, E. Surface functionalization of smectite-type clay by facile polymerization of β-cyclodextrin using citric acid cross linker: Application as sensing material for the electrochemical determination of paraquat. Appl. Clay Sci. 2019, 173, 97–106. [Google Scholar] [CrossRef]
- Pacheco, M.R.; Barbosa, S.C.; Quadrado, R.F.N.; Fajardo, A.R.; Dias, D. Glassy carbon electrode modified with carbon black and cross-linked alginate film: A new voltammetric electrode for paraquat determination. Anal. Bioanal. Chem. 2019, 411, 3269–3280. [Google Scholar] [CrossRef]
- Ghalkhani, M.; Maghsoudi, S.; Saeedi, R.; Khaloo, S.S. Ultrasensitive quantification of paraquat using a newly developed sensor based on silver nanoparticle-decorated carbon nanotubes. J. Iran. Chem. Soc. 2019, 16, 1301–1309. [Google Scholar] [CrossRef]
- Zhang, J.; Lin, Z.; Qin, Y.; Li, Y.; Liu, X.; Li, Q.; Huang, H. Fabricated Electrochemical Sensory Platform Based on the Boron Nitride Ternary Nanocomposite Film Electrode for Paraquat Detection. ACS Omega 2019, 4, 18398–18404. [Google Scholar] [CrossRef] [PubMed]
- Souza, D.C.; Orzari, L.O.; de Oliveira, P.R.; Kalinke, C.; Bonacin, J.A.; Malaspina, O.; Nocelli, R.C.F.; Janegitz, B.C. Electro-chemical sensor based on beeswax and carbon black thin biofilms for determination of paraquat in Apis mellifera Honey. Food Anal. Methods 2020, 14, 606–615. [Google Scholar] [CrossRef]
- Paramalinggam, T.; Yusoff, A.R.M.; Qureshi, M.S.; Shah, Z.A.; Sathishkumar, P.; Yusop, Z.; Khalid, M.; Khokhar, F.M. Determination of paraquat dichloride from water samples using differential pulse cathodic stripping voltammetry. Russ. J. Electrochem. 2018, 54, 1155–1163. [Google Scholar] [CrossRef]
- De Figueiredo-Filho, L.C.; Baccarin, M.; Janegitz, B.C.; Fatibello-Filho, O. A disposable and inexpensive bismuth film minisensor for a voltammetric determination of diquat and paraquat pesticides in natural water samples. Sens. Actuators B Chem. 2017, 240, 749–756. [Google Scholar] [CrossRef]
- Chuntib, P.; Themsirimongkon, S.; Saipanya, S.; Jakmunee, J. Sequential injection differential pulse voltammetric method based on screen printed carbon electrode modified with carbon nanotube/Nafion for sensitive determination of paraquat. Talanta 2017, 170, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, E.T.S.G.; Miserere, S.; Kubota, L.T.; Merkoçi, A. Simple on-plastic/paper inkjet-printed solid-state Ag/AgCl pseudoreference electrode. Anal. Chem. 2014, 86, 10531–10534. [Google Scholar] [CrossRef]
- Mocak, J.; Bond, A.M.; Mitchell, S.; Scollary, G. A statistical overview of standard (IUPAC and ACS) and new procedures for determining the limits of detection and quantification: Application to voltammetric and stripping techniques (Technical Report). Pure Appl. Chem. 1997, 69, 297–328. [Google Scholar] [CrossRef]
- Gosser, D.K. Cyclic Voltammetry: Simulation and Analysis of Reaction Mechanisms; VCH: New York, NY, USA, 1993; p. 165. [Google Scholar]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods, Fundamentals and Applications, 2nd ed.; Wiley: New York, NY, USA, 2001. [Google Scholar]
- Csóka, B.; Nagy, G. Determination of diffusion coefficient in gel and in aqueous solutions using scanning electrochemical microscopy. J. Biochem. Biophys. Methods 2004, 61, 57–67. [Google Scholar] [CrossRef]
- Nicholson, R.S. Theory and application of cyclic voltammetry for measurement of electrode reaction kinetics. Anal. Chem. 1965, 37, 1351–1355. [Google Scholar] [CrossRef]
- Lavagnini, I.; Antiochia, R.; Magno, F. An extended method for the practical evaluation of the standard rate constant from cyclic voltammetric data. Electroanalysis 2004, 16, 505–506. [Google Scholar] [CrossRef]
- Foster, C.; Metters, J.P.; Kampouris, D.K.; Banks, C.E. Ultraflexible screen-printed graphitic electroanalytical sensing platforms. Electroanalysis 2014, 26, 262–274. [Google Scholar] [CrossRef]
- Metters, J.P.; Kadara, R.O.; Banks, C.E. Fabrication of co-planar screen printed microband electrodes. Analist 2013, 138, 2516–2521. [Google Scholar] [CrossRef]
- Scremin, J.; Dos Santos, I.V.J.; Hughes, J.P.; Ferrari, A.G.-M.; Valderrama, E.; Zheng, W.; Zhong, X.; Zhao, X.; Sartori, E.J.R.; Crapnell, R.D.; et al. Platinum nanoparticle decorated vertically aligned graphene screen-printed electrodes: Electrochemical characterisation and exploration towards the hydrogen evolution reaction. Nanoscale 2020, 12, 18214–18224. [Google Scholar] [CrossRef]
- De Souza, D.; Machado, S.A.S. Study of the electrochemical behavior and sensitive detection of pesticides using microelectrodes allied to square-wave voltammetry. Electroanalysis 2006, 18, 862–872. [Google Scholar] [CrossRef]
- El Harmoudi, H.; Achak, M.; Farahi, A.; Lahrich, S.; El Gaini, L.; Abdennouri, M.; Bouzidi, A.; Bakasse, M.; El Mhammedi, M. Sensitive determination of paraquat by square wave anodic stripping voltammetry with chitin modified carbon paste electrode. Talanta 2013, 115, 172–177. [Google Scholar] [CrossRef]
- Farahi, A.; Achak, M.; El Gaini, L.; El Mhammedi, M.A.; Bakasse, M. Electrochemical determination of paraquat in citric fruit based on electrodeposition of silver particles onto carbon paste electrode. J. Food Drug Anal. 2015, 23, 463–471. [Google Scholar] [CrossRef]
- Laghrib, F.; Bakasse, M.; Lahrich, S.; El Mhammedi, M. Electrochemical sensors for improved detection of paraquat in food samples: A review. Mater. Sci. Eng. C 2020, 107, 110349. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.A.; Carreira, C.A.; Lee, H.J.; Silva, F.; Martins, A.; Pereira, C.M. Voltammetric determination of paraquat at DNA–gold nanoparticle composite electrodes. Electrochimica Acta 2010, 55, 7892–7896. [Google Scholar] [CrossRef]
- Díaz, T.G.; Cabanillas, A.G.; Salinas, F. Square-Wave and differential pulse oxidative voltammetric determination of diquat and paraquat in alkaline medium. Electroanalysis 2000, 12, 616–621. [Google Scholar] [CrossRef]
- Ye, X.; Gu, Y.; Wang, C. Fabrication of the Cu2O/polyvinyl pyrrolidone-graphene modified glassy carbon-rotating disk electrode and its application for sensitive detection of herbicide paraquat. Sens. Actuators B Chem. 2012, 173, 530–539. [Google Scholar] [CrossRef]






| Parameter | Evaluated Conditions | Best Conditions |
|---|---|---|
| SWV frequency (f) | 3–10 Hz | 3 Hz |
| SWV amplitude (a) | 10–70 mV | 60 mV |
| SWV potential increment (ΔE) | 1–5 mV | 2 mV |
| Electrode | Linear Range (µmol L−1) | LOD (µmol L−1) | Technique | Reference |
|---|---|---|---|---|
| CB-alginate film/GCE | 1.6–7.8 | 0.23 | SWV | [23] |
| AgNP/MWCNT/Nafion/GCE | 0.10–10 | 0.07 | AsDPV | [24] |
| BN/MoS2/AuNPs/GCE | 0.10–100 | 0.07 | DPV | [25] |
| HMDE | 0.25–1.7 | 0.04 | DPCSV | [27] |
| Bi/SPM | 0.12–4.2 | 0.01 | DPV | [28] |
| CNT-Nafion/SPE | 0.54–4.3 | 0.02 | SI-DPV | [29] |
| AuNPs/DNA/GCE | 5.0–3000 | 1.3 | DPV | [44] |
| GCE | 3.9–31.0 | 3.2 | SWV | [45] |
| Cu2O/PVP-GNs/GC-RDE | 1.0–200 | 0.26 | DPV | [46] |
| Paper-based silver sensor | 3.0–100 | 0.80 | SWV | This work |
| Sample | Added (µmol L−1) | Found 1 (µmol L−1) | Recovered % |
|---|---|---|---|
| Drinking water | 30.0 | 29 ± 4 | 97 ± 12 |
| Orange juice | 30.0 | 30 ± 1 | 103 ± 3 |
| Blood serum | 30.0 | 34 ± 2 | 113 ± 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deroco, P.B.; Wachholz Junior, D.; Kubota, L.T. Silver Inkjet-Printed Electrode on Paper for Electrochemical Sensing of Paraquat. Chemosensors 2021, 9, 61. https://doi.org/10.3390/chemosensors9040061
Deroco PB, Wachholz Junior D, Kubota LT. Silver Inkjet-Printed Electrode on Paper for Electrochemical Sensing of Paraquat. Chemosensors. 2021; 9(4):61. https://doi.org/10.3390/chemosensors9040061
Chicago/Turabian StyleDeroco, Patricia Batista, Dagwin Wachholz Junior, and Lauro Tatsuo Kubota. 2021. "Silver Inkjet-Printed Electrode on Paper for Electrochemical Sensing of Paraquat" Chemosensors 9, no. 4: 61. https://doi.org/10.3390/chemosensors9040061
APA StyleDeroco, P. B., Wachholz Junior, D., & Kubota, L. T. (2021). Silver Inkjet-Printed Electrode on Paper for Electrochemical Sensing of Paraquat. Chemosensors, 9(4), 61. https://doi.org/10.3390/chemosensors9040061