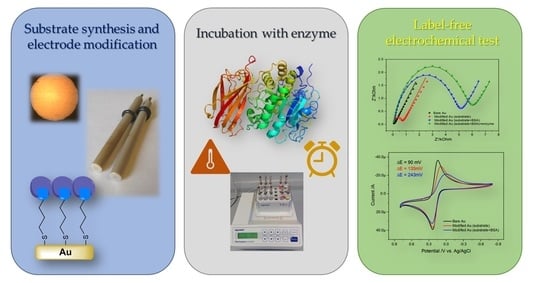
1. Introduction
Depositing peptides on conductive materials over the past few years has attracted the attention of many scientists. Highly efficient deposition of biomolecules on the surface, while maintaining their full activity and stability, is a decisive factor in biosensor technology. Accordingly, more and more research is focused on the development of electrochemical biosensors that express the ability for molecular recognition when detecting adsorbed molecules on the electrode surface through current changes with high selectivity and sensitivity [
1,
2,
3,
4,
5,
6,
7,
8,
9,
10].
Proprotein convertases (PCs) are a family of secretory serine endoproteases found in mammals, consisting of nine enzymes, including ubiquitously expressed furin [
11]. In the human body, they are responsible for a number of important cellular functions, and their dysregulation can lead to diabetes, obesity, metabolic syndrome, hypertension, inflammation, cancer, pain, depression, or even viral and parasitic infections [
12,
13,
14,
15,
16]. Therefore, the regulation of their concentration through the use of their selective inhibitors is an important and needed direction of research, which may lead to the development of new therapeutic agents with clinical application in the future. Detailed knowledge of the inhibition of individual convertases, as well as finding a selective inhibitor for individual enzymes, is an important element of research into new medicinal preparations. The technique used so far (competitive assays) allows to track the interaction of the inhibitor with the enzyme by spectrofluorimetric determination of the fluorophore released from the fluorogenic substrate by enzymatic hydrolysis. On this basis, the kinetics of the enzymatic reaction is assessed and the inhibition constant determined. This method has a limited sensitivity and requires the use of expensive reagents, including for the synthesis of a fluorogenic substrate [
14]. Electrochemical methods for the determination of analytes on modified electrodes are known for their high sensitivity, as well as the possibility to observe even small changes in their composition. Electrochemical studies of electrode surfaces provide additional information about their structure and the reactions taking place on them [
4,
10,
17,
18,
19].
The main goal of this work was to develop a measurement methodology that allows quantification of processes related to the evaluation of enzyme activity using electrochemical methods. Electrochemical techniques enable measurement in a label-free system. The presented research, apart from the synthesis of a peptide substrate (Arg-Val-Arg-Arg) and its thiolate derivative description, include the monitoring of the modification of the gold electrode at each step, but also the development of a new method of incubating the electrode with the enzyme—maintaining the conditions necessary for hydrolysis, as well as optimizing the conditions of the electrode modification process and its stability during storage. All this is to show that the developed method allows the observation of substrate-enzyme interactions electrochemically.
2. Materials and Methods
All chemicals were purchased from Sigma-Aldrich and used without further purification. Dimethylformamide (DMF), dichloromethane (DCM), 4-Methylmorpholine (NMM), diisopropylethylamine (DIPEA), 1-[Bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxid hexafluorophosphate (HATU), 1-Hydroxy-7-azabenzotriazole solution (HOAt), N,N’-diisopropylcarbodiimide (DIC), hexafluoroisopropanol (HFIP), trifluoroacetic acid (TFA); trifluoroethanol (TFE), triisopropylsilane (TIPS); bovine serum albumin (BSA); furin human 2000 unit/mL (furin).
2.1. Synthesis of Peptide Substrate Arg-Val-Arg-Arg
The peptides used in this work were synthesized by the solid-phase method with an automatic peptide synthesizer (Symphony, Gyros Protein Technologies, Uppsala, Sweden) using Fmoc/tBu strategy. The resin used in our study was chloro-(2′-chloro)trityl resin with the capacity of 0.8 mmol/g (Rapp Polymere, Germany). Loading of the first amino acid residue (Fmoc-Arg(Pbf)-OH) was conducted manually using twofold molar excess of Fmoc-amino acid and N,N-diisopropylethylamine (DIPEA, 1:1 eq) in DCM solution. After a one-hour reaction, methanol was added to the mixture and stirred for 15 min. The peptide-resin was drained, washed with DCM, DMF, and DCM, and dried in desiccator.
Fully protected peptide-resin was prepared according to standard procedures involving: (1) deprotection steps using 20% solution of piperidine in DMF (2.5 and 5 min), (2) two-time acylation of Fmoc-amino acid derivative (2.5 molar excess) using 1-[bis(dimethylamino)methylene]-1
H-1,2,3-triazolo[4–
b]pyridinium 3-oxido hexafluorophophate (HATU), 1-hydroxy-7-azabenzotriazole (HOAt) mixture in the presence of
N-methylmorpholine (NMM,1:1:1:2 eq) [
20]. DMF was used between each of the following steps to remove the excess of reagents. When synthesis of the peptide chain was completed, peptide-resin was washed with DCM and dried with nitrogen. MALDI-TOF (Bruker) mass spectrometry was used to check the identity of the synthesized compound.
Cleavage of the protected peptide was attained with the mixture of HFIP/TFE/DCM (3:2:5,
v/
v/
v) [
14]. After a two-hour reaction, the resin was drained and solution was concentrated to half of its starting volume. Fully protected peptide was precipitated with cold diethyl ether, centrifuged in 5 °C and lyophilized from 50% aqueous solution of
tert-butyl alcohol. MALDI-TOF mass spectrometry was used to confirm the identity of the obtained compound.
2.2. Synthesis of Peptide Substrate SH-(CH2)10-CO-(Arg-Val-Arg-Arg)
The peptidyl resin after swelling in the mixture of DCM and DMF (1:1) and Fmoc-deprotection reaction performed according to the procedure described above was used for a coupling reaction with 11-mercaptoundecanoic acid in the presence of DIC (1:1 eq). After 12 h of shaking, the resin was washed with mixture of DCM and DMF (1:1) and dried. The deprotected peptide was cleaved from the resin after 2 h reaction in the presence of TFA/TIPS/H
2O (95:2.5:2.5,
v/
v/
v) [
21]. After evaporation of the solvents, the desired product was precipitated by cold Et
2O, centrifuged, and lyophilized. The MALDI-TOF mass spectrometry was used to confirm the efficiency of the synthesis.
2.3. Modification Procedure
Before modification, the gold electrodes were prepared through a mechanical clea-ning process. Each time, the electrodes were polished on a polishing cloth with 0.05 µm alumina slurry. Then the electrodes were thoroughly rinsed with deionized water, ethyl alcohol, and dried under a stream of nitrogen. Prior to the modification process, each electrode was measured using CV and EIS techniques.
Then the electrode was immersed in the 5 mM degassed ethanol solution of SH-(CH2)10-CO-(Arg-Val-Arg-Arg) for 20 h at room temperature. The electrodes were then dried under a stream of nitrogen. To eliminate pinholes on the surface of the unmodified peptide substrate, the electrode was incubated in a 2 mM ethanolic solution of 1-octanethiol for 60 min. To block the non-specific binding occurring on the surface of the electrode, they was incubated in 5 mL of a 1% solution of BSA in 0.01 M PBS, pH 7.11, for 60 min at 4 °C.
2.4. Procedure of the Enzymatic Studies
In order to ensure conditions most similar to those used in the classical method (1 h in 37 °C), a new method of incubation of the modified electrodes with the tested enzyme was developed (
Figure 1). HEPES buffer solution (pH 7.5, 1 mM CaCl
2), required in furin activity studies [
14], was prepared in 2 mL Eppendorf tubes and one portion of enzyme (1U) was placed. A control sample containing no enzyme was prepared in an analogous manner. Then the modified gold electrodes were immersed in the Eppendorf tubes and the whole was sealed with a parafilm. They were then placed in the Eppendorf Thermomixer comfort chamber and incubated for one hour at 37 °C. After this time, electrodes were rinsed with Hepes buffer solution and dried with a stream of air and used for the electrochemical measurements.
2.5. Electrochemical Measurements
Electrochemical measurements were performed using an Autolab M204 Multi potentiostat/galvanostat equipped with a FRA module (Metrohm AG, the Netherlands). The measuring system consisted of a gold electrode (WE), a platinum plate as counter-electrode (CE), and 0.1 M NaCl Ag/AgCl as a reference electrode (RE). A 5 mM solution of [Fe(CN
6]
3−/4− in 0.5 M KCl (PBS buffered, pH 7.11) was used as the redox probe. The solutions before measurements were degassed by stream of argon. Voltammograms were recorded at a scan rate of 100 mV/s. Each measurement was repeated at least 3 times. EIS measurements were carried out by applying the electrode potential to the open circuit potential (OCP values = 0.180 V). Spectra were recorded in the range from 100 mHz to 10 kHz using RMS amplitude. EIS spectral matching was performed using the equivalent Randles circuit in Nova 2.1.4. software (Metrohm AG, The Netherlands) [
1,
22,
23].
2.6. Contact Angle Measurements
Measurements of the contact angle were made using Drop Shape Analyzer DSA100 (Krüss GmbH, Germany). Each time, a drop of 4 μL water was placed on the electrode surface using a syringe. An image of the droplet was taken with the help of a CCD camera. After the digital image analysis, the average contact angle was deduced by the Young-Laplace method from the angles measured at both sides of the drop-in equilibrium [
1,
22,
23]. Each measurement was repeated 20 times.
3. Results
3.1. Electrochemical Sensing Strategy of the Peptide Substrate—Enzyme Interaction
The electrochemical detection principle of the proprotein convertase interaction with a peptide substrate is schematically shown in
Figure 1 and
Figure 2. The binding affinity between the peptide substrate and the enzyme from the proprotein convertases family was used to obtain a specific electrochemical signal corresponding to the presence of this enzyme in the solution and allowing its quantitative determination. We chose the Arg-Val-Arg-Arg sequence, which is a furin recognition motif [
11]. In the first stage, a peptide substrate and 11-mercaptoundecanoic derivative (SH-(CH
2)
10-CO-(Arg-Val-Arg-Arg)) was used for modification of the gold electrode. The next stage involved blocking active sites by modifying with bovine serum albumine (BSA). These stages were optimized, which will also be described in this work in the Optimization of the experimental conditions section. Then the modified electrodes were tested for the interaction with furin.
3.2. Gold Electrode Modification with Peptide Substrate—SH-(CH2)10-CO-(Arg-Val-Arg-Arg)
Cyclic voltammetry served as a tool to characterize the electrode at every stage of its modification. For this purpose, an 5 mM ferro/ferricyanide redox system was used in 0.5 M KCl (PBS buffered, pH 7.11), which on a bare gold electrode is characterized by the pre-sence of two peaks—oxidation and reduction peak, with an anodic-to-cathodic peak ratio of about 1 and a peak separation (ΔE) of 90 mV (
Figure 3A, black line). From the CV measurements, we see that the deposited layer affects the voltammogram (
Figure 3A). The changes are observed both in peak separation and in the current response. The deposition of the peptide substrate in the peak-to-peak separation increased to about 135 mV, whereas the anodic/cathodic peak ratio decreased to 0.81. Further modification with BSA leads to significant changes in cyclic voltammogram. Peak separation increased almost twice (to 243 mV), and the observed anodic current decreased, leading to peak ratio about 0.66. Such electrochemical behavior indicates that the electron transfer process is partially blocked by compounds that modify the surface of the electrode. Due to the presence of positively charged amino acids in the modified layer, this effect can be changed by the electrostatic interactions of the charged layer with the charged [Fe(CN
6]
3−/4− redox system [
24,
25,
26,
27]. In order to further characterize the modified surface, measurements of electrochemical impedance spectroscopy were performed.
Due to the fact that electrochemical impedance spectroscopy is a method much more sensitive to changes in the double layer than cyclic voltammetry, it was used as a method of tracking the biosensor fabrication process and the interactions with the investigated enzyme.
Figure 3B presents the EIS spectra of a bare gold electrode immersed in a 5 mM solution of [Fe(CN
6]
3−/4− in 0.5 M KCl (PBS buffered, pH = 7.11) and after subsequent stages of modification. From the Nyquist plot (
Figure 3B), we definitely see the influence of the deposited molecules on the resistance of the surface. The bare electrode is characterized by a very small (~130 Ω) semicircle at high frequency related to a RC equivalent circuit, which corresponds to the combination of the charge transfer resistance (R
CT) with the double layer capacitance of the electrode and a low-frequency Warburg line at an angle of 45° representing the diffusion processes at the surface of the electrode. These parameters indicate a fast electron transfer [Fe(CN)
6]
3−/4− towards the electrode. After modification, the charge transfer resistance increased (
Table 1).
Bode plots (
Figure 3B,C) allow to examine the absolute impedance, |Z|, and the phase shift as a function of frequencies. At a very high frequency (>104 Hz), the solution resistance (Rs) dominates the impedance. The variation of the phase angle with frequency (
Figure 3B) shows that at very high frequency, the system behaves like a resistor (Rs). For all modified electrodes, the phase angle increased until it reached maximum at about 55–65°, due to the increase in the imaginary impedance component. Then, the phase angle decreased to 10° due to the influence of Rct; this value is less than the value of the reactance of the double layer. At very low frequencies (below 10 Hz), Warburg impedance prevails; therefore, the phase angle began to increase to 15–35°.
In addition, the wettability of the bare and modified electrodes was tested in order to further confirm the effectiveness of the modification process and determine its chemical nature (
Figure 4). In the case of gold electrodes, the water contact angle (WCA) decreased after modification with the peptide substrate by about 14°. The decrease in contact angle on modified Au electrodes revealed an increase in surface hydrophilicity due to the presence of amino groups in the modified layer. In turn, the total surface free energy γ
s increased after modification with the peptide substrate for Au electrodes from 26.52 to 33.93 mJ·m
−2. This increase was mainly due to an increase in the polar component γ
p. Analysis using the acid and basic components γ
+ and γ
- was more helpful because the alkaline component ranged from 5.86 to 13.75 mJ·m
−2 for Au electrodes (
Figure 4). This is consistent with the presence of amino groups in the structure of the deposited layer derived from basic amino acids.
3.3. Optimization of the Experimental Conditions
Several approaches have been developed to improve the chemical and electrochemical properties of modified gold electrodes. Experimental conditions, including concentration of peptide substrate immobilization, modification time, linker, and the presence of a additional blocking agent, have been optimized. The aim is to develop a sensor with greater sensitivity and without the use of a fluorescent label for determining substrate-enzyme interactions. In the case of gold electrodes, the most commonly used system are derivatives containing a thiol group, because they form a strong bond with the gold surface, and this process is relatively fast and efficient.
3.3.1. Effect of Peptide Concentration, Different Linker, and Time
The process of deposition of biomolecules on the surface of conductive materials requires the use of a linker that will allow for permanent and stable modification of the electrode. The linker used depends primarily on the type of electrode material, and thus on the chemistry of its surface, but also on the biomolecule that we want to deposit. In the case of gold electrodes, the best choice is to use a thiol linker. These systems are capable of forming self-organizing layers, and therefore allow to obtain layers of high ordering [
28,
29,
30,
31,
32,
33,
34].
In our research, we tested different linkers to determine which one would be the best platform for observing the enzyme-substrate reaction. The first group included derivatives of long-chain alkanethiols with a carboxyl group. Two modification methodologies were tested—the first one involved modifying the peptide substrate with an alkanethiol and then anchoring this product on the electrode surface, and the second one—modifying the electrode surface with an alkanethiol, and then attaching the peptide to it. The second group consisted of much smaller systems, namely 4-aminothiophenol and cysteamine. In this case, only the method of anchoring the peptide substrate to the previously thiolated surface was used. This method required the use of a coupling reagent
N-ethyl-
N-[dimethylaminopropyl]carbodiimide (EDC) to couple the amine and carboxyl groups and
N-hydroxysuccinimide (NHS) to make this process more efficient [
35,
36,
37]. Nevertheless, the conducted research clearly indicated that the first of the mentioned methods and groups of linkers turned out to be the best, allowing the observation of the greatest changes in the resistance of the electrode material. Therefore, in further stages of the research, a peptide substrate modified with alkanethiol was used, which was then attached to the electrode surface.
The next step was to select the appropriate concentration of the modified peptide for electrode modification. The limiting factor turned out to be the solubility of the peptide, so we used 5 mM as the highest possible concentration at which the peptide dissolved. On the other hand, the use of a lower concentration resulted in a small change in the resistance of the modified electrode, therefore indicating that the electrode surface was not saturated.
Moreover, the stability of the modified layers was also investigated, namely their resistance after prolonged storage in the PBS solution. This was to check whether the electrode could be stored between successive stages or whether the process should be carried out without longer time intervals (
Figure 5).
The results clearly indicate that the maximum electrode storage time is several hours to one day. During this time, the resistance increased by approx. 25%. The longer storage time resulted in a drastic increase in the electrode resistance, reaching as much as 250% increase after 72 h (
Figure 5).
3.3.2. Effect of Blocking Agent
The use of additional systems blocking free places on the electrode surface, previously modified with the substrate, is aimed at increasing the sensitivity of the sensor. In the presented research, alkanethiols were used due to their self-organizing properties, as well as the ease of modification of the gold surface. The presence of alkyl chains affects the arrangement of peptide chains on the electrode surface, and thus the ordering of the layer and the availability of the peptide for the enzyme in further stages of research.
Among the alkanethiols used, the most differentiating ones were octanethiol and undecanethiol. The conducted experiments have shown that, first of all, the application of any alkanethiol increases the sensitivity of the sensor. This is assessed on the basis of the differences in the change in charge transfer resistance between the modified electrode incubated with the enzyme and the electrode incubated without the enzyme (blank) (
Figure 6). Moreover, octanethiol turned out to be the optimal blocking agent, enhancing the observed effects. In the case of the system without alkanethiols, the difference in resistance between the electrode incubated with the enzyme and the control sample was about 500 Ω. In the case of undecanethiol, this value increased approximately two times, and in the case of octanethiol—four times. This effect is most likely due to the difference in the chain length of the thiols used—the undecane chain is already too long, while the octyl one seems to be optimal and has the most beneficial effect on the layer ordering. Therefore, only octanethiol was used in further experiments.
It is worth mentioning that the obtained results differed between individual electrodes—the modification process took place with different efficiency, which may be the result of their different morphology, but also of the gold electrode pre-treatment process. Therefore, it is very important that the entire experiment (as well as all tests) is carried out on one electrode, thus allowing the registration of the relative changes in the R
CT charge transfer resistance. Nevertheless, the process of interaction with the enzyme compared to a sample incubated in its absence is clearly evident in the form of large changes in charge transfer resistance. These differences are visible in the Nyquist plots presented in
Figure 7.
3.4. Enzyme-Substrate Interactions at the Electrode Surface
In this study, we used impedance spectroscopy to investigate the immobilization of peptide substrate with mercaptoundecanoic acid on the surface of gold electrode. A more complex circuit (Randles model) was used to interpret the experimental results; it additionally takes into consideration the diffusion of redox species through the double layer. This process takes place at very low frequencies (<10 Hz) and is described by Warburg impedance. The model also includes the solution resistance, the charge transfer resistance, and the double-layer capacitance. Data fitting was done using Nova software, and the quality of fitting was evaluated by the error value.
The electrical equivalent circuit (EEC) was selected based on the impedance tests obtained. The EEC with the abbreviation R(Q(RW)) consists of the R resistance and parallel connection of the solid phase element (CPE), imitating the heterogeneity on the electrode surface and the R
CT resistance of the charge transfer with the diffusion resistance W Warburg (
Figure 8).
From both the Nyquist and Bode plots (
Figure 8), we definitely see the influence of the deposited molecules on the resistance of the surface. The bare electrode is characteri-zed by a semicircle at high frequency related to a RC equivalent circuit, which corresponds to the combination of the charge transfer resistance (R
CT) with the double layer capacitance of the electrode and a low-frequency Warburg line at an angle of 45° representing the diffusion processes at the surface of the electrode. After modification, the charge transfer resistance increased (
Table 1). In addition, after the incubation of the electrodes with the enzyme, we observe changes in the impedance of the layer, while for an identical sample incubated without the enzyme (blank), such changes are not visible (
Figure 8).
Incubation of the modified electrode with the enzyme (furin) inhibits the charge transfer process, resulting in an increase in RCT. The observed behavior corresponds to the influence of a functionalized organic layer on the kinetics of charge transfer process. This allows observation of the interaction of the enzyme with the substrate resulting in its cleavage from the electrode surface at very low concentrations without any label used, thanks to which this technique might be considered competitive for the standard method.
4. Conclusions
The conducted research allowed to optimize both the method of obtaining a gold electrode modified with a peptide, as well as extract the methodology of incubating this electrode with the enzyme. In addition, the obtained results show that this method allows the observation of the enzyme-substrate interaction without the use of a label. Factors such as the thiol linker, the solubility of the substrate, the storage time between the individual steps, as well as the length of the aliphatic chain of the electrode surface blocking agent turned out to be the key elements determining the effectiveness of the obtained sensor. The obtained sensor consisted of a substrate Arg-Val-Arg-Arg modified with 11-mercaptoundecanoic acid, anchored to the surface of a gold electrode, which was then blocked with octanethiol. The material prepared in this way (without a label), incubated under the same conditions as in the standard (spectrofluorometric) method, in the presence of the enzyme (furin) and in its absence, showed clear changes in the charge transfer resistance, which is the analytical signal of our sensor.
Therefore, the sensor modified with a peptide turned out to be an interesting and competitive methodology for the electrochemical observation of the interaction of the substrate with an enzyme which belongs to the proprotein convertases family.
Author Contributions
Methodology, I.M. and A.W.; investigation, A.W. and P.N.; writing—original draft preparation, A.W.; writing—review and editing, A.W., I.M. and P.N.; visualization, A.W.; supervision, T.O. and A.P.; project administration, A.W.; funding acquisition, A.W. and A.P. All authors have read and agreed to the published version of the manuscript.
Funding
The National Science Centre, Poland, is acknowledged for financial support through Miniatura 1 Project DEC-2017/01/X/ST5/00828.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Niedziałkowski, P.; Bojko, M.; Ryl, J.; Wcisło, A.; Spodzieja, M.; Magiera-Mularz, K.; Guzik, K.; Dubin, G.; Holak, T.A.; Ossowski, T.; et al. Ultrasensitive Electrochemical Determination of the Cancer Biomarker Protein SPD-L1 Based on a BMS-8-Modified Gold Electrode. Bioelectrochemistry 2021, 139, 107742. [Google Scholar] [CrossRef]
- Niedzialkowski, P.; Slepski, P.; Wysocka, J.; Chamier-Cieminska, J.; Burczyk, L.; Sobaszek, M.; Wcislo, A.; Ossowski, T.; Bogdanowicz, R.; Ryl, J. Multisine Impedimetric Probing of Biocatalytic Reactions for Label-Free Detection of DEFB1 Gene: How to Verify That Your Dog Is Not Human? Sens. Actuators B Chem. 2020, 323, 128664. [Google Scholar] [CrossRef]
- Gooding, J.J.; Pugliano, L.; Hibbert, D.B.; Erokhin, P. Amperometric Biosensor with Enzyme Amplification Fabricated Using Self-Assembled Monolayers of Alkanethiols: The Influence of the Spatial Distribution of the Enzymes. Electrochem. Commun. 2000, 2, 217–221. [Google Scholar] [CrossRef]
- Randviir, E.P.; Banks, C.E. Electrochemical Impedance Spectroscopy: An Overview of Bioanalytical Applications. Anal. Methods 2013, 5, 1098. [Google Scholar] [CrossRef]
- Bertók, T.; Katrlík, J.; Gemeiner, P.; Tkac, J. Electrochemical Lectin Based Biosensors as a Label-Free Tool in Glycomics. Microchim. Acta 2013, 180, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Arugula, M.A.; Simonian, A. Novel Trends in Affinity Biosensors: Current Challenges and Perspectives. Meas. Sci. Technol. 2014, 25, 032001. [Google Scholar] [CrossRef]
- Tersch, C.; Lisdat, F. Label-Free Detection of Protein–DNA Interactions Using Electrochemical Impedance Spectroscopy. Electrochim. Acta 2011, 56, 7673–7679. [Google Scholar] [CrossRef]
- Akiba, U.; Anzai, J. Recent Progress in Electrochemical Biosensors for Glycoproteins. Sensors 2016, 16, 2045. [Google Scholar] [CrossRef] [Green Version]
- Liang, B.; Guo, X.; Fang, L.; Hu, Y.; Yang, G.; Zhu, Q.; Wei, J.; Ye, X. Study of Direct Electron Transfer and Enzyme Activity of Glucose Oxidase on Graphene Surface. Electrochem. Commun. 2015, 50, 1–5. [Google Scholar] [CrossRef]
- Gooding, J.J.; Darwish, N. The Rise of Self-Assembled Monolayers for Fabricating Electrochemical Biosensors—An Interfacial Perspective. Chem. Rec. 2012, 12, 92–105. [Google Scholar] [CrossRef]
- Seidah, N.G.; Prat, A. The Biology and Therapeutic Targeting of the Proprotein Convertases. Nat. Rev. Drug Discov. 2012, 11, 367–383. [Google Scholar] [CrossRef] [PubMed]
- Seidah, N.G. The Proprotein Convertases, 20 Years Later. In Proprotein Convertases; Mbikay, M., Seidah, N.G., Eds.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2011; pp. 23–57. ISBN 978-1-61779-203-8. [Google Scholar]
- Małuch, I.; Makowska, M.; Tomczykowska, M.; Walewska, A.; Sikorska, E.; Prahl, A. Rola konwertaz probiałkowych w chorobach nowotworowych ze szczególnym uwzględnieniem enzymu PACE4. Postepy Biochem. 2017, 63, 179–184. [Google Scholar] [PubMed]
- Małuch, I.; Levesque, C.; Kwiatkowska, A.; Couture, F.; Ly, K.; Desjardins, R.; Neugebauer, W.A.; Prahl, A.; Day, R. Positional Scanning Identifies the Molecular Determinants of a High Affinity Multi-Leucine Inhibitor for Furin and PACE4. J. Med. Chem. 2017, 60, 2732–2744. [Google Scholar] [CrossRef]
- Lewandowska-Goch, M.A.; Kwiatkowska, A.; Łepek, T.; Ly, K.; Navals, P.; Gagnon, H.; Dory, Y.L.; Prahl, A.; Day, R. Design and Structure–Activity Relationship of a Potent Furin Inhibitor Derived from Influenza Hemagglutinin. ACS Med. Chem. Lett. 2021, 12, 365–372. [Google Scholar] [CrossRef]
- Jin, W.; Fuki, I.V.; Seidah, N.G.; Benjannet, S.; Glick, J.M.; Rader, D.J. Proprotein Covertases Are Responsible for Proteolysis and Inactivation of Endothelial Lipase. J. Biol. Chem. 2005, 280, 36551–36559. [Google Scholar] [CrossRef] [Green Version]
- Lin, D.; Tang, T.; Jed Harrison, D.; Lee, W.E.; Jemere, A.B. A Regenerating Ultrasensitive Electrochemical Impedance Immunosensor for the Detection of Adenovirus. Biosens. Bioelectron. 2015, 68, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Noh, M.F.M.; Tothill, I.E. Development and Characterisation of Disposable Gold Electrodes, and Their Use for Lead(II) Analysis. Anal. Bioanal. Chem. 2006, 386, 2095–2106. [Google Scholar] [CrossRef]
- Jayanthi, V.S.P.K.S.A.; Das, A.B.; Saxena, U. Recent Advances in Biosensor Development for the Detection of Cancer Biomarkers. Biosens. Bioelectron. 2017, 91, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Małuch, I.; Stachurski, O.; Kosikowska-Adamus, P.; Makowska, M.; Bauer, M.; Wyrzykowski, D.; Hać, A.; Kamysz, W.; Deptuła, M.; Pikuła, M.; et al. Double-Headed Cationic Lipopeptides: An Emerging Class of Antimicrobials. Int. J. Mol. Sci. 2020, 21, 8944. [Google Scholar] [CrossRef]
- Czupryniak, J.; Niedziałkowski, P.; Karbarz, M.; Ossowski, T.; Stojek, Z. Lysine and Arginine Oligopeptides Tagged with Anthraquinone: Electrochemical Properties. Electroanalysis 2012, 24, 975–982. [Google Scholar] [CrossRef]
- Cirocka, A.; Zarzeczańska, D.; Wcisło, A.; Ryl, J.; Bogdanowicz, R.; Finke, B.; Ossowski, T. Tuning of the Electrochemical Properties of Transparent Fluorine-Doped Tin Oxide Electrodes by Microwave Pulsed-Plasma Polymerized Allylamine. Electrochim. Acta 2019, 313, 432–440. [Google Scholar] [CrossRef]
- Swebocki, T.; Niedziałkowski, P.; Cirocka, A.; Szczepańska, E.; Ossowski, T.; Wcisło, A. In Pursuit of Key Features for Constructing Electrochemical Biosensors–Electrochemical and Acid-Base Characteristic of Self-Assembled Monolayers on Gold. Supramol. Chem. 2020, 32, 1–11. [Google Scholar] [CrossRef]
- Kokab, T.; Shah, A.; Iftikhar, F.J.; Nisar, J.; Akhter, M.S.; Khan, S.B. Amino Acid-Fabricated Glassy Carbon Electrode for Efficient Simultaneous Sensing of Zinc(II), Cadmium(II), Copper(II), and Mercury(II) Ions. ACS Omega 2019, 4, 22057–22068. [Google Scholar] [CrossRef]
- Trzeciakiewicz, H.; Esteves-Villanueva, J.; Soudy, R.; Kaur, K.; Martic-Milne, S. Electrochemical Characterization of Protein Adsorption onto YNGRT-Au and VLGXE-Au Surfaces. Sensors 2015, 15, 19429–19442. [Google Scholar] [CrossRef] [Green Version]
- Yan, X.; Tang, J.; Tanner, D.; Ulstrup, J.; Xiao, X. Direct Electrochemical Enzyme Electron Transfer on Electrodes Modified by Self-Assembled Molecular Monolayers. Catalysts 2020, 10, 1458. [Google Scholar] [CrossRef]
- Zeng, M.; Zhou, T.; Su, Z.; Pan, W. Electrochemically Prepared Poly(L-Lysine) and 3-Hydroxyphenylboronic Acid Composite as a Conventional Adhesion Material for Rice Suspension Cells. Electrochem. Commun. 2020, 115, 106737. [Google Scholar] [CrossRef]
- Celestin, M.; Krishnan, S.; Bhansali, S.; Stefanakos, E.; Goswami, D.Y. A Review of Self-Assembled Monolayers as Potential Terahertz Frequency Tunnel Diodes. NANO Res. 2014, 7, 589–625. [Google Scholar] [CrossRef]
- Eckermann, A.L.; Feld, D.J.; Shaw, J.A.; Meade, T.J. Electrochemistry of Redox-Active Self-Assembled Monolayers. Coord. Chem. Rev. 2010, 254, 1769–1802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maksymovych, P.; Voznyy, O.; Dougherty, D.B.; Sorescu, D.C.; Yates, J.T. Gold Adatom as a Key Structural Component in Self-Assembled Monolayers of Organosulfur Molecules on Au(111). Prog. Surf. Sci. 2010, 85, 206–240. [Google Scholar] [CrossRef]
- Kaur, I.; Zhao, X.; Bryce, M.R.; Schauer, P.A.; Low, P.J.; Kataky, R. Modification of Electrode Surfaces by Self-Assembled Monolayers of Thiol-Terminated Oligo(Phenyleneethynylene)s. ChemPhysChem 2013, 14, 431–440. [Google Scholar] [CrossRef] [Green Version]
- Couto, R.A.S.; Gonçalves, L.M.; Góes, M.S.; Rodrigues, C.M.P.; Quinaz, M.B.; Rodrigues, J.A. SAM-Based Immunosensor for the Analysis of Thyroxine (T4). J. Electrochem. Soc. 2017, 164, B103–B106. [Google Scholar] [CrossRef]
- Love, J.C.; Estroff, L.A.; Kriebel, J.K.; Nuzzo, R.G.; Whitesides, G.M. Self-Assembled Monolayers of Thiolates on Metals as a Form of Nanotechnology. Chem. Rev. 2005, 105, 1103–1170. [Google Scholar] [CrossRef]
- Gooding, J.J.; Hibbert, D.B. The Application of Alkanethiol Self-Assembled Monolayers to Enzyme Electrodes. TrAC Trends Anal. Chem. 1999, 18, 525–533. [Google Scholar] [CrossRef]
- Pruneanu, S.; Boughriet, A.; Henderson, A.; Malins, C.; Ali, Z.; Olenic, L. Impedimetric Measurements for Monitoring Avidin-Biotin Interaction on Self-Assembled Monolayer. Part. Sci. Technol. 2008, 26, 136–144. [Google Scholar] [CrossRef]
- Malecka, K.; Michalczuk, L.; Radecka, H.; Radecki, J. Ion-Channel Genosensor for the Detection of Specific DNA Sequences Derived from Plum Pox Virus in Plant Extracts. Sensors 2014, 14, 18611–18624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, J.A.; Hsu, W.-L.; Liao, W.-C.; Chiu, J.-K.; Chen, M.-L.; Chang, H.-C.; Li, C.-C. Ultrasensitive Electrochemical Detection of Biotin Using Electrically Addressable Site-Oriented Antibody Immobilization Approach via Aminophenyl Boronic Acid. Biosens. Bioelectron. 2010, 26, 1021–1027. [Google Scholar] [CrossRef] [PubMed]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).