Monocytic Cell Adhesion to Oxidised Ligands: Relevance to Cardiovascular Disease
Abstract
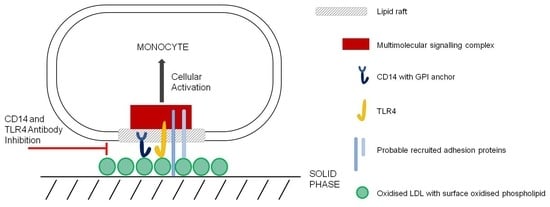
:1. Introduction
2. Materials and Methods
2.1. Human Tissues and Ethical Committee Statement
2.2. U937 Cell Culture
2.3. Monocyte Isolation
2.4. Isolation of Human LDL and Oxidation
2.5. Plate Sensitisation
2.6. Fluorescent Static Solid Phase Adhesion Assay
2.7. Surface Expression of Oxidised Phospholipid, MDA and HSP60 on HUVEC
2.8. Immunohistochemistry (IHC)
2.9. Adhesion under Static and Flow Conditions
2.10. Data Analysis and Statistical Analysis
3. Results
3.1. Ox-LDL as a Monocytic Cell Adhesion Ligand, Actions of Inhibitory Antibodies
3.2. HSP60 as a Monocytic Cell Adhesion Molecule, Actions of Inhibitory Antibodies
3.3. Effects of Membrane Active Agents
3.4. Variants of the Assay
3.5. Comparison of PBMC Adhesion to oxLDL under Static and Flow Conditions
3.6. Expression of Oxidised Lipids and HSP60 on HUVEC
3.7. Oxidised Lipids and HSP60 in the Endothelium of Human Atherosclerotic Plaques
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Libby, P.; Ridker, P.M.; Hansson, G.K. Inflammation in Atherosclerosis: From Pathophysiology to Practice. J. Am. Coll. Cardiol. 2009, 54, 2129–2138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poston, R.N. Atherosclerosis: Integration of its pathogenesis as a self-perpetuating propagating inflammation: A review. Cardiovasc. Endocrinol. Metab. 2019, 8, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, S.M.; Sugiyama, M.G.; Fung, K.Y.; Gao, Y.; Wang, C.; Levy, A.S.; Azizi, P.; Roufaiel, M.; Zhu, S.-N.; Neculai, D.; et al. A novel assay uncovers an unexpected role for SR-BI in LDL transcytosis. Cardiovasc. Res. 2015, 108, 268–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Sessa, W.C.; Fernández-Hernando, C. Endothelial Transcytosis of Lipoproteins in Atherosclerosis. Front. Cardiovasc. Med. 2018, 5, 130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watson, A.D.; Leitinger, N.; Navab, M.; Faull, K.F.; Hörkkö, S.; Witztum, J.L.; Palinski, W.; Schwenke, D.; Salomon, R.G.; Sha, W.; et al. Structural identification by mass spectrometry of oxidized phospholipids in minimally oxidized low density lipoprotein that induce monocyte/endothelial interactions and evidence for their presence in vivo. J. Biol. Chem. 1997, 272, 13597–13607. [Google Scholar] [CrossRef] [Green Version]
- Friedman, P.; Hörkkö, S.; Steinberg, D.; Witztum, J.L.; Dennis, E.A. Correlation of Antiphospholipid Antibody Recognition with the Structure of Synthetic Oxidized Phospholipids. J. Biol. Chem. 2002, 277, 7010–7020. [Google Scholar] [CrossRef] [Green Version]
- Palinski, W.; Ylä-Herttuala, S.; Rosenfeld, M.E.; Butler, S.W.; ASocher, S.; Parthasarathy, S.; Curtiss, L.K.; Witztum, J.L. Antisera and monoclonal antibodies specific for epitopes generated during oxidative modification of low density lipoprotein. Arterioscler. Off. J. Am. Heart Assoc. Inc. 1990, 10, 325–335. [Google Scholar] [CrossRef] [Green Version]
- Skålén, K.; Gustafsson, M.; Rydberg, E.K.; Hultén, L.M.; Wiklund, O.; Innerarity, T.L.; Borén, J. Subendothelial retention of atherogenic lipoproteins in early atherosclerosis. Nature 2002, 417, 750–754. [Google Scholar] [CrossRef]
- Simionescu, N.; Vasile, E.; Lupu, F.; Popescu, G.; Simionescu, M. Prelesional events in atherogenesis. Accumulation of extracellular cholesterol-rich liposomes in the arterial intima and cardiac valves of the hyperlipidemic rabbit. Am. J. Pathol. 1986, 123, 109–125. [Google Scholar]
- Fukuchi, M.; Watanabe, J.; Kumagai, K.; Baba, S.; Shinozaki, T.; Miura, M.; Kagaya, Y.; Shirato, K. Normal and Oxidized Low Density Lipoproteins Accumulate Deep in Physiologically Thickened Intima of Human Coronary Arteries. Lab. Investig. 2002, 82, 1437–1447. [Google Scholar] [CrossRef] [Green Version]
- Cole, A.L.; Subbanagounder, G.; Mukhopadhyay, S.; Berliner, J.A.; Vora, D.K. Oxidized phospholipid-induced endothelial cell/monocyte interaction is mediated by a cAMP-dependent R-Ras/PI3-kinase pathway. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1384–1390. [Google Scholar] [CrossRef]
- Murohara, T.; Scalia, R.; Lefer, A.M. Lysophosphatidylcholine promotes P-selectin expression in platelets and endothelial cells. Possible involvement of protein kinase C activation and its inhibition by nitric oxide donors. Circ. Res. 1996, 78, 780–789. [Google Scholar] [CrossRef]
- Vora, D.K.; Fang, Z.T.; Liva, S.M.; Tyner, T.R.; Parhami, F.; Watson, A.D.; Drake, T.A.; Territo, M.C.; Berliner, J.A. Induction of P-selectin by oxidized lipoproteins. Separate effects on synthesis and surface expression. Circ. Res. 1997, 80, 810–818. [Google Scholar] [CrossRef]
- Morgan, J.; Smith, J.A.; Wilkins, G.M.; Leake, D.S. Oxidation of low density lipoprotein by bovine and porcine aortic endothelial cells and porcine endocardial cells in culture. Atherosclerosis 1993, 102, 209–216. [Google Scholar] [CrossRef]
- Lamb, D.J.; Mitchinson, M.J.; Leake, D.S. Transition metal ions within human atherosclerotic lesions can catalyse the oxidation of low density lipoprotein by macrophages. FEBS Lett. 1995, 374, 12–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chávez-Sánchez, L.; Chávez-Rueda, K.; Legorreta-Haquet, M.V.; Zenteno, E.; Ledesma-Soto, Y.; Montoya-Díaz, E.; Tesoro-Cruz, E.; Madrid-Miller, A.; Blanco-Favela, F. The activation of CD14, TLR4, and TLR2 by mmLDL induces IL-1β, IL-6, and IL-10 secretion in human monocytes and macrophages. Lipids Health Dis. 2010, 9, 117. [Google Scholar] [CrossRef] [Green Version]
- Duewell, P.; Kono, H.; Rayner, K.J.; Sirois, C.M.; Vladimer, G.; Bauernfeind, F.G.; Abela, G.S.; Franchi, L.; Nuñez, G.; Schnurr, M.; et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 2010, 464, 1357–1361, Erratum in Nature 2010, 466, 652. [Google Scholar] [CrossRef] [Green Version]
- Miller, Y.I.; Shyy, J.Y. Context-Dependent Role of Oxidized Lipids and Lipoproteins in Inflammation. Trends Endocrinol. Metab. 2017, 28, 143–152. [Google Scholar] [CrossRef] [Green Version]
- Poston, R.N.; Haskard, D.O.; Coucher, J.R.; Gall, N.P.; Johnson-Tidey, R.R. Expression of intercellular adhesion molecule-1 in atherosclerotic plaques. Am. J. Pathol. 1992, 140, 665–673. [Google Scholar]
- Reape, T.J.; Groot, P.H. Chemokines and atherosclerosis. Atherosclerosis 1999, 147, 213–225. [Google Scholar] [CrossRef]
- Yin, M.; Li, C.; Jiang, J.; Le, J.; Luo, B.; Yang, F.; Fang, Y.; Yang, M.; Deng, Z.; Ni, W.; et al. Cell adhesion molecule-mediated therapeutic strategies in atherosclerosis: From a biological basis and molecular mechanism to drug delivery nanosystems. Biochem. Pharmacol. 2021, 186, 114471. [Google Scholar] [CrossRef] [PubMed]
- Ciesielska, A.; Matyjek, M.; Kwiatkowska, K. TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling. Cell. Mol. Life Sci. 2020, 78, 1233–1261. [Google Scholar] [CrossRef] [PubMed]
- Triantafilou, M.; Triantafilou, K. Lipopolysaccharide recognition: CD14, TLRs and the LPS-activation cluster. Trends Immunol. 2002, 23, 301–304. [Google Scholar] [CrossRef] [PubMed]
- Giocondi, M.-C.; Milhiet, P.E.; Dosset, P.; Le Grimellec, C. Use of Cyclodextrin for AFM Monitoring of Model Raft Formation. Biophys. J. 2004, 86, 861–869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gironi, B.; Oliva, R.; Petraccone, L.; Paolantoni, M.; Morresi, A.; Del Vecchio, P.; Sassi, P. Solvation properties of raft-like model membranes. Biochim. Biophys. Acta BBA-Biomembr. 2019, 1861, 183052. [Google Scholar] [CrossRef] [PubMed]
- De Ménorval, M.-A.; Mir, L.M.; Fernández, M.L.; Reigada, R. Effects of Dimethyl Sulfoxide in Cholesterol-Containing Lipid Membranes: A Comparative Study of Experiments In Silico and with Cells. PLoS ONE 2012, 7, e41733. [Google Scholar] [CrossRef] [Green Version]
- Płóciennikowska, A.; Hromada-Judycka, A.; Borzęcka, K.; Kwiatkowska, K. Co-operation of TLR4 and raft proteins in LPS-induced pro-inflammatory signaling. Cell. Mol. Life Sci. 2015, 72, 557–581. [Google Scholar] [CrossRef] [Green Version]
- Beekhuizen, H.; Blokland, I.; Tilburg, A.J.C.-V.; Koning, F.; Van Furth, R. CD14 contributes to the adherence of human monocytes to cytokine-stimulated endothelial cells. J. Immunol. 1991, 147, 3761–3767. [Google Scholar]
- Poston, R.N.; Johnson-Tidey, R.R. Localized adhesion of monocytes to human atherosclerotic plaques demonstrated in vitro: Implications for atherogenesis. Am. J. Pathol. 1996, 149, 73–80. [Google Scholar]
- Stocker, R.; Keaney, J.F., Jr. Role of Oxidative Modifications in Atherosclerosis. Physiol. Rev. 2004, 84, 1381–1478. [Google Scholar] [CrossRef]
- Marchio, P.; Guerra-Ojeda, S.; Vila, J.M.; Aldasoro, M.; Victor, V.M.; Mauricio, M.D. Targeting Early Atherosclerosis: A Focus on Oxidative Stress and Inflammation. Oxid. Med. Cell Longev. 2019, 2019, 8563845. [Google Scholar] [CrossRef] [Green Version]
- Kol, A.; Lichtman, A.H.; Finberg, R.W.; Libby, P.; Kurt-Jones, E.A. Cutting Edge: Heat Shock Protein (HSP) 60 Activates the Innate Immune Response: CD14 Is an Essential Receptor for HSP60 Activation of Mononuclear Cells. J. Immunol. 2000, 164, 13–17. [Google Scholar] [CrossRef] [Green Version]
- Ohashi, K.; Burkart, V.; Flohé, S.; Kolb, H. Cutting Edge: Heat Shock Protein 60 Is a Putative Endogenous Ligand of the Toll-Like Receptor-4 Complex. J. Immunol. 2000, 164, 558–561. [Google Scholar] [CrossRef] [Green Version]
- Xu, Q.; Schett, G.; Seitz, C.S.; Hu, Y.; Gupta, R.S.; Wick, G. Surface staining and cytotoxic activity of heat-shock protein 60 antibody in stressed aortic endothelial cells. Circ. Res. 1994, 75, 1078–1085. [Google Scholar] [CrossRef]
- Soltys, B.J.; Gupta, R.S. Cell surface localization of the 60 kDa heat shock chaperonin protein (hsp60) in mammalian cells. Cell Biol. Int. 1997, 21, 315–320. [Google Scholar] [CrossRef]
- Xu, Q.; Kleindienst, R.; Waitz, W.; Dietrich, H.; Wick, G. Increased expression of heat shock protein 65 coincides with a population of infiltrating T lymphocytes in atherosclerotic lesions of rabbits specifically responding to heat shock protein 65. J. Clin. Investig. 1993, 91, 2693–2702. [Google Scholar] [CrossRef] [Green Version]
- Kleindienst, R.; Xu, Q.; Willeit, J.; Waldenberger, F.R.; Weimann, S.; Wick, G. Immunology of atherosclerosis. Demonstration of heat shock protein 60 expression and T lymphocytes bearing alpha/beta or gamma/delta receptor in human atherosclerotic lesions. Am. J. Pathol. 1993, 142, 1927–1937. [Google Scholar]
- Gao, B.; Tsan, M.F. Recombinant Human Heat Shock Protein 60 Does Not Induce the Release of Tumor Necrosis Factor α from Murine Macrophages. J. Biol. Chem. 2003, 278, 22523–22529. [Google Scholar] [CrossRef] [Green Version]
- Levels, J.H.M.; Abraham, P.R.; Ende, A.V.D.; van Deventer, S.J.H. Distribution and Kinetics of Lipoprotein-Bound Endotoxin. Infect. Immun. 2001, 69, 2821–2828. [Google Scholar] [CrossRef] [Green Version]
- Shao, B.; Munford, R.S.; Kitchens, R.L.; Varley, A.W. Hepatic uptake and deacylation of the LPS in bloodborne LPS-lipoprotein complexes. Innate Immun. 2012, 18, 825–833. [Google Scholar] [CrossRef] [Green Version]
- Pedrinaci, S.; Ruiz-Cabello, F.; Gomez, O.; Collado, A.; Garrido, F. Protein kinase C-mediated regulation of the expression of CD14 and CD11/CD18 in U937 cells. Int. J. Cancer 1990, 45, 294–298. [Google Scholar] [CrossRef]
- Tsouknos, A.; Nash, G.B.; Rainger, G.E. Monocytes initiate a cycle of leukocyte recruitment when cocultured with endothelial cells. Atherosclerosis 2003, 170, 49–58. [Google Scholar] [CrossRef]
- Wilkins, G.M.; Leake, D.S. The effect of inhibitors of free radical generating-enzymes on low-density lipoprotein oxidation by macrophages. Biochim. Biophys. Acta 1994, 1211, 69–78. [Google Scholar] [CrossRef]
- Evans, D.J.; Norton, P.; Ivanyi, J. Distribution in tissue sections of the human groEL stress-protein homologue. APMIS 1990, 98, 437–441. [Google Scholar] [CrossRef]
- Siow, R.C.M. Culture of Human Endothelial Cells from Umbilical Veins. Methods Mol. Biol 2012, 806, 265–274. [Google Scholar] [CrossRef]
- Iqbal, A.J.; Krautter, F.; Blacksell, I.A.; Wright, R.D.; Austin-Williams, S.N.; Voisin, M.; Hussain, M.T.; Law, H.L.; Niki, T.; Hirashima, M.; et al. Galectin-9 mediates neutrophil capture and adhesion in a CD44 and β2 integrin-dependent manner. FASEB J. 2022, 36, e22065. [Google Scholar] [CrossRef]
- Nishi, N.; Itoh, A.; Fujiyama, A.; Yoshida, N.; Araya, S.-I.; Hirashima, M.; Shoji, H.; Nakamura, T. Development of highly stable galectins: Truncation of the linker peptide confers protease-resistance on tandem-repeat type galectins. FEBS Lett. 2005, 579, 2058–2064. [Google Scholar] [CrossRef] [Green Version]
- Nishi, N.; Abe, A.; Iwaki, J.; Yoshida, H.; Itoh, A.; Shoji, H.; Kamitori, S.; Hirabayashi, J.; Nakamura, T. Functional and structural bases of a cysteine-less mutant as a long-lasting substitute for galectin-1. Glycobiology 2008, 18, 1065–1073. [Google Scholar] [CrossRef] [Green Version]
- Maxeiner, H.; Husemann, J.; Thomas, C.A.; Loike, J.D.; El Khoury, J.; Silverstein, S.C. Complementary roles for scavenger receptor A and CD36 of human monocyte-derived macrophages in adhesion to surfaces coated with oxidized low-density lipoproteins and in secretion of H2O2. J. Exp. Med. 1998, 188, 2257–2265. [Google Scholar] [CrossRef] [Green Version]
- Nourshargh, S.; Alon, R. Leukocyte Migration into Inflamed Tissues. Immunity 2014, 41, 694–707. [Google Scholar] [CrossRef] [Green Version]
- Johnson-Tidey, R.R.; McGregor, J.L.; Taylor, P.R.; Poston, R.N. Increase in the adhesion molecule P-selectin in endothelium overlying atherosclerotic plaques. Coexpression with intercellular adhesion molecule-1. Am. J. Pathol. 1994, 144, 952–961. [Google Scholar]
- Tandon, N.N.; Kralisz, U.; AJamieson, G. Identification of glycoprotein IV (CD36) as a primary receptor for platelet-collagen adhesion. J. Biol. Chem. 1989, 264, 7576–7583. [Google Scholar] [CrossRef]
- Silverstein, R.L.; Febbraio, M. CD36 and atherosclerosis. Curr. Opin. Lipidol. 2000, 11, 483–491. [Google Scholar] [CrossRef]
- Patten, D.A.; Shetty, S. More Than Just a Removal Service: Scavenger Receptors in Leukocyte Trafficking. Front. Immunol. 2018, 9, 2904. [Google Scholar] [CrossRef] [Green Version]
- Pfeiffer, A.; Böttcher, A.; Orsó, E.; Kapinsky, M.; Nagy, P.; Bodnár, A.; Spreitzer, I.; Liebisch, G.; Drobnik, W.; Gempel, K.; et al. Lipopolysaccharide and ceramide docking to CD14 provokes ligand-specific receptor clustering in rafts. Eur. J. Immunol. 2001, 31, 3153–3164. [Google Scholar] [CrossRef]
- Zanoni, I.; Ostuni, R.; Marek, L.R.; Barresi, S.; Barbalat, R.; Barton, G.M.; Granucci, F.; Kagan, J.C. CD14 Controls the LPS-Induced Endocytosis of Toll-Like Receptor 4. Cell 2011, 147, 868–880. [Google Scholar] [CrossRef]
- Dustin, M.L. The Immunological Synapse. Cancer Immunol. Res. 2014, 2, 1023–1033. [Google Scholar] [CrossRef] [Green Version]
- Ao, M.; Wu, L.; Zhou, X.; Chen, Y. Methyl-β-Cyclodextrin Impairs the Monocyte-Adhering Ability of Endothelial Cells by Down-Regulating Adhesion Molecules and Caveolae and Reorganizing the Actin Cytoskeleton. Biol. Pharm. Bull. 2016, 39, 1029–1034. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Zhou, Y.; Zhang, W.; Qin, Y.; Wei, B.; Sun, Y.; Chen, Y. Methyl-β-cyclodextrin suppresses the monocyte-endothelial adhesion triggered by lipopolysaccharide (LPS) or oxidized low-density lipoprotein (oxLDL). Pharm. Biol. 2021, 59, 1034–1042. [Google Scholar] [CrossRef]
- Del Pozo, M.A. Integrin signaling and lipid rafts. Cell Cycle 2004, 3, 725–728. [Google Scholar]
- Shih, P.T.; Elices, M.J.; Fang, Z.T.; Ugarova, T.P.; Strahl, D.; Territo, M.C.; Frank, J.S.; Kovach, N.L.; Cabanas, C.; Berliner, J.A.; et al. Minimally modified low-density lipoprotein induces monocyte adhesion to endothelial connecting segment-1 by activating beta1 integrin. J. Clin. Investig. 1999, 103, 613–625. [Google Scholar] [CrossRef] [Green Version]
- Niwa, S.; Totsuka, T.; Hayashi, S. Inhibitory effect of fluvastatin, an HMG-CoA reductase inhibitor, on the expression of adhesion molecules on human monocyte cell line. Int. J. Immunopharmacol. 1996, 18, 669–675. [Google Scholar] [CrossRef]
- Kawakami, A.; Tanaka, A.; Nakajima, K.; Shimokado, K.; Yoshida, M. Atorvastatin Attenuates Remnant Lipoprotein-Induced Monocyte Adhesion to Vascular Endothelium under Flow Conditions. Circ. Res. 2002, 91, 263–271. [Google Scholar] [CrossRef] [Green Version]
- Cerda, A.; Rodrigues, A.C.; Alves, C.; Genvigir, F.D.V.; Fajardo, C.M.; Dorea, E.L.; Gusukuma, M.C.; Pinto, G.A.; Hirata, M.H.; Hirata, R.D.C. Modulation of Adhesion Molecules by Cholesterol-Lowering Therapy in Mononuclear Cells from Hypercholesterolemic Patients. Cardiovasc. Ther. 2015, 33, 168–176. [Google Scholar] [CrossRef] [Green Version]
- Weber, C.; Erl, W.; Weber, K.S.C.; Weber, P.C. Effects of Oxidized Low Density Lipoprotein, Lipid Mediators and Statins on Vascular Cell Interactions. Clin. Chem. Lab. Med. CCLM 1999, 37, 243–254. [Google Scholar] [CrossRef] [Green Version]
- Gironi, B.; Kahveci, Z.; McGill, B.; Lechner, B.-D.; Pagliara, S.; Metz, J.; Morresi, A.; Palombo, F.; Sassi, P.; Petrov, P.G. Effect of DMSO on the Mechanical and Structural Properties of Model and Biological Membranes. Biophys. J. 2020, 119, 274–286. [Google Scholar] [CrossRef]
- DeBons, A.F.; Fani, K.; AJimenez, F.; Maayan, M.L. Inhibition of cholesterol-induced atherosclerosis in rabbits by dimethyl sulfoxide. J. Pharmacol. Exp. Ther. 1987, 243, 745–757. [Google Scholar]
- Kollerup, M.B.; Hilscher, M.; Zetner, D.; Rosenberg, J. Adverse reactions of dimethyl sulfoxide in humans: A systematic review. F1000Research 2018, 7, 1746. [Google Scholar] [CrossRef]
- Ravandi, A.; Babaei, S.; Leung, R.; Monge, J.C.; Hoppe, G.; Hoff, H.; Kamido, H.; Kuksis, A. Phospholipids and oxophospholipids in atherosclerotic plaques at different stages of plaque development. Lipids 2004, 39, 97–109. [Google Scholar] [CrossRef]
- Yoshida, H.; Kisugi, R. Mechanisms of LDL oxidation. Clin. Chim. Acta 2010, 411, 1875–1882. [Google Scholar] [CrossRef]
- Ghaffari, S.; Nabi, F.N.; Sugiyama, M.G.; Lee, W.L. Estrogen Inhibits LDL (Low-Density Lipoprotein) Transcytosis by Human Coronary Artery Endothelial Cells via GPER (G-Protein–Coupled Estrogen Receptor) and SR-BI (Scavenger Receptor Class B Type 1). Arterioscler. Thromb. Vasc. Biol. 2018, 38, 2283–2294. [Google Scholar] [CrossRef] [Green Version]
- Libby, P.; Pasterkamp, G.; Crea, F.; Jang, I.-K. Reassessing the Mechanisms of Acute Coronary Syndromes. Circ. Res. 2019, 124, 150–160. [Google Scholar] [CrossRef]
- Swystun, L.L.; Liaw, P.C. The role of leukocytes in thrombosis. Blood 2016, 128, 753–762. [Google Scholar] [CrossRef] [Green Version]
- Silverstein, R.L.; Li, W.; Park, Y.M.; Rahaman, S.O. Mechanisms of cell signaling by the scavenger receptor CD36: Implications in atherosclerosis and thrombosis. Trans. Am. Clin. Clim. Assoc. 2010, 121, 206–220. [Google Scholar]
- Bolick, D.T.; Srinivasan, S.; Whetzel, A.; Fuller, L.C.; Hedrick, C.C. 12/15 lipoxygenase mediates monocyte adhesion to aortic endothelium in apolipoprotein E-deficient mice through activation of RhoA and NF-kappaB. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1260–1266. [Google Scholar] [CrossRef]
- Fu, G.; Deng, M.; Neal, M.D.; Billiar, T.R.; Scott, M.J. Platelet–Monocyte Aggregates: Understanding Mechanisms and Functions in Sepsis. Shock 2021, 55, 156–166. [Google Scholar] [CrossRef]
- Lewis, J.C.; Jones, N.L.; Hermanns, M.; Röhrig, O.; Klein, C.L.; Kirkpatrick, C. Tissue Factor Expression during Coculture of Endothelial Cells and Monocytes. Exp. Mol. Pathol. 1995, 62, 207–218. [Google Scholar] [CrossRef]
- Palabrica, T.; Lobb, R.; Furie, B.C.; Aronovitz, M.; Benjamin, C.; Hsu, Y.-M.; Sajer, S.A.; Furie, B. Leukocyte accumulation promoting fibrin deposition is mediated in vivo by P-selectin on adherent platelets. Nature 1992, 359, 848–851. [Google Scholar] [CrossRef]
- Michelson, A.D.; Barnard, M.R.; Krueger, L.A.; Valeri, C.R.; Furman, M.I. Circulating monocyte-platelet aggregates are a more sensitive marker of in vivo platelet activation than platelet surface P-Selectin: Studies in baboons, human coronary intervention, and human acute myocardial infarction. Circulation 2001, 104, 1533–1537. [Google Scholar] [CrossRef]
- Azumi, H.; Hirata, K.-I.; Ishida, T.; Kojima, Y.; Rikitake, Y.; Takeuchi, S.; Inoue, N.; Kawashima, S.; Hayashi, Y.; Itoh, H.; et al. Immunohistochemical localization of endothelial cell-derived lipase in atherosclerotic human coronary arteries. Cardiovasc. Res. 2003, 58, 647–654. [Google Scholar] [CrossRef] [Green Version]
- Auerbach, B.J.; Bisgaier, C.L.; Wölle, J.; Saxena, U. Oxidation of Low Density Lipoproteins Greatly Enhances Their Association with Lipoprotein Lipase Anchored to Endothelial Cell Matrix. J. Biol. Chem. 1996, 271, 1329–1335. [Google Scholar] [CrossRef] [Green Version]
- Obunike, J.C.; Paka, S.; Pillarisetti, S.; Goldberg, I.J. Lipoprotein lipase can function as a monocyte adhesion protein. Arter. Thromb. Vasc. Biol. 1997, 17, 1414–1420. [Google Scholar] [CrossRef]
- Hayashida, K.; Kume, N.; Minami, M.; Kita, T. Lectin-like oxidized LDL receptor-1 (LOX-1) supports adhesion of mononuclear leukocytes and a monocyte-like cell line THP-1 cells under static and flow conditions. FEBS Lett. 2002, 511, 133–138. [Google Scholar] [CrossRef] [Green Version]
- Duryee, M.J.; Clemens, D.L.; Opperman, P.J.; Thiele, G.M.; Duryee, L.M.; Garvin, R.P.; Anderson, D.R. Malondialdehyde-Acetaldehyde Modified (MAA) Proteins Differentially Effect the Inflammatory Response in Macrophage, Endothelial Cells and Animal Models of Cardiovascular Disease. Int. J. Mol. Sci. 2021, 22, 12948. [Google Scholar] [CrossRef]
- Geloen, A.; Helin, L.; Geeraert, B.; Malaud, E.; Holvoet, P.; Marguerie, G. CD36 Inhibitors Reduce Postprandial Hypertriglyceridemia and Protect against Diabetic Dyslipidemia and Atherosclerosis. PLoS ONE 2012, 7, e37633. [Google Scholar] [CrossRef] [Green Version]
- Gargalovic, P.S.; Gharavi, N.M.; Clark, M.J.; Pagnon, J.; Yang, W.-P.; He, A.; Truong, A.; Baruch-Oren, T.; Berliner, J.A.; Kirchgessner, T.G.; et al. The Unfolded Protein Response Is an Important Regulator of Inflammatory Genes in Endothelial Cells. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2490–2496. [Google Scholar] [CrossRef] [Green Version]
- Mendel, I.; Feige, E.; Yacov, N.; Salem, Y.; Levi, I.; Propheta-Meiran, O.; Shoham, A.; Ishai, E.; George, J.; Harats, D.; et al. VB-201, an oxidized phospholipid small molecule, inhibits CD14- and Toll-like receptor-2-dependent innate cell activation and constrains atherosclerosis. Clin. Exp. Immunol. 2013, 175, 126–137. [Google Scholar] [CrossRef]
- Feige, E.; Yacov, N.; Salem, Y.; Levi, I.; Mendel, I.; Propheta-Meiran, O.; Shoham, A.; Hait-Darshan, R.; Polonsky, O.; George, J.; et al. Inhibition of monocyte chemotaxis by VB-201, a small molecule lecinoxoid, hinders atherosclerosis development in ApoE−/− mice. Atherosclerosis 2013, 229, 430–439. [Google Scholar] [CrossRef] [Green Version]
- Montecucco, F.; Lenglet, S.; Carbone, F.; Boero, S.; Pelli, G.; Burger, F.; Roth, A.; Bertolotto, M.; Nencioni, A.; Cea, M.; et al. Treatment with KLEPTOSE® CRYSMEB reduces mouse atherogenesis by impacting on lipid profile and Th1 lymphocyte response. Vasc. Pharmacol. 2015, 72, 197–208. [Google Scholar] [CrossRef]
- Zimmer, S.; Grebe, A.; Bakke, S.S.; Bode, N.; Halvorsen, B.; Ulas, T.; Skjelland, M.; De Nardo, D.; Labzin, L.I.; Kerksiek, A.; et al. Cyclodextrin promotes atherosclerosis regression via macrophage reprogramming. Sci. Transl. Med. 2016, 8, 333ra50. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Zhang, X.; Yu, B.; Peng, X.; Liu, Y.; Wang, A.; Zhao, D.; Pang, D.; Ouyang, H.; Tang, X. Cyclodextrin Ameliorates the Progression of Atherosclerosis via Increasing High-Density Lipoprotein Cholesterol Plasma Levels and Anti-inflammatory Effects in Rabbits. J. Cardiovasc. Pharmacol. 2019, 73, 334–342. [Google Scholar] [CrossRef]
- Kim, H.; Han, J.; Park, J.-H. Cyclodextrin polymer improves atherosclerosis therapy and reduces ototoxicity. J. Control. Release 2020, 319, 77–86. [Google Scholar] [CrossRef]
- Kilsdonk, E.P.C.; Yancey, P.G.; Stoudt, G.W.; Bangerter, F.W.; Johnson, W.J.; Phillips, M.C.; Rothblat, G.H. Cellular Cholesterol Efflux Mediated by Cyclodextrins. J. Biol. Chem. 1995, 270, 17250–17256. [Google Scholar] [CrossRef] [Green Version]
- Bakke, S.S.; Aune, M.H.; Niyonzima, N.; Pilely, K.; Ryan, L.; Skjelland, M.; Garred, P.; Aukrust, P.; Halvorsen, B.; Latz, E.; et al. Cyclodextrin Reduces Cholesterol Crystal–Induced Inflammation by Modulating Complement Activation. J. Immunol. 2017, 199, 2910–2920. [Google Scholar] [CrossRef] [Green Version]
- Pilely, K.; Bakke, S.S.; Palarasah, Y.; Skjoedt, M.-O.; Bartels, E.D.; Espevik, T.; Garred, P. Alpha-cyclodextrin inhibits cholesterol crystal-induced complement-mediated inflammation: A potential new compound for treatment of atherosclerosis. Atherosclerosis 2019, 283, 35–42. [Google Scholar] [CrossRef] [Green Version]
- Oesterle, A.; Laufs, U.; Liao, J.K. Pleiotropic Effects of Statins on the Cardiovascular System. Circ. Res. 2017, 120, 229–243. [Google Scholar] [CrossRef]







| Primary Antibodies | Immunoglobulin Class | Human Target Antigen | Supplier |
|---|---|---|---|
| EO6 | IgM | Oxidised phospholipid | Avanti |
| HTA125 | IgG2a | TLR4 | BioRad |
| II-13 | IgG2a | HSP60 | Abcam |
| LK-1 | IgG1 | HSP60 | Merck/Sigma |
| MDA2 | IgG2a | Malondialdehyde | Abcam |
| ML30 | IgG1 | HSP60 | Reference [44] |
| MOPC104E | IgM | Negative control | Merck/Sigma |
| MOPC21 | IgG1 | Negative control | Merck/Sigma |
| polyclonal | - | Apolipoprotein B | Dako/Invitrogen |
| UCHM1 | IgG2a | CD14 | BioRad |
| UPC10 | IgG2a | Negative control | Merck/Sigma |
| W6/32 | IgG2a | MHC/HLA Class 1 | Abcam |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poston, R.N.; Chughtai, J.; Ujkaj, D.; Louis, H.; Leake, D.S.; Cooper, D. Monocytic Cell Adhesion to Oxidised Ligands: Relevance to Cardiovascular Disease. Biomedicines 2022, 10, 3083. https://doi.org/10.3390/biomedicines10123083
Poston RN, Chughtai J, Ujkaj D, Louis H, Leake DS, Cooper D. Monocytic Cell Adhesion to Oxidised Ligands: Relevance to Cardiovascular Disease. Biomedicines. 2022; 10(12):3083. https://doi.org/10.3390/biomedicines10123083
Chicago/Turabian StylePoston, Robin N., Jenna Chughtai, Desara Ujkaj, Huguette Louis, David S. Leake, and Dianne Cooper. 2022. "Monocytic Cell Adhesion to Oxidised Ligands: Relevance to Cardiovascular Disease" Biomedicines 10, no. 12: 3083. https://doi.org/10.3390/biomedicines10123083
APA StylePoston, R. N., Chughtai, J., Ujkaj, D., Louis, H., Leake, D. S., & Cooper, D. (2022). Monocytic Cell Adhesion to Oxidised Ligands: Relevance to Cardiovascular Disease. Biomedicines, 10(12), 3083. https://doi.org/10.3390/biomedicines10123083