Role of Polyphenol-Derived Phenolic Acid in Mitigation of Inflammasome-Mediated Anxiety and Depression
Abstract
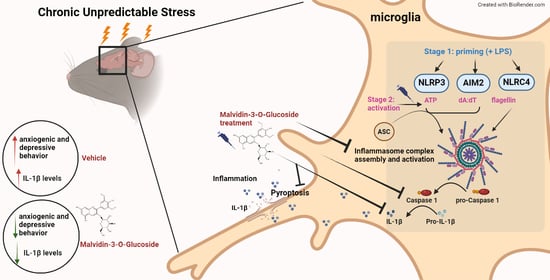
:1. Introduction
2. Materials and Methods
2.1. In Vitro Experiments
2.1.1. Preparation of Murine Primary Cortical Microglia Culture
2.1.2. Polyphenols Screening In Vitro
2.1.3. Determination of the MG Cytotoxicity
2.1.4. Microglia Activation
2.1.5. Pyroptosis Assessment
2.1.6. Dose–Response Curve for NLRP3 Inhibition
2.1.7. Activation of the NLRC4 and AIM2 Inflammasomes
2.1.8. Inhibition of the Caspase-1 Activity
2.1.9. THP1 Macrophages
2.2. In Vivo Studies
2.2.1. Animals
2.2.2. Malvidin-3-O-Glucoside (MG) Treatment
2.2.3. Behavioral Studies
Chronic Unpredictable Stress (CUS) Protocol
Elevated Plus Maze (EPM)
Forced Swim Test (FST)
2.2.4. MG bioavailability: Pharmacokinetics
Sample Preparation
LC–MS Method
2.2.5. Quantitative PCR
2.3. ELISA Assay
2.4. Statistical Analysis
3. Results
3.1. The Natural Polyphenol MG Reduces NLRP3–Mediated IL–1β Production in Murine Primary Cortical Microglia
3.2. MG Is Not Cytotoxic and Elicits Anti–Pyroptotic Properties in Murine Primary Cortical Microglia
3.3. MG Potently Inhibits NLRP3, NLRC4, and AIM2 Inflammasome Assembly
3.4. MG Does Not Interfere with The transcriptional Priming of Inflammasome Components
3.5. MG Treatment Decreases Anxiety and Depressive-Related Behavior in a Mouse Model of Chronic Unpredictable Stress (CUS)
3.6. Il-1β Is Downregulated after MG Treatment in a Mouse Model of Chronic Unpredictable Stress (CUS)
3.7. MG Is Rapidly Degraded in Brain and Plasma after Intragastric Administration: Pharmacokinetics Study
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Landbergis, P.A. The changing organization of work and the safety and health of working people: A commentary. J. Occup. Environ. Med. 2003, 45, 61–72. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S. Allostasis and allostatic load: Implications for neuropsychopharmacology. Neuropsychopharmacology 2000, 22, 108–124. [Google Scholar] [CrossRef]
- Liu, Y.Z.; Wang, Y.X.; Jiang, C.L. Inflammation: The Common Pathway of Stress-Related Diseases. Front. Hum. Neurosci. 2017, 11, 316. [Google Scholar] [CrossRef] [PubMed]
- Norman, G.J.; Karelina, K.; Zhang, N.; Walton, J.C.; Morris, J.S.; Devries, A.C. Stress and IL-1b contribute to the development of depressive-like behavior following peripheral nerve injury. Mol. Psychiatry 2010, 15, 404–414. [Google Scholar] [CrossRef] [Green Version]
- Krizanova, O.; Babula, P.; Pacak, K. Stress, catecholaminergic system and cancer. Stress 2016, 19, 419–428. [Google Scholar] [CrossRef]
- Kuo, L.E.; Czarnecka, M.; Kitlinska, J.B.; Tilan, J.U.; Kvetnanský, R.; Zukowska, Z. Chronic stress, combined with a high-fat/high-sugar diet, shifts sympathetic signaling toward neuropeptide Y and leads to obesity and the metabolic syndrome. Ann. N. Y. Acad. Sci. 2008, 1148, 232–237. [Google Scholar] [CrossRef] [Green Version]
- Mikolajczyk, R.T.; El Ansari, W.; Maxwell, A.E. Food consumption frequency and perceived stress and depressive symptoms among students in three European countries. Nutr. J. 2009, 8, 31. [Google Scholar] [CrossRef] [Green Version]
- Kunjathoor, V.V.; Tseng, A.A.; Medeiros, L.A.; Khan, T.; Moore, K.J. β-Amyloid promotes accumulation of lipid peroxides by inhibiting CD36-mediated clearance of oxidized lipoproteins. J. Neuroinflamm. 2004, 1, 23. [Google Scholar] [CrossRef] [Green Version]
- García-Bueno, B.; Caso, J.R.; Leza, J.C. Stress as a neuroinflammatory condition in brain: Damaging and protective mechanisms. Neurosci. Biobehav. Rev. 2008, 32, 1136–1151. [Google Scholar] [CrossRef]
- Munhoz, C.D.; García-Bueno, B.; Madrigal, J.L.M.; Lepsch, L.B.; Scavone, C.; Leza, J.C. Stress-induced neuroinflammation: Mechanisms and new pharmacological targets. Braz. J. Med. Biol. Res. 2008, 41, 1037–1046. [Google Scholar] [CrossRef]
- Rohleder, N. Stimulation of systemic low-grade inflammation by psychosocial stress. Psychosom. Med. 2014, 76, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Danesh, J.; Whincup, P.; Walker, M.; Lennon, L.; Thomson, A.; Appleby, P.; Gallimore, J.R.; Pepys, M.B. Low-grade inflammation and coronary heart disease: Prospective study and updated meta-analyses. BMJ 2000, 321, 199–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dowlati, Y.; Herrmann, N.; Swardfager, W.; Liu, H.; Sham, L.; Reim, E.K.; Lanctot, K.L. A meta-analysis of cytokines in major depression. Biol. Psychiatry 2010, 67, 446–457. [Google Scholar] [CrossRef] [PubMed]
- Howren, M.B.; Lamkin, D.M.; Suls, J. Associations of depression with C reactive protein, IL-1, and IL-6: A meta-analysis. Psychosom. Med. 2009, 71, 171–186. [Google Scholar] [CrossRef] [Green Version]
- Norman, R.S. The macrophage theory of depression. Med. Hypotheses 1991, 35, 298–306. [Google Scholar]
- Schiepers, O.J.G.; Wichers, M.C.; Maes, M. Cytokines and major depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2005, 29, 201–217. [Google Scholar] [CrossRef]
- Johnson, J.D.; Campisi, J.; Sharkey, C.M.; Kennedy, S.L.; Nickerson, M.; Greenwood, B.N.; Fleshner, M. Catecholamines mediate stress-induced increases in peripheral and central inflammatory cytokines. Neuroscience 2005, 135, 1295–1307. [Google Scholar] [CrossRef]
- Wohleb, E.S.; Delpech, J.C. Dynamic cross-talk between microglia and peripheral monocytes underlies stress-induced neuroinflammation and behavioral consequences. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 79, 40–48. [Google Scholar] [CrossRef]
- Miller, A.H.; Maletic, V.; Raison, C.L. Inflammation and its discontents: The role of cytokines in the pathophysiology of major depression. Biol. Psychiatry 2009, 65, 732–741. [Google Scholar] [CrossRef] [Green Version]
- Medzhitov, R.; Janeway, C., Jr. Innate immunity. N. Engl. J. Med. 2000, 343, 338–344. [Google Scholar] [CrossRef]
- Sebastian-Valverde, M.; Pasinetti, G.M. The NLRP3 Inflammasome as a Critical Actor in the Inflammaging Process. Cells 2020, 9, 1552. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.Y.; Nuñez, G. Sterile inflammation: Sensing and reacting to damage. Nat. Rev. Immunol. 2010, 10, 826–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Wang, H.; Kouadir, M.; Song, H.; Shi, F. Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death Dis. 2019, 10, 128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chear, C.T.; Nallusamy, R.; Canna, S.W.; Chan, K.C.; Baharin, M.F.; Hishamshah, M.; Ghani, H.; Ripen, A.M.; Mohamad, S.B. A novel de novo NLRC4 mutation reinforces the likely pathogenicity of specific LRR domain mutation. Clin. Immunol. 2020, 211, 108328. [Google Scholar] [CrossRef]
- Duncan, J.A.; Bergstralh, D.T.; Wang, Y.; Willingham, S.B.; Ye, Z.; Zimmermann, A.G.; Ting, J.P.Y. Cryopyrin/NALP3 binds ATP/dATP, is an ATPase, and requires ATP binding to mediate inflammatory signaling. Proc. Natl. Acad. Sci. USA 2007, 104, 8041–8046. [Google Scholar] [CrossRef] [Green Version]
- Lamkanfi, M.; Dixit, V.M. Inflammasomes and their roles in health and disease. Annu. Rev. Cell Dev. Biol. 2012, 28, 137–161. [Google Scholar] [CrossRef] [Green Version]
- Strowig, T.; Henao-Mejia, J.; Elinav, E.; Flavell, R. Inflammasomes in health and disease. Nature 2012, 481, 278–286. [Google Scholar] [CrossRef]
- Fernandes-Alnemri, T.; Yu, J.W.; Datta, P.; Wu, J.; Alnemri, E.S. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature 2009, 458, 509–513. [Google Scholar] [CrossRef] [Green Version]
- Lamkanfi, M.; Dixit, V.M. Mechanisms and functions of inflammasomes. Cell 2014, 157, 1013–1022. [Google Scholar] [CrossRef] [Green Version]
- Martinon, F.; Burns, K.; Tschopp, J. The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol. Cell 2002, 10, 417–426. [Google Scholar] [CrossRef]
- Jorgensen, I.; Miao, E.A. Pyroptotic cell death defends against intracellular pathogens. Immunol. Rev. 2015, 265, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Miao, E.A.; Miao, E.A.; Leaf, I.A.; Treuting, P.M.; Mao, D.P.; Dors, M.; Sarkar, A.; Warren, S.E.; Wewers, M.D.; Aderem, A. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat. Immunol. 2010, 11, 1136–1142. [Google Scholar] [CrossRef] [PubMed]
- Aglietti, R.A.; Estevez, A.; Gupta, A.; Gonzalez Ramirez, M.; Liu, P.S.; Kayagaki, N.; Ciferri, C.; Dixit, V.M.; Dueber, E.C. GsdmD p30 elicited by caspase-11 during pyroptosis forms pores in membranes. Proc. Natl. Acad. Sci. USA 2016, 113, 7858–7863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, J.; Zhao, Y.; Wang, K.; Shi, X.; Wang, Y.; Huang, H.; Zhuang, Y.; Cai, T.; Wang, F.; Shao, F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 2015, 526, 660–665. [Google Scholar] [CrossRef]
- Sebastian-Valverde, M.; Wu, H.; Al Rahim, M.; Sanchez, R.; Kumar, K.; De Vita, R.J.; Pasinetti, G.M. Discovery and characterization of small-molecule inhibitors of NLRP3 and NLRC4 inflammasomes. J. Biol. Chem. 2021, 296, 100597. [Google Scholar] [CrossRef]
- Bagchi, D.; Bagchi, M.; Stohs, S.J.; Das, D.K.; Ray, S.D.; Kuszynski, C.A.; Joshi, S.S.; Pruess, H.G. Free radicals and grape seed proanthocyanidin extract: Importance in human health and disease prevention. Toxicology 2000, 148, 187–197. [Google Scholar] [CrossRef]
- Montagut, G.; Onnockx, S.; Vaqué, M.; Bladé, C.; Blay, M.; Fernández-Larrea, J.; Pujadas, G.; Salvadó, M.J.; Arola, L.; Pirson, I.; et al. Oligomers of a grape-seed procyanidin extract activate the insulin receptor and key targets of the insulin signaling pathway differently from insulin. J. Nutr. Biochem. 2010, 21, 476–481. [Google Scholar] [CrossRef]
- Song, X.; Siriwardhana, N.; Rathore, K.; Lin, D.; Wang, H.C. Grape seed proanthocyanidin suppression of breast cell carcinogenesis induced by chronic exposure to combined 4-(methylnitorsamino)-1- (3-pyridyl)-1-butanone and benzo[a]pyrene. Mol. Carcinog. 2010, 49, 450–463. [Google Scholar] [CrossRef] [Green Version]
- Ferruzzi, M.G.; Lobo, J.K.; Janle, E.M.; Whittaker, N.; Cooper, B.; Simon, J.E.; Wu, Q.L.; Welch, C.; Ho, L.; Weaver, C.; et al. Bioavailability of gallic acid and catechins from grape seed polyphenol extract is improved by repeated dosing in rats: Implications for treatment in Alzheimer’s disease. J. Alzheimer’s Dis. 2009, 18, 113–124. [Google Scholar] [CrossRef] [Green Version]
- Ho, L.; Yemul, S.; Wang, J.; Pasinetti, G.M. Grape seed polyphenolic extract as a potential novel therapeutic agent in tauopathies. J. Alzheimer’s Dis. 2009, 16, 433–439. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Ho, L.; Zhao, W.; Ono, K.; Rosensweig, C.; Chen, L.; Humala, N.; Teplow, D.B.; Pasinetti, G.M. Grape-derived polyphenolics prevent abeta oligomerization and attenuate cognitive deterioration in a mouse model of Alzheimer’s disease. J. Neurosci. 2008, 28, 6388–6392. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Thomas, P.; Zhong, J.H.; Bi, F.F.; Kosaraju, S.; Pollard, A.; Fenech, M.; Zhou, X.F. Consumption of grape seed extract prevents amyloid-beta deposition and attenuates inflammation in brain of an Alzheimer’s disease mouse. Neurotox. Res. 2009, 15, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.Y.; Liu, Y.M.; Wang, J.; Wang, X.N.; Li, C.Y. Anti-Inflammatory Effect of the Blueberry Anthocyanins Malvidin-3-Glucoside and Malvidin-3-Galactoside in Endothelial Cells. Molecules 2014, 19, 12827–12841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, W.Y.; Wang, J.; Liu, Y.M.; Zheng, Q.S.; Li, C.Y. Inhibitory effect of Malvidin on TNF-α-induced inflammatory response in endothelial cells. Eur. J. Pharmacol. 2014, 723, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Esposito, D.; Chen, A.; Grace, M.H.; Komarnytsky, S.; Lila, M.A. Inhibitory effects of wild blueberry anthocyanins and other flavonoids on biomarkers of acute and chronic inflammation in vitro. J. Agric. Food Chem. 2014, 62, 7022–7028. [Google Scholar] [CrossRef]
- Bronstein, R.; Torres, L.; Nissen, J.C.; Tsirka, S.E. Culturing microglia from the neonatal and adult central nervous system. J. Vis. Exp. 2013, 78, 50647. [Google Scholar] [CrossRef]
- Saura, J.; Tusell, J.M.; Serratosa, J. High-yield isolation of murine microglia by mild trypsinization. Glia 2003, 44, 183–189. [Google Scholar] [CrossRef]
- Wang, J.; Bi, W.; Cheng, A.; Freire, D.; Vempati, P.; Zhao, W.; Gong, B.; Janle, E.; Chen, T.Y.; Ferruzzi, M.; et al. Targeting multiple pathogenic mechanisms with polyphenols for the treatment of Alzheimer’s disease-experimental approach and therapeutic implications. Front. Aging Neurosci. 2014, 6, 42. [Google Scholar]
- Vingtdeux, V.; Giliberto, L.; Zhao, H.; Chandakkar, P.; Wu, Q.; Simon, J.E.; Janle, E.M.; Lobo, J.; Ferruzzi, M.G.; Davies, P.; et al. AMP-activated protein kinase signaling activation by resveratrol modulates amyloid-beta peptide metabolism. J. Biol. Chem. 2010, 285, 9100–9113. [Google Scholar] [CrossRef] [Green Version]
- Krikorian, R.; Shidler, M.D.; Nash, T.A.; Kalt, W.; Vinqvist-Tymchuk, M.R.; Shukitt-Hale, B.; Joseph, J.A. Blueberry supplementation improves memory in older adults. J. Agric. Food Chem. 2010, 58, 3996–4000. [Google Scholar] [CrossRef] [Green Version]
- Kanneganti, T.D. The inflammasome: Firing up innate immunity. Immunol. Rev. 2015, 265, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westfall, S.; Caracci, F.; Zhao, D.; Wu, Q.L.; Frolinger, T.; Simon, J.; Pasinetti, G.M. Microbiota metabolites modulate the T helper 17 to regulatory T cell (Th17/Treg) imbalance promoting resilience to stress-induced anxiety- and depressive-like behaviors. Brain Behav. Immun. 2021, 91, 350–368. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.L.; Charney, D.S.; Lewis, L.; Golden, R.N.; Gorman, J.M.; Krishnan, K.R.; Nemeroff, C.B.; Bremner, J.D.; Carney, R.M.; Coyne, J.C.; et al. Mood disorders in the medically ill: Scientific review and recommendations. Biol. Psychiatry 2005, 58, 175–189. [Google Scholar] [CrossRef] [PubMed]
- Hyun, J.W.; Chung, H.S. Cyanidin and malvidin from Oryza sativa cv. Heugjinjubyeo mediate cytotoxicity against human monocytic leukemia cells by arrest of G(2)/M phase and induction of apoptosis. J. Agric. Food Chem. 2004, 52, 2213–2217. [Google Scholar] [CrossRef] [PubMed]
- Patterson, S.J.; Fischer, J.G.; Dulebohn, R.V. DNA damage in HT-29 colon cancer cells is enhanced by high concentrations of the anthocyanin malvidin. FASEB J. 2008, 22, 890.10. [Google Scholar] [CrossRef]
- Song, A.Q.; Gao, B.; Fan, J.J.; Zhu, Y.J.; Wang, Y.L.; Xu, L.Z.; Wu, W.N. NLRP1 inflammasome contributes to chronic stress-induced depressive-like behaviors in mice. J. Neuroinflamm. 2020, 17, 178. [Google Scholar] [CrossRef]
- Dong, Y.; Li, S.; Lu, Y.; Li, X.; Liao, Y.; Peng, Z.; Li, Y.; Hou, L.; Yuan, Z.; Cheng, J. Stress-induced NLRP3 inflammasome activation negatively regulates fear memory in mice. J. Neuroinflamm. 2020, 17, 205. [Google Scholar] [CrossRef]
- Fan, R.; You, M.; Toney, A.M.; Kim, J.; Giraud, D.; Xian, Y.; Ye, F.; Gu, L.; Ramer-Tait, A.E.; Chung, S. Red Raspberry Polyphenols Attenuate High-Fat Diet-Driven Activation of NLRP3 Inflammasome and its Paracrine Suppression of Adipogenesis via Histone Modifications. Mol. Nutr. Food Res. 2020, 64, e1900995. [Google Scholar] [CrossRef]
- Wang, D.X.; Zhang, M.; Wang, T.T.; Cai, M.; Qian, F.; Sun, Y.; Wang, Y.J. Green tea polyphenols prevent lipopolysaccharide-induced inflammatory liver injury in mice by inhibiting NLRP3 inflammasome activation. Food Funct. 2019, 10, 3898–3908. [Google Scholar] [CrossRef]
- Iwata, M.; Ota, K.T.; Duman, R.S. The inflammasome: Pathways linking psychological stress, depression, and systemic illnesses. Brain Behav. Immun. 2013, 31, 105–114. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Liu, L.; Liu, Y.Z.; Shen, X.L.; Wu, T.Y.; Zhang, T.; Wang, W.; Wang, Y.X.; Jiang, C.L. NLRP3 Inflammasome Mediates Chronic Mild Stress-Induced Depression in Mice via Neuroinflammation. Int. J. Neuropsychopharmacol. 2015, 18, pyv006. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Wang, J.; Bi, W.; Ferruzzi, M.; Yemul, S.; Freire, D.; Mazzola, P.; Ho, L.; Dubner, L.; Pasinetti, G.M. Novel application of brain-targeting polyphenol compounds in sleep deprivation-induced cognitive dysfunction. Neurochem. Int. 2015, 89, 191–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Hodes, G.E.; Zhang, H.; Zhang, S.; Zhao, W.; Golden, S.A.; Bi, W.; Menard, C.; Kana, V.; Leboeuf, M.; et al. Epigenetic modulation of inflammation and synaptic plasticity promotes resilience against stress in mice. Nat. Commun. 2018, 9, 477. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Ho, L.; Zhao, Z.; Seror, I.; Humala, N.; Dickstein, D.L.; Thiyagarajan, M.; Percival, S.S.; Talcott, S.T.; Pasinetti, G.M. Moderate consumption of Cabernet Sauvignon attenuates Abeta neuropathology in a mouse model of Alzheimer’s disease. FASEB J. 2006, 20, 2313–2320. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Santa-Maria, I.; Ho, L.; Ksiezak-Reding, H.; Ono, K.; Teplow, D.B.; Pasinetti, G.M. Grape derived polyphenols attenuate tau neuropathology in a mouse model of Alzheimer’s disease. J. Alzheimer’s Dis. 2010, 22, 653–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasinetti, G.M. Novel role of red wine-derived polyphenols in the prevention of Alzheimer’s disease dementia and brain pathology: Experimental approaches and clinical implications. Planta Med. 2012, 78, 1614–1619. [Google Scholar]
- Rezaee, N.; Fernando, B.; Hone, E.; Sohrabi, H.R.; Johnson, S.K.; Gunzburg, S.; Martins, R.N. Potential of Sorghum Polyphenols to Prevent and Treat Alzheimer’s Disease: A Review Article. Front. Aging Neurosci. 2021, 13, 72949. [Google Scholar] [CrossRef]
- Clifford, M.N. Diet-derived phenols in plasma and tissues and their implications for health. Planta Med. 2004, 70, 1103–1114. [Google Scholar] [CrossRef] [Green Version]
- Saura-Calixto, F.; Serrano, J.; Goñi, I. Intake and bioaccessibility of total polyphenols in a whole diet. Food Chem. 2007, 101, 492–501. [Google Scholar] [CrossRef] [Green Version]
- Frolinger, T.; Sims, S.; Smith, C.; Wang, J.; Cheng, H.; Faith, J.; Ho, L.; Hao, K.; Pasinetti, G.M. The gut microbiota composition affects dietary polyphenols-mediated cognitive resilience in mice by modulating the bioavailability of phenolic acids. Sci. Rep. 2019, 9, 3546. [Google Scholar] [CrossRef] [Green Version]
- Calani, L.; Dall’Asta, M.; Derlindati, E.; Scazzina, F.; Bruni, R.; Del Rio, D. Colonic metabolism of polyphenols from coffee, green tea, and hazelnut skins. J. Clin. Gastroenterol. 2012, 46, S95–S99. [Google Scholar] [CrossRef] [PubMed]
- Monagas, M.; Urpi-Sarda, M.; Sanchez-Patan, F.; Llorach, R.; Garrido, I.; Gomez-Cordoves, C.; Andres-Lacueva, C.; Bartolome, B. Insights into the metabolism and microbial biotransformation of dietary flavan-3-ols and the bioactivity of their metabolites. Food Funct. 2010, 1, 233–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Ho, L.; Faith, J.; Ono, K.; Janle, E.M.; Lachcik, P.J.; Cooper, B.R.; Jannasch, A.H.; D’Arcy, B.R.; Williams, B.A.; et al. Role of intestinal microbiota in the generation of polyphenol-derived phenolic acid mediated attenuation of Alzheimer’s disease β-amyloid oligomerization. Mol. Nutr. Food Res. 2015, 59, 1025–1040. [Google Scholar] [CrossRef] [PubMed]
- Cruz, L.; Fernandes, I.; Évora, A.; de Freitas, V.; Mateus, N. Synthesis of the Main Red Wine Anthocyanin Metabolite: Malvidin-3-O-β-Glucuronide Synthesis of the Main Red Wine Anthocyanin Metabolite: Malvidin-3-O-β-Glucuronide. Synlett 2017, 28, 593–596. [Google Scholar] [CrossRef] [Green Version]








| Name | Nature | Company | Purity |
|---|---|---|---|
| Ferulic acid (FA) | Phenolic acid | MP Biochemicals (Irvine, CA, USA) | No information |
| Vanillic acid (VA) | Phenolic acid | Sigma Aldrich (St. Louis, MO, USA) | (HPLC) ≥ 97% |
| 3′,4′-dihydrocaffeic acid (DHCA) | Phenolic acid | Sigma Aldrich (St. Louis, MO, USA) | (titration by NaOH) 97.5-102.5% |
| Epicatechin (EPI) | Polyphenol | Thermo Fisher Scientific (Waltham, MA, USA | ≈60% |
| Catechin (CA) | Polyphenol | Sigma Aldrich (St. Louis, MO, USA) | (HPLC) ≥ 96% |
| Resveratrol (Resv) | Polyphenol | Sigma Aldrich (St. Louis, MO, USA) | (HPLC) ≥ 98.5% |
| OMe-quercetin-glucuronide (3MQG) | Polyphenol metabolite | Donated | No information |
| Hippuric acid (HA) | Phenolic acid | Sigma Aldrich (St. Louis, MO, USA) | (titration by NaOH) 97.5-102.5% |
| 3- (3′-hydroxyphenyl)propionic acid (3HPPA) | Phenolic acid | Alfa Aesar (Tewksbury, MA, USA) | (HPLC) ≥ 98% |
| 3-hydroxypropionic acid (3HPA) | Phenolic acid | Sigma Aldrich (St. Louis, MO, USA) | (TLC) ≥ 99% |
| Quercetin-glucuronide (QG) | Polyphenol metabolite | Sigma Aldrich (St. Louis, MO, USA) | (LC/MS-ELSD) ≥ 95% |
| Malvidin-3-O-glucoside (MG) | Polyphenol | Millipore Sigma (Burlington, MA, USA) and Extrasynthese (Rhone, France) | (HPLC) ≥ 95% |
| 3-hydroxybenzoic acid (3HBA) | Phenolic acid | Sigma Aldrich (St. Louis, MO, USA) | (HPLC) ≥ 98.5% |
| Stress 1 | Stress 2 | |
|---|---|---|
| Day | 9:00 a.m. | 4:00 p.m. |
| 1 | Crowd 1 h | Cold swim 5 m |
| 2 | Restrain 1 h | Lights on overnight |
| 3 | No food 7 h | Cage shake 20 m |
| 4 | Wet bedding 7 h | Restrain 1 h |
| 5 | Hot drier 10 m | Cage tilt 17 h |
| 6 | No water 7 h | Lights on overnight |
| 7 | Cold swim 5 m | Crowd 1 h |
| 8 | Restrain 1 h | No bedding 17 h |
| 9 | Cage shake 20 m | No food 17 h |
| 10 | Hot drier 10 m | Crowd 1 h |
| 11 | Cold swim 5 m | Restrain 1 h |
| 12 | Wet bedding 7 h | No bedding 17 h |
| 13 | Crowd 1 h | Cage tilt 17 h |
| 14 | Hot drier 10 m | Cage shake 1 h |
| 15 | Restrain 2 h | No food 17 h |
| 16 | Cage shake 1 h | Foot shock |
| 17 | Tail suspension 1 h | Cage tilt 17 h + Lights on |
| 18 | Cold swim 5 m | No bedding 17 h |
| 19 | Hot drier 10 m | Cage tilt 17 h + Lights on |
| 20 | Cage shake 1 h | No food + Lights on |
| 21 | Wet bedding 7 h | Restrain + Predator odor 1 h |
| 22 | Hot drier 10 m | Cage tilt 17 h |
| 23 | Crowd 2 h | Tail suspension 1 h |
| 24 | Restrain + Predator odor 2 h | Cage shake 1 h |
| 25 | Hot drier 10 m | Crowd 1 h |
| 26 | Cage shake 1 h | Lights on overnight |
| 27 | Restrain + Predator odor 1 h | No bedding 17 h |
| 28 | Hot drier 10 m | Cage tilt 17 h |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iban-Arias, R.; Sebastian-Valverde, M.; Wu, H.; Lyu, W.; Wu, Q.; Simon, J.; Pasinetti, G.M. Role of Polyphenol-Derived Phenolic Acid in Mitigation of Inflammasome-Mediated Anxiety and Depression. Biomedicines 2022, 10, 1264. https://doi.org/10.3390/biomedicines10061264
Iban-Arias R, Sebastian-Valverde M, Wu H, Lyu W, Wu Q, Simon J, Pasinetti GM. Role of Polyphenol-Derived Phenolic Acid in Mitigation of Inflammasome-Mediated Anxiety and Depression. Biomedicines. 2022; 10(6):1264. https://doi.org/10.3390/biomedicines10061264
Chicago/Turabian StyleIban-Arias, Ruth, Maria Sebastian-Valverde, Henry Wu, Weiting Lyu, Qingli Wu, Jim Simon, and Giulio Maria Pasinetti. 2022. "Role of Polyphenol-Derived Phenolic Acid in Mitigation of Inflammasome-Mediated Anxiety and Depression" Biomedicines 10, no. 6: 1264. https://doi.org/10.3390/biomedicines10061264
APA StyleIban-Arias, R., Sebastian-Valverde, M., Wu, H., Lyu, W., Wu, Q., Simon, J., & Pasinetti, G. M. (2022). Role of Polyphenol-Derived Phenolic Acid in Mitigation of Inflammasome-Mediated Anxiety and Depression. Biomedicines, 10(6), 1264. https://doi.org/10.3390/biomedicines10061264