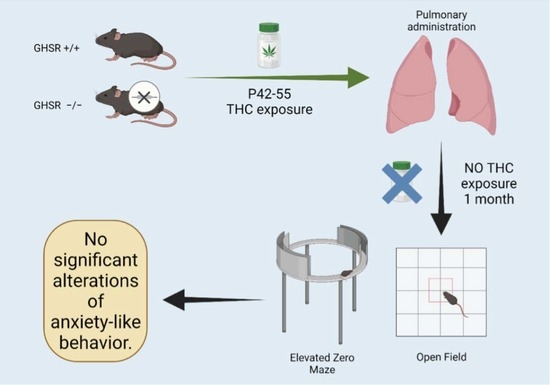
Impaired Ghrelin Signaling Does Not Lead to Alterations of Anxiety-like Behaviors in Adult Mice Chronically Exposed to THC during Adolescence
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
2.3. THC Administration
2.4. Behavioral Assessments
2.4.1. Open Field
2.4.2. Elevated Zero Maze
2.5. Statistics
3. Results
3.1. Open Field
3.2. Elevated Zero Maze
4. Discussion
Limitations and Future Studies
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spear, L.P. The adolescent brain and age-related behavioral manifestations. Neurosci. Biobehav. Rev. 2000, 24, 417–463. [Google Scholar] [CrossRef] [PubMed]
- Dahl, R.E. Adolescent development and the regulation of behavior and emotion: Introduction to part VIII. Ann. N. Y. Acad. Sci. 2004, 1021, 294–295. [Google Scholar] [CrossRef] [PubMed]
- Andersen, S.L. Trajectories of brain development: Point of vulnerability or window of opportunity? Neurosci. Biobehav. Rev. 2003, 27, 3–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miech, R.; Johnston, L.; O’Malley, P.M. Prevalence and Attitudes Regarding Marijuana Use Among Adolescents Over the Past Decade. Pediatrics 2017, 140, e20170982. [Google Scholar] [CrossRef] [Green Version]
- Curran, H.V.; Freeman, T.P.; Mokrysz, C.; Lewis, D.A.; Morgan, C.J.; Parsons, L.H. Keep off the grass? Cannabis, cognition and addiction. Nat. Rev. Neurosci. 2016, 17, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Silins, E.; Horwood, L.J.; Patton, G.C.; Fergusson, D.M.; Olsson, C.A.; Hutchinson, D.M.; Spry, E.; Toumbourou, J.W.; Degenhardt, L.; Swift, W.; et al. Young adult sequelae of adolescent cannabis use: An integrative analysis. Lancet Psychiatry 2014, 1, 286–293. [Google Scholar] [CrossRef] [Green Version]
- Volkow, N.D. Effects of Cannabis Use on Human Behavior-Reply. JAMA Psychiatry 2016, 73, 996. [Google Scholar] [CrossRef]
- Castellanos-Ryan, N.; Pingault, J.B.; Parent, S.; Vitaro, F.; Tremblay, R.E.; Seguin, J.R. Adolescent cannabis use, change in neurocognitive function, and high-school graduation: A longitudinal study from early adolescence to young adulthood. Dev. Psychopathol. 2017, 29, 1253–1266. [Google Scholar] [CrossRef] [Green Version]
- Volkow, N.D.; Swanson, J.M.; Evins, A.E.; DeLisi, L.E.; Meier, M.H.; Gonzalez, R.; Bloomfield, M.A.; Curran, H.V.; Baler, R. Effects of Cannabis Use on Human Behavior, Including Cognition, Motivation, and Psychosis: A Review. JAMA Psychiatry 2016, 73, 292–297. [Google Scholar] [CrossRef]
- Crean, R.D.; Crane, N.A.; Mason, B.J. An evidence based review of acute and long-term effects of cannabis use on executive cognitive functions. J Addict. Med. 2011, 5, 1. [Google Scholar] [CrossRef] [Green Version]
- Simpson, A.K.; Magid, V. Cannabis Use Disorder in Adolescence. Child Adolesc. Psychiatr. Clin. N. Am. 2016, 25, 431–443. [Google Scholar] [CrossRef]
- Connor, J.P.; Stjepanovic, D.; Le Foll, B.; Hoch, E.; Budney, A.J.; Hall, W.D. Cannabis use and cannabis use disorder. Nat. Rev. Dis. Prim. 2021, 7, 16. [Google Scholar] [CrossRef]
- Portugalov, A.; Akirav, I. Do Adolescent Exposure to Cannabinoids and Early Adverse Experience Interact to Increase the Risk of Psychiatric Disorders: Evidence from Rodent Models. Int. J. Mol. Sci. 2021, 22, 730. [Google Scholar] [CrossRef]
- Reilly, D.; Didcott, P.; Swift, W.; Hall, W. Long-term cannabis use: Characteristics of users in an Australian rural area. Addiction 1998, 93, 837–846. [Google Scholar] [CrossRef]
- Kedzior, K.K.; Laeber, L.T. A positive association between anxiety disorders and cannabis use or cannabis use disorders in the general population--a meta-analysis of 31 studies. BMC Psychiatry 2014, 14, 136. [Google Scholar] [CrossRef] [Green Version]
- Mechoulam, R.; Parker, L.A. The endocannabinoid system and the brain. Annu. Rev. Psychol. 2013, 64, 21–47. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.C.; Mackie, K. An Introduction to the Endogenous Cannabinoid System. Biol. Psychiatry 2016, 79, 516–525. [Google Scholar] [CrossRef] [Green Version]
- Viveros, M.P.; Llorente, R.; Suarez, J.; Llorente-Berzal, A.; Lopez-Gallardo, M.; de Fonseca, F.R. The endocannabinoid system in critical neurodevelopmental periods: Sex differences and neuropsychiatric implications. J. Psychopharmacol. 2012, 26, 164–176. [Google Scholar] [CrossRef]
- Mato, S.; Del Olmo, E.; Pazos, A. Ontogenetic development of cannabinoid receptor expression and signal transduction functionality in the human brain. Eur. J. Neurosci. 2003, 17, 1747–1754. [Google Scholar] [CrossRef]
- Lopez-Larson, M.P.; Bogorodzki, P.; Rogowska, J.; McGlade, E.; King, J.B.; Terry, J.; Yurgelun-Todd, D. Altered prefrontal and insular cortical thickness in adolescent marijuana users. Behav. Brain Res. 2011, 220, 164–172. [Google Scholar] [CrossRef]
- Churchwell, J.C.; Lopez-Larson, M.; Yurgelun-Todd, D.A. Altered frontal cortical volume and decision making in adolescent cannabis users. Front. Psychol. 2010, 1, 225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yucel, M.; Solowij, N.; Respondek, C.; Whittle, S.; Fornito, A.; Pantelis, C.; Lubman, D.I. Regional brain abnormalities associated with long-term heavy cannabis use. Arch. Gen. Psychiatry 2008, 65, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Ashtari, M.; Avants, B.; Cyckowski, L.; Cervellione, K.L.; Roofeh, D.; Cook, P.; Gee, J.; Sevy, S.; Kumra, S. Medial temporal structures and memory functions in adolescents with heavy cannabis use. J. Psychiatry Res. 2011, 45, 1055–1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kojima, M.; Hosoda, H.; Date, Y.; Nakazato, M.; Matsuo, H.; Kangawa, K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 1999, 402, 656–660. [Google Scholar] [CrossRef] [PubMed]
- Mason, B.L.; Wang, Q.; Zigman, J.M. The central nervous system sites mediating the orexigenic actions of ghrelin. Annu. Rev. Physiol. 2014, 76, 519–533. [Google Scholar] [CrossRef] [Green Version]
- Naleid, A.M.; Grace, M.K.; Cummings, D.E.; Levine, A.S. Ghrelin induces feeding in the mesolimbic reward pathway between the ventral tegmental area and the nucleus accumbens. Peptides 2005, 26, 2274–2279. [Google Scholar] [CrossRef]
- Abizaid, A.; Hougland, J.L. Ghrelin Signaling: GOAT and GHS-R1a Take a LEAP in Complexity. Trends Endocrinol. Metab. 2020, 31, 107–117. [Google Scholar] [CrossRef]
- Carlini, V.P.; Monzon, M.E.; Varas, M.M.; Cragnolini, A.B.; Schioth, H.B.; Scimonelli, T.N.; de Barioglio, S.R. Ghrelin increases anxiety-like behavior and memory retention in rats. Biochem. Biophys. Res. Commun. 2002, 299, 739–743. [Google Scholar] [CrossRef]
- Lutter, M.; Sakata, I.; Osborne-Lawrence, S.; Rovinsky, S.A.; Anderson, J.G.; Jung, S.; Birnbaum, S.; Yanagisawa, M.; Elmquist, J.K.; Nestler, E.J.; et al. The orexigenic hormone ghrelin defends against depressive symptoms of chronic stress. Nat. Neurosci. 2008, 11, 752–753. [Google Scholar] [CrossRef] [Green Version]
- Jerlhag, E.; Egecioglu, E.; Dickson, S.L.; Andersson, M.; Svensson, L.; Engel, J.A. Ghrelin stimulates locomotor activity and accumbal dopamine-overflow via central cholinergic systems in mice: Implications for its involvement in brain reward. Addict. Biol. 2006, 11, 45–54. [Google Scholar] [CrossRef]
- Tang-Christensen, M.; Vrang, N.; Ortmann, S.; Bidlingmaier, M.; Horvath, T.L.; Tschop, M. Central administration of ghrelin and agouti-related protein (83–132) increases food intake and decreases spontaneous locomotor activity in rats. Endocrinology 2004, 145, 4645–4652. [Google Scholar] [CrossRef]
- Spencer, S.J.; Xu, L.; Clarke, M.A.; Lemus, M.; Reichenbach, A.; Geenen, B.; Kozicz, T.; Andrews, Z.B. Ghrelin regulates the hypothalamic-pituitary-adrenal axis and restricts anxiety after acute stress. Biol. Psychiatry 2012, 72, 457–465. [Google Scholar] [CrossRef]
- Chuang, J.C.; Perello, M.; Sakata, I.; Osborne-Lawrence, S.; Savitt, J.M.; Lutter, M.; Zigman, J.M. Ghrelin mediates stress-induced food-reward behavior in mice. J. Clin. Investig. 2011, 121, 2684–2692. [Google Scholar] [CrossRef]
- Alen, F.; Crespo, I.; Ramirez-Lopez, M.T.; Jagerovic, N.; Goya, P.; de Fonseca, F.R.; de Heras, R.G.; Orio, L. Ghrelin-induced orexigenic effect in rats depends on the metabolic status and is counteracted by peripheral CB1 receptor antagonism. PLoS ONE 2013, 8, e60918. [Google Scholar] [CrossRef] [Green Version]
- Tucci, S.A.; Rogers, E.K.; Korbonits, M.; Kirkham, T.C. The cannabinoid CB1 receptor antagonist SR141716 blocks the orexigenic effects of intrahypothalamic ghrelin. Br. J. Pharmacol. 2004, 143, 520–523. [Google Scholar] [CrossRef] [Green Version]
- Edwards, A.; Abizaid, A. Driving the need to feed: Insight into the collaborative interaction between ghrelin and endocannabinoid systems in modulating brain reward systems. Neurosci. Biobehav. Rev. 2016, 66, 33–53. [Google Scholar] [CrossRef]
- Sustkova-Fiserova, M.; Charalambous, C.; Khryakova, A.; Certilina, A.; Lapka, M.; Slamberova, R. The Role of Ghrelin/GHS-R1A Signaling in Nonalcohol Drug Addictions. Int. J. Mol. Sci. 2022, 23, 761. [Google Scholar] [CrossRef]
- Kalafateli, A.L.; Vallof, D.; Jornulf, J.W.; Heilig, M.; Jerlhag, E. A cannabinoid receptor antagonist attenuates ghrelin-induced activation of the mesolimbic dopamine system in mice. Physiol. Behav. 2018, 184, 211–219. [Google Scholar] [CrossRef]
- Abizaid, A.; Liu, Z.W.; Andrews, Z.B.; Shanabrough, M.; Borok, E.; Elsworth, J.D.; Roth, R.H.; Sleeman, M.W.; Picciotto, M.R.; Tschop, M.H.; et al. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J. Clin. Investig. 2006, 116, 3229–3239. [Google Scholar] [CrossRef]
- Charan, J.; Kantharia, N.D. How to calculate sample size in animal studies? J. Pharmacol. Pharmacother. 2013, 4, 303–306. [Google Scholar] [CrossRef]
- Manwell, L.A.; Charchoglyan, A.; Brewer, D.; Matthews, B.A.; Heipel, H.; Mallet, P.E. A vapourized Delta(9)-tetrahydrocannabinol (Delta(9)-THC) delivery system part I: Development and validation of a pulmonary cannabinoid route of exposure for experimental pharmacology studies in rodents. J. Pharmacol. Toxicol. Methods 2014, 70, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Manwell, L.A.; Ford, B.; Matthews, B.A.; Heipel, H.; Mallet, P.E. A vapourized Delta(9)-tetrahydrocannabinol (Delta(9)-THC) delivery system part II: Comparison of behavioural effects of pulmonary versus parenteral cannabinoid exposure in rodents. J. Pharmacol. Toxicol. Methods 2014, 70, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Bailey, K.R.; Crawley, J.N. Anxiety-Related Behaviors in Mice Methods of Behavior Analysis in Neuroscience; Taylor & Francis Group, LLC.: Boca Raton, FL, USA, 2009. [Google Scholar]
- File, S.E. The use of social interaction as a method for detecting anxiolytic activity of chlordiazepoxide-like drugs. J. Neurosci. Methods 1980, 2, 219–238. [Google Scholar] [CrossRef] [PubMed]
- Prut, L.; Belzung, C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: A review. Eur. J. Pharmacol. 2003, 463, 3–33. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, R.J.; Dalvi, A. Anxiety, defence and the elevated plus-maze. Neurosci. Biobehav. Rev. 1997, 21, 801–810. [Google Scholar] [CrossRef]
- Kaplan, J.S.; Wagner, J.K.; Reid, K.; McGuinness, F.; Arvila, S.; Brooks, M.; Stevenson, H.; Jones, J.; Risch, B.; McGillis, T.; et al. Cannabidiol Exposure During the Mouse Adolescent Period Is Without Harmful Behavioral Effects on Locomotor Activity, Anxiety, and Spatial Memory. Front. Behav. Neurosci. 2021, 15, 711639. [Google Scholar] [CrossRef]
- Zuo, Y.; Iemolo, A.; Montilla-Perez, P.; Li, H.R.; Yang, X.; Telese, F. Chronic adolescent exposure to cannabis in mice leads to sex-biased changes in gene expression networks across brain regions. Neuropsychopharmacology 2022, 47, 2071–2080. [Google Scholar] [CrossRef]
- Chen, H.T.; Mackie, K. Adolescent Delta(9)-Tetrahydrocannabinol Exposure Selectively Impairs Working Memory but Not Several Other mPFC-Mediated Behaviors. Front. Psychiatry 2020, 11, 576214. [Google Scholar] [CrossRef]
- Hamidullah, S.; Lutelmowski, C.D.; Creighton, S.D.; Luciani, K.R.; Frie, J.A.; Winters, B.D.; Khokhar, J.Y. Effects of vapourized THC and voluntary alcohol drinking during adolescence on cognition, reward, and anxiety-like behaviours in rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 106, 110141. [Google Scholar] [CrossRef]
- Brust, V.; Schindler, P.M.; Lewejohann, L. Lifetime development of behavioural phenotype in the house mouse (Mus musculus). Front. Zool. 2015, 12 (Suppl. S1), S17. [Google Scholar] [CrossRef]
- Bilkei-Gorzo, A.; Albayram, O.; Draffehn, A.; Michel, K.; Piyanova, A.; Oppenheimer, H.; Dvir-Ginzberg, M.; Racz, I.; Ulas, T.; Imbeault, S.; et al. A chronic low dose of Delta(9)-tetrahydrocannabinol (THC) restores cognitive function in old mice. Nat. Med. 2017, 23, 782–787. [Google Scholar] [CrossRef]
- Renard, J.; Szkudlarek, H.J.; Kramar, C.P.; Jobson, C.E.L.; Moura, K.; Rushlow, W.J.; Laviolette, S.R. Adolescent THC Exposure Causes Enduring Prefrontal Cortical Disruption of GABAergic Inhibition and Dysregulation of Sub-Cortical Dopamine Function. Sci. Rep. 2017, 7, 11420. [Google Scholar] [CrossRef] [Green Version]
- Mills, K.L.; Lalonde, F.; Clasen, L.S.; Giedd, J.N.; Blakemore, S.J. Developmental changes in the structure of the social brain in late childhood and adolescence. Soc. Cogn. Affect. Neurosci. 2014, 9, 123–131. [Google Scholar] [CrossRef] [Green Version]
- Zbucki, R.L.; Sawicki, B.; Hryniewicz, A.; Winnicka, M.M. Cannabinoids enhance gastric X/A-like cells activity. Folia Histochem. Cytobiol. 2008, 46, 219–224. [Google Scholar] [CrossRef] [Green Version]
- Charalambous, C.; Havlickova, T.; Lapka, M.; Puskina, N.; Slamberova, R.; Kuchar, M.; Sustkova-Fiserova, M. Cannabinoid-Induced Conditioned Place Preference, Intravenous Self-Administration, and Behavioral Stimulation Influenced by Ghrelin Receptor Antagonism in Rats. Int. J. Mol. Sci. 2021, 22, 2397. [Google Scholar] [CrossRef]
- Charalambous, C.; Lapka, M.; Havlickova, T.; Syslova, K.; Sustkova-Fiserova, M. Alterations in Rat Accumbens Dopamine, Endocannabinoids and GABA Content During WIN55,212-2 Treatment: The Role of Ghrelin. Int. J. Mol. Sci. 2020, 22, 210. [Google Scholar] [CrossRef]
- Lillo, J.; Lillo, A.; Zafra, D.A.; Miralpeix, C.; Rivas-Santisteban, R.; Casals, N.; Navarro, G.; Franco, R. Identification of the Ghrelin and Cannabinoid CB(2) Receptor Heteromer Functionality and Marked Upregulation in Striatal Neurons from Offspring of Mice under a High-Fat Diet. Int. J. Mol. Sci. 2021, 22, 8928. [Google Scholar] [CrossRef]
- Zamberletti, E.; Prini, P.; Speziali, S.; Gabaglio, M.; Solinas, M.; Parolaro, D.; Rubino, T. Gender-dependent behavioral and biochemical effects of adolescent delta-9-tetrahydrocannabinol in adult maternally deprived rats. Neuroscience 2012, 204, 245–257. [Google Scholar] [CrossRef]
- Lecca, S.; Luchicchi, A.; Scherma, M.; Fadda, P.; Muntoni, A.L.; Pistis, M. Delta(9)-Tetrahydrocannabinol During Adolescence Attenuates Disruption of Dopamine Function Induced in Rats by Maternal Immune Activation. Front. Behav. Neurosci. 2019, 13, 202. [Google Scholar] [CrossRef]
- De Felice, M.; Laviolette, S.R. Reversing the Psychiatric Effects of Neurodevelopmental Cannabinoid Exposure: Exploring Pharmacotherapeutic Interventions for Symptom Improvement. Int. J. Mol. Sci. 2021, 22, 7861. [Google Scholar] [CrossRef]
- Patton, G.C.; Coffey, C.; Carlin, J.B.; Degenhardt, L.; Lynskey, M.; Hall, W. Cannabis use and mental health in young people: Cohort study. BMJ 2002, 325, 1195–1198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayatbakhsh, M.R.; Najman, J.M.; Jamrozik, K.; Mamun, A.A.; Alati, R.; Bor, W. Cannabis and anxiety and depression in young adults: A large prospective study. J. Am. Acad. Child Adolesc. Psychiatry 2007, 46, 408–417. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sestan-Pesa, M.; Shanabrough, M.; Horvath, T.L.; Miletta, M.C. Impaired Ghrelin Signaling Does Not Lead to Alterations of Anxiety-like Behaviors in Adult Mice Chronically Exposed to THC during Adolescence. Biomedicines 2023, 11, 144. https://doi.org/10.3390/biomedicines11010144
Sestan-Pesa M, Shanabrough M, Horvath TL, Miletta MC. Impaired Ghrelin Signaling Does Not Lead to Alterations of Anxiety-like Behaviors in Adult Mice Chronically Exposed to THC during Adolescence. Biomedicines. 2023; 11(1):144. https://doi.org/10.3390/biomedicines11010144
Chicago/Turabian StyleSestan-Pesa, Matija, Marya Shanabrough, Tamas L. Horvath, and Maria Consolata Miletta. 2023. "Impaired Ghrelin Signaling Does Not Lead to Alterations of Anxiety-like Behaviors in Adult Mice Chronically Exposed to THC during Adolescence" Biomedicines 11, no. 1: 144. https://doi.org/10.3390/biomedicines11010144
APA StyleSestan-Pesa, M., Shanabrough, M., Horvath, T. L., & Miletta, M. C. (2023). Impaired Ghrelin Signaling Does Not Lead to Alterations of Anxiety-like Behaviors in Adult Mice Chronically Exposed to THC during Adolescence. Biomedicines, 11(1), 144. https://doi.org/10.3390/biomedicines11010144