Serum Cytokines and Growth Factors in Subjects with Type 1 Diabetes: Associations with Time in Ranges and Glucose Variability
Abstract
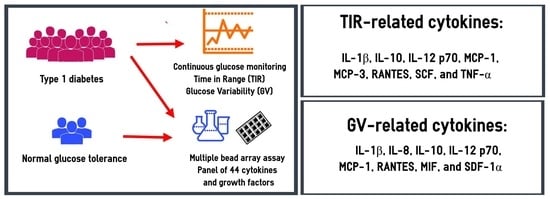
:1. Introduction
2. Materials and Methods
2.1. Design
2.2. Methods
2.3. Statistical Analysis
3. Results
3.1. Clinical Characteristics of the Study Participants
3.2. Serum Cytokines and Growth Factors in Subjects with NGT and T1D
3.3. Serum Cytokines and Growth Factors in Subjects with T1D: Relationships with TIRs
3.4. Serum Concentrations of Cytokines and Growth Factors in Subjects with T1D: Relationships with GV
3.5. Serum Concentrations of Cytokines and Growth Factors in Subjects with T1D: Other Relationships
3.6. Multiple Regression Models
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gregory, G.A.; Robinson, T.I.G.; Linklater, S.E.; Wang, F.; Colagiuri, S.; De Beaufort, C.; Donaghue, K.C.; Magliano, D.J.; Maniam, J.; Orchard, T.J.; et al. Global Incidence, Prevalence, and Mortality of Type 1 Diabetes in 2021 with Projection to 2040: A Modelling Study. Lancet Diabetes Endocrinol. 2022, 10, 741–760. [Google Scholar] [CrossRef] [PubMed]
- Garofolo, M.; Gualdani, E.; Giannarelli, R.; Aragona, M.; Campi, F.; Lucchesi, D.; Daniele, G.; Miccoli, R.; Francesconi, P.; Del Prato, S.; et al. Microvascular Complications Burden (Nephropathy, Retinopathy and Peripheral Polyneuropathy) Affects Risk of Major Vascular Events and All-Cause Mortality in Type 1 Diabetes: A 10-Year Follow-up Study. Cardiovasc. Diabetol. 2019, 18, 159. [Google Scholar] [CrossRef] [PubMed]
- Bjerg, L.; Gudbjörnsdottir, S.; Franzén, S.; Carstensen, B.; Witte, D.R.; Jørgensen, M.E.; Svensson, A.-M. Duration of Diabetes-Related Complications and Mortality in Type 1 Diabetes: A National Cohort Study. Int. J. Epidemiol. 2021, 50, 1250–1259. [Google Scholar] [CrossRef]
- Bebu, I.; Braffett, B.H.; De Boer, I.H.; Aiello, L.P.; Bantle, J.P.; Lorenzi, G.M.; Herman, W.H.; Gubitosi-Klug, R.A.; Perkins, B.A.; Lachin, J.M.; et al. Relationships Between the Cumulative Incidences of Long-Term Complications in Type 1 Diabetes: The DCCT/EDIC Study. Diabetes Care 2023, 46, 361–368. [Google Scholar] [CrossRef]
- Diabetes Control and Complications Trial Research Group; Nathan, D.M.; Genuth, S.; Lachin, J.; Cleary, P.; Crofford, O.; Davis, M.; Rand, L.; Siebert, C. The Effect of Intensive Treatment of Diabetes on the Development and Progression of Long-Term Complications in Insulin-Dependent Diabetes Mellitus. N. Engl. J. Med. 1993, 329, 977–986. [Google Scholar] [CrossRef]
- The Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Study Research Group. Intensive Diabetes Treatment and Cardiovascular Outcomes in Type 1 Diabetes: The DCCT/EDIC Study 30-Year Follow-Up. Diabetes Care 2016, 39, 686–693. [Google Scholar] [CrossRef]
- Helmink, M.A.G.; Hageman, S.H.J.; Visseren, F.L.J.; De Ranitz-Greven, W.L.; De Valk, H.W.; Van Sloten, T.T.; Westerink, J. Variability in Benefit from Intensive Insulin Therapy on Cardiovascular Events in Individuals with Type 1 Diabetes: A Post Hoc Analysis of the DCCT/EDIC Study. Diabet. Med. 2023, 40, e15183. [Google Scholar] [CrossRef]
- Goth, A.; Lengyel, L.; Nadasdi, N.; Savely, C. Renal Lesions Due to Fluctuations in Blood Sugar Levels. Acta Med. Scand. 1957, 158, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Kovatchev, B. Glycemic Variability: Risk Factors, Assessment, and Control. J. Diabetes Sci. Technol. 2019, 13, 627–635. [Google Scholar] [CrossRef]
- Sun, B.; Luo, Z.; Zhou, J. Comprehensive Elaboration of Glycemic Variability in Diabetic Macrovascular and Microvascular Complications. Cardiovasc. Diabetol. 2021, 20, 9. [Google Scholar] [CrossRef]
- Wadén, J.; Forsblom, C.; Thorn, L.M.; Gordin, D.; Saraheimo, M.; Groop, P.-H.; Finnish Diabetic Nephropathy Study Group A1C. Variability Predicts Incident Cardiovascular Events, Microalbuminuria, and Overt Diabetic Nephropathy in Patients with Type 1 Diabetes. Diabetes 2009, 58, 2649–2655. [Google Scholar] [CrossRef]
- Li, F.; Zhang, L.; Shen, Y.; Liu, H.-H.; Zhang, Z.-Y.; Hu, G.; Wang, R.-X. Higher Glucose Fluctuation Is Associated with a Higher Risk of Cardiovascular Disease: Insights from Pooled Results among Patients with Diabetes. J. Diabetes 2023, 15, 368–381. [Google Scholar] [CrossRef]
- Battelino, T.; Danne, T.; Bergenstal, R.M.; Amiel, S.A.; Beck, R.; Biester, T.; Bosi, E.; Buckingham, B.A.; Cefalu, W.T.; Close, K.L.; et al. Clinical Targets for Continuous Glucose Monitoring Data Interpretation: Recommendations From the International Consensus on Time in Range. Diabetes Care 2019, 42, 1593–1603. [Google Scholar] [CrossRef]
- El Malahi, A.; Van Elsen, M.; Charleer, S.; Dirinck, E.; Ledeganck, K.; Keymeulen, B.; Crenier, L.; Radermecker, R.; Taes, Y.; Vercammen, C.; et al. Relationship Between Time in Range, Glycemic Variability, HbA1c, and Complications in Adults with Type 1 Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2022, 107, e570–e581. [Google Scholar] [CrossRef]
- Ranjan, A.G.; Rosenlund, S.V.; Hansen, T.W.; Rossing, P.; Andersen, S.; Nørgaard, K. Improved Time in Range Over 1 Year Is Associated With Reduced Albuminuria in Individuals With Sensor-Augmented Insulin Pump-Treated Type 1 Diabetes. Diabetes Care 2020, 43, 2882–2885. [Google Scholar] [CrossRef]
- Šoupal, J.; Škrha, J., Jr.; Fajmon, M.; Horová, E.; Mráz, M.; Škrha, J.; Prázný, M. Glycemic variability is higher in type 1 diabetes patients with microvascular complications irrespective of glycemic control. Diabetes Technol. Ther. 2014, 16, 198–203. [Google Scholar] [CrossRef]
- Naaman, S.H.; Mizrak, H.I.; Lind, N.; Laursen, J.C.; Kufaishi, H.; Christensen, M.M.B.; Ranjan, A.G.; Nørgaard, K.; Hansen, C.S. Cardiovascular Autonomic Neuropathy Is Associated With Increased Glucose Variability in People With Type 1 Diabetes. Diabetes Care 2022, 45, 2461–2465. [Google Scholar] [CrossRef]
- Saik, O.V.; Klimontov, V.V. Bioinformatic Reconstruction and Analysis of Gene Networks Related to Glucose Variability in Diabetes and Its Complications. Int. J. Mol. Sci. 2020, 21, 8691. [Google Scholar] [CrossRef]
- Klimontov, V.V.; Saik, O.V.; Korbut, A.I. Glucose Variability: How Does It Work? Int. J. Mol. Sci. 2021, 22, 7783. [Google Scholar] [CrossRef]
- Capone, F.; Guerriero, E.; Sorice, A.; Colonna, G.; Ciliberto, G.; Costantini, S. Serum Cytokinome Profile Evaluation: A Tool to Define New Diagnostic and Prognostic Markers of Cancer Using Multiplexed Bead-Based Immunoassays. Mediat. Inflamm. 2016, 2016, 3064643. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Di, Y.P. Fast and efficient measurement of clinical and biological samples using immunoassay-based multiplexing systems. Methods Mol. Biol. 2020, 2102, 129–147. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, X.; Liao, N.; Wen, F. Assessment of biomarkers using multiplex assays in aqueous humor of patients with diabetic retinopathy. BMC Ophthalmol. 2017, 17, 176. [Google Scholar] [CrossRef]
- Klimontov, V.V.; Korbut, A.I.; Orlov, N.B.; Dashkin, M.V.; Konenkov, V.I. Multiplex Bead Array Assay of a Panel of Circulating Cytokines and Growth Factors in Patients with Albuminuric and Non-AlbuminuricDiabetic Kidney Disease. J. Clin. Med. 2020, 9, 3006. [Google Scholar] [CrossRef]
- Lee, M.Y.; Park, S.; Song, J.Y.; Ra, H.; Baek, J.U.; Baek, J. Inflammatory cytokines and retinal nonperfusion area in quiescent proliferative diabetic retinopathy. Cytokine 2022, 154, 155774. [Google Scholar] [CrossRef]
- Danne, T.; Nimri, R.; Battelino, T.; Bergenstal, R.M.; Close, K.L.; DeVries, J.H.; Garg, S.; Heinemann, L.; Hirsch, I.; Amiel, S.A.; et al. International Consensus on Use of Continuous Glucose Monitoring. Diabetes Care 2017, 40, 1631–1640. [Google Scholar] [CrossRef]
- Hill, N.R.; Oliver, N.S.; Choudhary, P.; Levy, J.C.; Hindmarsh, P.; Matthews, D.R. Normal Reference Range for Mean Tissue Glucose and Glycemic Variability Derived from Continuous Glucose Monitoring for Subjects without Diabetes in Different Ethnic Groups. Diabetes Technol. Ther. 2011, 13, 921–928. [Google Scholar] [CrossRef]
- Kovatchev, B.; Cobelli, C. Glucose Variability: Timing, Risk Analysis, and Relationship to Hypoglycemia in Diabetes. Diabetes Care 2016, 39, 502–510. [Google Scholar] [CrossRef]
- Dogan, Y.; Akarsu, S.; Ustundag, B.; Yilmaz, E.; Gurgoze, M.K. Serum IL-1beta, IL-2, and IL-6 in Insulin-Dependent Diabetic Children. Mediat. Inflamm. 2006, 2006, 59206. [Google Scholar] [CrossRef]
- Gabbay, M.A.L.; Sato, M.N.; Duarte, A.J.S.; Dib, S.A. Serum Titres of Anti-Glutamic Acid Decarboxylase-65 and Anti-IA-2 Autoantibodies Are Associated with Different Immunoregulatory Milieu in Newly Diagnosed Type 1 Diabetes Patients. Clin. Exp. Immunol. 2012, 168, 60–67. [Google Scholar] [CrossRef]
- Fatima, N.; Faisal, S.M.; Zubair, S.; Ajmal, M.; Siddiqui, S.S.; Moin, S.; Owais, M. Role of Pro-Inflammatory Cytokines and Biochemical Markers in the Pathogenesis of Type 1 Diabetes: Correlation with Age and Glycemic Condition in Diabetic Human Subjects. PLoS ONE 2016, 11, e0161548. [Google Scholar] [CrossRef]
- Chen, Y.-L.; Qiao, Y.-C.; Pan, Y.-H.; Xu, Y.; Huang, Y.-C.; Wang, Y.-H.; Geng, L.-J.; Zhao, H.-L.; Zhang, X.-X. Correlation between Serum Interleukin-6 Level and Type 1 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Cytokine 2017, 94, 14–20. [Google Scholar] [CrossRef]
- Tangjittipokin, W.; Umjai, P.; Khemaprasit, K.; Charoentawornpanich, P.; Chanprasert, C.; Teerawattanapong, N.; Narkdontri, T.; Santiprabhob, J. Vitamin D Pathway Gene Polymorphisms, Vitamin D Level, and Cytokines in Children with Type 1 Diabetes. Gene 2021, 791, 145691. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xu, L.; Zhao, W.; Pan, J.; Lu, J.; Lu, H.; Yan, J.; Weng, J.; Liu, F. Serum IL-17A Concentration and a IL17RA Single Nucleotide Polymorphism Contribute to the Risk of Autoimmune Type 1 Diabetes. Diabetes Metab. Res. Rev. 2022, 38, e3547. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, B.Z.; Hanifi-Moghaddam, P.; Eerligh, P.; van der Slik, A.R.; Kolb, H.; Kharagjitsingh, A.V.; Pereira Arias, A.M.; Ronkainen, M.; Knip, M.; Bonfanti, R.; et al. Association of Interferon-Gamma and Interleukin 10 Genotypes and Serum Levels with Partial Clinical Remission in Type 1 Diabetes. Clin. Exp. Immunol. 2006, 145, 480–484. [Google Scholar] [CrossRef]
- Ismail, N.A.; Abd El Baky, A.N.; Ragab, S.; Hamed, M.; Hashish, M.A.; Shehata, A. Monocyte Chemoattractant Protein 1 and Macrophage Migration Inhibitory Factor in Children with Type 1 Diabetes. J. Pediatr. Endocrinol. Metab. 2016, 29, 641–645. [Google Scholar] [CrossRef]
- Qiao, Y.-C.; Chen, Y.-L.; Pan, Y.-H.; Tian, F.; Xu, Y.; Zhang, X.-X.; Zhao, H.-L. The Change of Serum Tumor Necrosis Factor Alpha in Patients with Type 1 Diabetes Mellitus: A Systematic Review and Meta-Analysis. PLoS ONE 2017, 12, e0176157. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Latif, M.; Abdel-Moneim, A.A.; El-Hefnawy, M.H.; Khalil, R.G. Comparative and Correlative Assessments of Cytokine, Complement and Antibody Patterns in Paediatric Type 1 Diabetes. Clin. Exp. Immunol. 2017, 190, 110–121. [Google Scholar] [CrossRef]
- Jamali, Z.; Nazari, M.; Khoramdelazad, H.; Hakimizadeh, E.; Mahmoodi, M.; Karimabad, M.N.; Hassanshahi, G.; Rezaeian, M.; Balaei, P.; Darakhshan, S.; et al. Expression of CC Chemokines CCL2, CCL5, and CCL11 Is Associated with Duration of Disease and Complications in Type-1 Diabetes: A Study on Iranian Diabetic Patients. Clin. Lab. 2013, 59, 993–1001. [Google Scholar] [CrossRef]
- Purohit, S.; Sharma, A.; Hopkins, D.; Steed, L.; Bode, B.; Anderson, S.W.; Reed, J.C.; Steed, R.D.; Yang, T.; She, J.-X. Large-Scale Discovery and Validation Studies Demonstrate Significant Reductions in Circulating Levels of IL8, IL-1Ra, MCP-1, and MIP-1β in Patients With Type 1 Diabetes. J. Clin. Endocrinol. Metab. 2015, 100, E1179–E1187. [Google Scholar] [CrossRef]
- Pérez, F.; Oyarzún, A.; Carrasco, E.; Angel, B.; Albala, C.; Santos, J.L. Plasma levels of interleukin-1beta, interleukin-2 and interleukin-4 in recently diagnosed type 1 diabetic children and their association with beta-pancreatic autoantibodies. Rev. Med. Chil. 2004, 132, 413–420. [Google Scholar] [CrossRef]
- Antonelli, A.; Fallahi, P.; Ferrari, S.M.; Pupilli, C.; d’Annunzio, G.; Lorini, R.; Vanelli, M.; Ferrannini, E. Serum Th1 (CXCL10) and Th2 (CCL2) Chemokine Levels in Children with Newly Diagnosed Type 1 Diabetes: A Longitudinal Study. Diabet Med. 2008, 25, 1349–1353. [Google Scholar] [CrossRef] [PubMed]
- Hanifi-Moghaddam, P.; Schloot, N.C.; Kappler, S.; Seissler, J.; Kolb, H. An Association of Autoantibody Status and Serum Cytokine Levels in Type 1 Diabetes. Diabetes 2003, 52, 1137–1142. [Google Scholar] [CrossRef] [PubMed]
- Alnek, K.; Kisand, K.; Heilman, K.; Peet, A.; Varik, K.; Uibo, R. Increased Blood Levels of Growth Factors, Proinflammatory Cytokines, and Th17 Cytokines in Patients with Newly Diagnosed Type 1 Diabetes. PLoS ONE 2015, 10, e0142976. [Google Scholar] [CrossRef]
- Wu, H.-P.; Kuo, S.-F.; Wu, S.-Y.; Chuang, D.-Y. High Interleukin-12 Production from Stimulated Peripheral Blood Mononuclear Cells of Type 2 Diabetes Patients. Cytokine 2010, 51, 298–304. [Google Scholar] [CrossRef]
- Gonzalez, Y.; Herrera, M.T.; Soldevila, G.; Garcia-Garcia, L.; Fabián, G.; Pérez-Armendariz, E.M.; Bobadilla, K.; Guzmán-Beltrán, S.; Sada, E.; Torres, M. High Glucose Concentrations Induce TNF-α Production through the down-Regulation of CD33 in Primary Human Monocytes. BMC Immunol. 2012, 13, 19. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Xia, C.-Q.; Butfiloski, E.; Clare-Salzler, M. Effect of High Glucose on Cytokine Production by Human Peripheral Blood Immune Cells and Type I Interferon Signaling in Monocytes: Implications for the Role of Hyperglycemia in the Diabetes Inflammatory Process and Host Defense against Infection. Clin. Immunol. 2018, 195, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhao, M.; Li, J.; Luo, W.; Huang, J.; Huang, G.; Xie, Z.; Xiao, Y.; Huang, J.; Li, X.; et al. Elevated Glucose Metabolism Driving Pro-Inflammatory Response in B Cells Contributes to the Progression of Type 1 Diabetes. Clin. Immunol. 2023, 255, 109729. [Google Scholar] [CrossRef]
- Wu, M.; Wu, Y.; Xu, K.; Lin, L. Protective Effects of 1,25 Dihydroxyvitamin D3 against High-Glucose-Induced Damage in Human Umbilical Vein Endothelial Cells Involve Activation of Nrf2 Antioxidant Signaling. J. Vasc. Res. 2021, 58, 267–276. [Google Scholar] [CrossRef]
- Zhang, M.; Jin, X.; Zhang, Z.; Li, B.; Yang, G. Vildagliptin Protects Endothelial Cells against High Glucose-Induced Damage. Biomed. Pharmacother. 2018, 108, 1790–1796. [Google Scholar] [CrossRef]
- Xu, L.; Shao, F. Sitagliptin Protects Renal Glomerular Endothelial Cells against High Glucose-Induced Dysfunction and Injury. Bioengineered 2022, 13, 655–666. [Google Scholar] [CrossRef]
- Cheng, G.; Li, L. High-Glucose-Induced Apoptosis, ROS Production and pro-Inflammatory Response in Cardiomyocytes Is Attenuated by Metformin Treatment via PP2A Activation. J. Biosci. 2020, 45, 126. [Google Scholar] [CrossRef]
- Maedler, K.; Sergeev, P.; Ris, F.; Oberholzer, J.; Joller-Jemelka, H.I.; Spinas, G.A.; Kaiser, N.; Halban, P.A.; Donath, M.Y. Glucose-Induced Beta Cell Production of IL-1beta Contributes to Glucotoxicity in Human Pancreatic Islets. J. Clin. Investig. 2002, 110, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Li-Bo, Y.; Wen-Bo, Q.; Xiao-Hong, L.; You-Lun, F.; Tie, Z. Intermittent High Glucose Promotes Expression of Proinflammatory Cytokines in Monocytes. Inflamm. Res. 2011, 60, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Piconi, L.; Quagliaro, L.; Assaloni, R.; Da Ros, R.; Maier, A.; Zuodar, G.; Ceriello, A. Constant and Intermittent High Glucose Enhances Endothelial Cell Apoptosis through Mitochondrial Superoxide Overproduction. Diabetes Metab. Res. Rev. 2006, 22, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Quagliaro, L.; Piconi, L.; Assaloni, R.; Da Ros, R.; Maier, A.; Zuodar, G.; Ceriello, A. Intermittent High Glucose Enhances ICAM-1, VCAM-1 and E-Selectin Expression in Human Umbilical Vein Endothelial Cells in Culture: The Distinct Role of Protein Kinase C and Mitochondrial Superoxide Production. Atherosclerosis 2005, 183, 259–267. [Google Scholar] [CrossRef]
- Hu, Z.; Fang, W.; Liu, Y.; Liang, H.; Chen, W.; Wang, H. Acute Glucose Fluctuation Promotes RAGE Expression via Reactive Oxygen Species-mediated NF-κB Activation in Rat Podocytes. Mol. Med. Rep. 2021, 23, 330. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Xu, Y.; Dai, Z.; Sun, Y. Intermittent High Glucose Stimulate MCP-l, IL-18, and PAI-1, but Inhibit Adiponectin Expression and Secretion in Adipocytes Dependent of ROS. Cell Biochem. Biophys. 2009, 55, 173–180. [Google Scholar] [CrossRef]
- Wu, N.; Shen, H.; Liu, H.; Wang, Y.; Bai, Y.; Han, P. Acute Blood Glucose Fluctuation Enhances Rat Aorta Endothelial Cell Apoptosis, Oxidative Stress and pro-Inflammatory Cytokine Expression in Vivo. Cardiovasc. Diabetol. 2016, 15, 109. [Google Scholar] [CrossRef]
- Al-Rashed, F.; Sindhu, S.; Arefanian, H.; Al Madhoun, A.; Kochumon, S.; Thomas, R.; Al-Kandari, S.; Alghaith, A.; Jacob, T.; Al-Mulla, F.; et al. Repetitive Intermittent Hyperglycemia Drives the M1 Polarization and Inflammatory Responses in THP-1 Macrophages Through the Mechanism Involving the TLR4-IRF5 Pathway. Cells 2020, 9, 1892. [Google Scholar] [CrossRef]
- Ratter, J.M.; Rooijackers, H.M.M.; Tack, C.J.; Hijmans, A.G.M.; Netea, M.G.; de Galan, B.E.; Stienstra, R. Proinflammatory Effects of Hypoglycemia in Humans With or Without Diabetes. Diabetes 2017, 66, 1052–1061. [Google Scholar] [CrossRef] [PubMed]
- Ceriello, A.; Novials, A.; Ortega, E.; Canivell, S.; La Sala, L.; Pujadas, G.; Esposito, K.; Giugliano, D.; Genovese, S. Glucagon-like Peptide 1 Reduces Endothelial Dysfunction, Inflammation, and Oxidative Stress Induced by Both Hyperglycemia and Hypoglycemia in Type 1 Diabetes. Diabetes Care 2013, 36, 2346–2350. [Google Scholar] [CrossRef] [PubMed]
- Ceriello, A.; Novials, A.; Ortega, E.; Canivell, S.; Pujadas, G.; La Sala, L.; Bucciarelli, L.; Rondinelli, M.; Genovese, S. Vitamin C Further Improves the Protective Effect of GLP-1 on the Ischemia-Reperfusion-like Effect Induced by Hyperglycemia Post-Hypoglycemia in Type 1 Diabetes. Cardiovasc. Diabetol. 2013, 12, 97. [Google Scholar] [CrossRef] [PubMed]
- Fleiner, H.F.; Radtke, M.; Ryan, L.; Moen, T.; Grill, V. Circulating Immune Mediators Are Closely Linked in Adult-Onset Type 1 Diabetes as Well as in Non-Diabetic Subjects. Autoimmunity 2014, 47, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Wallace, A.S.; Chang, A.R.; Shin, J.-I.; Reider, J.; Echouffo-Tcheugui, J.B.; Grams, M.E.; Selvin, E. Obesity and Chronic Kidney Disease in US Adults With Type 1 and Type 2 Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2022, 107, 1247–1256. [Google Scholar] [CrossRef] [PubMed]
- Belete, R.; Ataro, Z.; Abdu, A.; Sheleme, M. Global Prevalence of Metabolic Syndrome among Patients with Type I Diabetes Mellitus: A Systematic Review and Meta-Analysis. Diabetol. Metab. Syndr. 2021, 13, 25. [Google Scholar] [CrossRef]



| Parameter | Median (25; 75 Percentile) |
|---|---|
| Demographic and general clinical parameters | |
| Age, years | 33 (24; 43) |
| Smokers, n (%) | 23 (18%) |
| BMI, kg/m2 | 23 (20; 26) |
| Waist-to-hip ratio | 0.81 (0.76; 0.91) |
| Diabetes-related parameters and associated diseases | |
| Diabetes duration, years | 15 (10; 23) |
| Daily insulin dose, IU/kg | 0.7 (0.5; 0.9) |
| Diabetic retinopathy, n (%) | 79 (61%) |
| Chronic kidney disease, n (%) | 75 (58%) |
| Diabetic neuropathy, n (%) | 96 (74%) |
| Arterial hypertension, n (%) | 39 (30%) |
| Coronary artery disease, n (%) | 7 (5.3%) |
| Peripheral artery disease, n (%) | 18 (14%) |
| Laboratory parameters | |
| HbA1c, % | 7.9 (6.8; 9.6) |
| HbA1c, mmol/L | 67 (51; 81) |
| Total cholesterol, mmol/L | 5.1 (4.2; 6.3) |
| LDL-cholesterol, mmol/L | 3.0 (2.4; 3.7) |
| HDL-cholesterol, mmol/L | 1.5 (1.3; 1.8) |
| Triglycerides, mmol/L | 1.0 (0.7; 1.3) |
| hsCRP, mmol/L | 1.3 (0.7; 2.8) |
| eGFR (CKD-EPI formula, 2009), mL/min/1.73 m2 | 94 (82; 105) |
| UACR, mg/mmol | 0.5 (0.3; 1.1) |
| Hemoglobin, g/L | 139 (125; 150) |
| RBC, ×1012 | 4.7 (4.4; 5.0) |
| WBC, ×109 | 5.4 (4.8; 6.9) |
| Parameter | Median (25; 75 Percentile) |
|---|---|
| Mean glucose, mmol/L | 7.7 (6.6; 9.3) |
| TIR, % | 72 (57; 88) |
| TAR L-1, % | 17 (7.4; 25) |
| TAR L-2, % | 3.5 (0.3; 11) |
| TBR L-1, % | 1.5 (0; 2.1) |
| TBR L-2, % | 0.2 (0; 1.2) |
| CV, % | 33 (27; 38) |
| MAGE, mmol/L | 4.3 (3.1; 5.2) |
| MAG, mmol × h−1 × L−1 | 1.7 (2.1; 2.4) |
| Molecule | Group | p | |
|---|---|---|---|
| TIR > 70% (n = 69) | TIR ≤ 70% (n = 61) | ||
| IL-1α | 0.93 (0; 6.42) ** | 0 (0; 3.82) *** | 0.01 |
| IL-1β | 3.07 (2.53; 3.90) *** | 3.79 (3.06; 4.31) *** | 0.0004 |
| IL-1Ra | 296 (4.46; 461) | 405 (7.25; 478) ** | 0.08 |
| IL-2 | 7.94 (3.42; 9.55) | 6.12 (1.07; 9.56) | 0.09 |
| IL-2Rα | 18 (3.24; 63) *** | 30 (4.96; 54) *** | 0.68 |
| IL-3 | 0.53 (0.45; 0.65) *** | 0.56 (0.49; 0.70)*** | 0.07 |
| IL-4 | 3.82 (1.10; 5.88) *** | 1.53 (0.42; 3.95) *** | 0.01 |
| IL-5 | 42 (5.14; 77) | 9.89 (4.14; 117) | 0.96 |
| IL-6 | 4.32 (2.40; 5.86) | 5.54 (3.84; 7.03) *** | 0.007 |
| IL-7 | 10 (3.91; 18) ** | 11 (3.78; 18) *** | 0.78 |
| IL-8 | 6.53 (2.28; 9.38) | 3.83 (1.52; 6.90) * | 0.07 |
| IL-9 | 182 (5.22; 251) | 6.64 (2.92; 235) | 0.07 |
| IL-10 | 5.29 (3.83; 6.05) | 4.75 (2.90; 5.29) * | 0.001 |
| IL-12 p40 | 3.62 (0; 9.66) | 0.28 (0; 9.42) | 0.25 |
| IL-12 p70 | 4.10 (3.41; 5.41) | 6.56 (3.41; 14) ** | 0.02 |
| IL-16 | 9.12 (3.74; 36) * | 34 (6.08; 490) *** | 0.02 |
| IL-17A | 10 (4.46; 15) * | 8.95 (6.09; 13) ** | 0.94 |
| IL-18 | 8.34 (3.29; 23) | 8.01 (4.02; 32) | 0.35 |
| LIF | 8.58 (0; 49) *** | 26 (8.58; 59) | 0.02 |
| G-CSF | 14 (2.46; 72) ** | 38 (5.48; 74) * | 0.16 |
| GM-CSF | 4.66 (0.83; 6.04) | 1.08 (0; 5.08) *** | 0.002 |
| M-CSF | 9.29 (3.67; 18) | 12 (4.4; 21) *** | 0.25 |
| GRO-α | 0 (0; 5.46) | 0 (0; 0.75) | 0.2 |
| IFN-α2 | 0 (0; 1.62) * | 0 (0; 1.62) | 0.71 |
| IFN-γ | 1.79 (1.09; 3.06) *** | 1.94 (1.07; 2.53) *** | 0.87 |
| IP-10 | 145 (4.57; 333) | 235 (6.09; 471) | 0.13 |
| MCP-1 | 6.83 (3.11; 30) * | 24.39 (6.48; 41) *** | 0.01 |
| MCP-3 | 0 (0; 5.70) ** | 6.05 (0.91; 6.74) *** | 0.0006 |
| MIF | 8.44 (0; 149) | 0.44 (0; 28) ** | 0.005 |
| MIG | 110 (6.23; 177) | 117 (5.95; 200) | 0.84 |
| MIP-1α | 1.54 (1.18; 2.22) | 1.81 (1.50; 2.58) | 0.07 |
| MIP-1β | 193 (4.51; 224) | 7.98 (4.07; 232) | 0.61 |
| RANTES | 2437 (3.14; 11,840) | 9590 (6.99; 14,106) | 0.02 |
| TNF-α | 4.25 (0; 12) | 6.77 (3.29; 79) *** | 0.002 |
| TNF-β | 176 (4.30; 226) | 208 (6.72; 289) * | 0.04 |
| TRAIL | 7.47 (5.35; 13) | 8.49 (3.79; 13) | 0.95 |
| SCF | 19 (3.10; 78) | 69 (6.48; 99) * | 0.06 |
| SCGF-β | 9.01 (4.57; 117,138) | 9.85 (3.87; 126,518) | 0.92 |
| SDF-1α | 1276 (4.63; 1611) | 1339 (1132; 1543) | 0.26 |
| bFGF | 20 (3.82; 28) | 8.33 (7.06; 108) | 0.75 |
| PDGF-BB | 385 (5.66; 1307) | 730 (4.78; 1402) | 0.58 |
| HGF | 159 (5.42; 326) | 291 (6.28; 407) * | 0.06 |
| β-NGF | 0 (0; 1.24) * | 1.24 (0; 4.69) | 0.02 |
| VEGF | 152 (6.11; 208) | 6.11 (2.87; 214) | 0.22 |
| Molecule | Group | p | |
|---|---|---|---|
| CV < 36% (n = 72) | CV ≥ 36% (n = 58) | ||
| IL-1α | 0.46 (0; 4.85) *** | 0.51 (0; 4.05) *** | 0.74 |
| IL-1β | 3.60 (2.62; 4.10) *** | 3.48 (2.71; 4.10) *** | 0.94 |
| IL-1Ra | 324 (5.81; 464) | 376 (5.81; 478) * | 0.69 |
| IL-2 | 7.78 (2.35; 9.93) | 7.20 (1.07; 9.24) | 0.16 |
| IL-2Rα | 21 (4.96; 59) *** | 25 (4.96; 54) *** | 0.99 |
| IL-3 | 0.57 (0.46; 0.69) *** | 0.53 (0.49; 0.63) *** | 0.23 |
| IL-4 | 4.89 (2.40; 6.26) *** | 5.17 (3.33; 6.69) *** | 0.33 |
| IL-5 | 39 (5.03; 98) | 38 (4.63; 90) | 0.40 |
| IL-6 | 3.08 (0.90; 4.86) ** | 2.73 (0.17; 5.29) ** | 0.76 |
| IL-7 | 11 (4.03; 18) *** | 10 (3.78; 18) ** | 0.63 |
| IL-8 | 5.69 (2.43; 9.26) | 4.41 (1.52; 8.10) | 0.15 |
| IL-9 | 7.85 (3.71; 244) | 11 (4.35; 258) | 0.55 |
| IL-10 | 4.96 (3.83; 5.66) | 4.75 (2.90; 5.66) | 0.45 |
| IL-12 p40 | 2.92 (0; 14) | 0.38 (0; 6.09) * | 0.33 |
| IL-12 p70 | 4.33 (3.41; 13) | 4.45 (3.41; 13) | 0.73 |
| IL-16 | 31 (5.74; 45) ** | 9.12 (4.87; 38) * | 0.26 |
| IL-17A | 9.15 (4.85; 15) * | 9.36 (5.77; 15) ** | 0.57 |
| IL-18 | 8.85 (3.86; 24) | 7.37 (3.89; 25) | 0.98 |
| LIF | 8.58 (0.43; 49) *** | 22 (4.92; 54) *** | 0.29 |
| G-CSF | 38 (3.30; 87) | 22 (3.37; 60) | 0.53 |
| GM-CSF | 2.85 (0.24; 5.40) *** | 2.94 (0; 6.28) * | 0.96 |
| M-CSF | 9.78 (3.89; 18) ** | 9.66 (3.89; 20) ** | 0.77 |
| GRO-α | 0 (0; 4.34) | 0 (0; 0.75) | 0.45 |
| IFN-α2 | 0 (0; 1.62) | 0 (0; 3.35) * | 0.27 |
| IFN-γ | 1.87 (1.08; 2.98) *** | 1.94 (1.07; 2.48) *** | 0.67 |
| IP-10 | 180 (5.62; 442) | 193 (6.09; 448) | 0.87 |
| MCP-1 | 6.91 (3.18; 30) * | 24 (6.75; 43) *** | 0.007 |
| MCP-3 | 5.34 (0; 6.63) *** | 4.74 (0; 6.28) *** | 0.72 |
| MIF | 14 (0; 144) | 1.59 (0; 20) ** | 0.04 |
| MIG | 115 (6.23; 217) | 112 (6.11; 193) | 0.79 |
| MIP-1α | 1.63 (1.35; 3.06) | 1.74 (1.41; 2.24) | 0.65 |
| MIP-1β | 173 (3.95; 224) | 96 (4.99; 230) | 0.58 |
| RANTES | 9.85 (4.07; 12,458) | 9868 (7.22; 13,970) | 0.03 |
| TNF-α | 5.21 (0.67; 62) | 6.53 (2.77; 60) * | 0.61 |
| TNF-β | 170 (4.77; 236) | 208 (6.96; 266) * | 0.18 |
| TRAIL | 7.78 (5.12; 12) | 7.98 (3.79; 13) | 0.93 |
| SCF | 14 (3.65; 88) | 69 (6.68; 99) * | 0.18 |
| SCGF-β | 10,314 (4.04; 115,521) | 8.39 (4.65; 130,694) | 0.64 |
| SDF-1α | 1298 (6.48; 1580) | 1314 (8.44; 1569) | 0.55 |
| bFGF | 20 (5.26; 34) | 8.33 (5.64; 28) | 0.36 |
| PDGF-BB | 190 (3.80; 1212) | 794 (5.38; 1544) | 0.11 |
| HGF | 219 (5.33; 328) | 288 (6.72; 403) * | 0.19 |
| β-NGF | 0.00 (0; 4.07) ** | 0 (0; 3.18) ** | 0.53 |
| VEGF | 167 (3.39; 226) | 6.18 (3.31; 198) | 0.21 |
| Molecule | Crude OR (95% CI), p-Value | Adjusted OR (95% CI), p-Value |
|---|---|---|
| TIR ≤ 70% | ||
| IL-1β, 1 pg/mL | 1.78 (1.18–2.67), p = 0.006 | 1.69 (1.12–2.55), p = 0.01 |
| IL-4, 1 pg/mL | 0.82 (0.71–0.95), p = 0.007 | 0.82 (0.7–0.96), p = 0.01 |
| IL-10, 1 pg/mL | 0.7 (0.56–0.89), p = 0.003 | 0.73 (0.56–0.94), p = 0.01 |
| IL-12 (p70), 1 pg/mL | 1.08 (1.02–1.15), p = 0.006 | 1.08 (1.01–1.15), p = 0.02 |
| MCP-3, 1 pg/mL | 1.16 (1.04–1.29), p = 0.006 | 1.14 (1.02–1.28), p = 0.03 |
| MIF, 100 pg/mL | 0.76 (0.6–0.97), p = 0.03 | 0.79 (0.62–1.01), p = 0.06 |
| TNF-α, 10 pg/mL | 1.14 (1.03–1.27), p = 0.01 | 1.12 (1–1.25), p = 0.04 |
| GM-CSF, 1 pg/mL | 0.82 (0.72–0.94), p = 0.005 | 0.84 (0.73–0.97), p = 0.02 |
| HGF, 100 pg/mL | 1.21 (1.02–1.44), p = 0.03 | 1.23 (1.02–1.47), p = 0.03 |
| CV ≥ 36% | ||
| MIF, 100 pg/mL | 0.78 (0.62–0.99), p = 0.04 | 0.78 (0.61–0.99), p = 0.04 |
| PDGF-BB, 1000 pg/mL | 1.56 (1.05–2.32), p = 0.03 | 1.58 (1.05–2.37), p = 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klimontov, V.V.; Mavlianova, K.R.; Orlov, N.B.; Semenova, J.F.; Korbut, A.I. Serum Cytokines and Growth Factors in Subjects with Type 1 Diabetes: Associations with Time in Ranges and Glucose Variability. Biomedicines 2023, 11, 2843. https://doi.org/10.3390/biomedicines11102843
Klimontov VV, Mavlianova KR, Orlov NB, Semenova JF, Korbut AI. Serum Cytokines and Growth Factors in Subjects with Type 1 Diabetes: Associations with Time in Ranges and Glucose Variability. Biomedicines. 2023; 11(10):2843. https://doi.org/10.3390/biomedicines11102843
Chicago/Turabian StyleKlimontov, Vadim V., Kamilla R. Mavlianova, Nikolai B. Orlov, Julia F. Semenova, and Anton I. Korbut. 2023. "Serum Cytokines and Growth Factors in Subjects with Type 1 Diabetes: Associations with Time in Ranges and Glucose Variability" Biomedicines 11, no. 10: 2843. https://doi.org/10.3390/biomedicines11102843
APA StyleKlimontov, V. V., Mavlianova, K. R., Orlov, N. B., Semenova, J. F., & Korbut, A. I. (2023). Serum Cytokines and Growth Factors in Subjects with Type 1 Diabetes: Associations with Time in Ranges and Glucose Variability. Biomedicines, 11(10), 2843. https://doi.org/10.3390/biomedicines11102843