The Relationship between TNF-a, IL-35, VEGF and Cutaneous Microvascular Dysfunction in Young Patients with Uncomplicated Type 1 Diabetes
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Study Design and Population
2.2. Evaluation of Cutaneous Microcirculation
2.3. Laboratory Analyses
2.4. Statistical Analysis
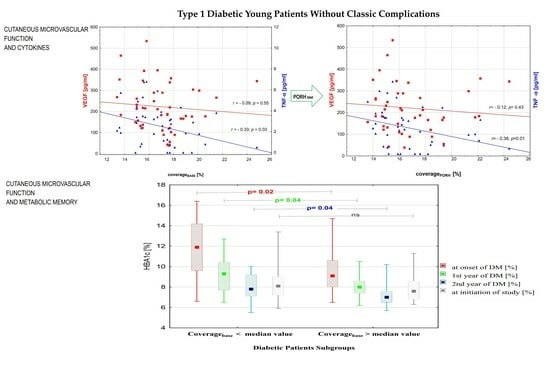
3. Results
3.1. Subgroup Comparison
3.2. Correlations between Variables
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. 2. Classification and Diagnosis of Diabetes: Standards of Care in Diabetes-2023. Diabetes Care 2023, 46 (Suppl. S1), S19–S40. [Google Scholar] [CrossRef] [PubMed]
- Cnop, M.; Welsh, N.; Jonas, J.C.; Jorns, A.; Lenzen, S.; Eizirik, D.L. Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: Many differences, few similarities. Diabetes 2005, 54 (Suppl. S2), S97–S107. [Google Scholar] [CrossRef]
- Kota, S.K.; Meher, L.K.; Jammula, S.; Kota, S.K.; Krishna, S.V.; Modi, K.D. Aberrant angiogenesis: The gateway to diabetic complications. Indian J. Endocrinol. Metab. 2012, 16, 918–930. [Google Scholar] [CrossRef] [PubMed]
- Simons, M. Angiogenesis: Where do we stand now? Circulation 2005, 111, 1556–1566. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.M.; Liang, J.; Xia, J.P.; Li, L.; Zheng, L.; Wang, Y.L.; Li, Y.H.; Li, Y.; Lu, Y. Interleukin 35: Protective role and mechanism in type 1 diabetes. Cent. Eur. J. Immunol. 2023, 48, 48–53. [Google Scholar] [CrossRef]
- Yang, X.F. Factors regulating apoptosis and homeostasis of CD4+ CD25(high) FOXP3+ regulatory T cells are new therapeutic targets. Front. Biosci. A J. Virtual Libr. 2008, 13, 1472–1499. [Google Scholar] [CrossRef]
- Li, X.; Mai, J.; Virtue, A.; Yin, Y.; Gong, R.; Sha, X.; Gutchigian, S.; Frisch, A.; Hodge, I.; Jiang, X.; et al. IL-35 is a novel responsive anti-inflammatory cytokine—A new system of categorizing anti-inflammatory cytokines. PLoS ONE 2012, 7, e33628. [Google Scholar]
- Lin, Y.; Huang, Y.; Lu, Z.; Luo, C.; Shi, Y.; Zeng, Q.; Cao, Y.; Liu, L.; Wang, X.; Ji, Q. Decreased plasma IL-35 levels are related to the left ventricular ejection fraction in coronary artery diseases. PLoS ONE 2012, 7, e52490. [Google Scholar] [CrossRef]
- Sha, X.; Meng, S.; Li, X.; Xi, H.; Maddaloni, M.; Pascual, D.W.; Shan, H.; Jiang, X.; Wang, H.; Yang, X.F. Interleukin-35 Inhibits Endothelial Cell Activation by Suppressing MAPK-AP-1 Pathway. J. Biol. Chem. 2015, 290, 19307–19318. [Google Scholar] [CrossRef]
- Du, W.X.; He, Y.; Jiang, H.Y.; Ai, Q.; Yu, J.L. Interleukin 35: A novel candidate biomarker to diagnose early onset sepsis in neonates. Clin. Chim. Acta Int. J. Clin. Chem. 2016, 462, 90–95. [Google Scholar] [CrossRef]
- Bettini, M.; Castellaw, A.H.; Lennon, G.P.; Burton, A.R.; Vignali, D.A. Prevention of autoimmune diabetes by ectopic pancreatic β-cell expression of interleukin-35. Diabetes 2012, 61, 1519–1526. [Google Scholar] [CrossRef]
- Mondanelli, G.; Volpi, C.; Bianchi, R.; Allegrucci, M.; Talesa, V.N.; Grohmann, U.; Belladonna, M.L. Islet antigen-pulsed dendritic cells expressing ectopic IL-35Ig protect nonobese diabetic mice from autoimmune diabetes. Cytokine 2015, 75, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, F.; Johnson, M.C.; Li, C.; Samulski, R.J.; Wang, B.; Tisch, R. β-cell-specific IL-35 therapy suppresses ongoing autoimmune diabetes in NOD mice. Eur. J. Immunol. 2017, 47, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Aiello, L.P.; Wong, J.S. Role of vascular endothelial growth factor in diabetic vascular complications. Kidney Int. Suppl. 2000, 77, S113–S119. [Google Scholar] [CrossRef] [PubMed]
- Simons, M. Angiogenesis, arteriogenesis, and diabetes: Paradigm reassessed? J. Am. Coll. Cardiol. 2005, 46, 835–837. [Google Scholar] [CrossRef]
- Cao, Y.; Linden, P.; Shima, D.; Browne, F.; Folkman, J. In vivo angiogenic activity and hypoxia induction of heterodimers of placenta growth factor/vascular endothelial growth factor. J. Clin. Investig. 1996, 98, 2507–2511. [Google Scholar] [CrossRef]
- Semeran, K.; Pawłowski, P.; Lisowski, Ł.; Szczepaniak, I.; Wójtowicz, J.; Ławicki, S.; Bakunowicz-Łazarczyk, A.; Bossowski, A. Plasma levels of IL-17, VEGF, and adrenomedullin and S-cone dysfunction of the retina in children and adolescents without signs of retinopathy and with varied duration of diabetes. Mediat. Inflamm. 2013, 2013, 274726. [Google Scholar] [CrossRef]
- Bergkamp, S.C.; Wahadat, M.J.; Salah, A.; Kuijpers, T.W.; Smith, V.; Tas, S.W.; van den Berg, J.M.; Kamphuis, S.; Schonenberg-Meinema, D. Dysregulated endothelial cell markers in systemic lupus erythematosus: A systematic review and meta-analysis. J. Inflamm. 2023, 20, 18. [Google Scholar] [CrossRef]
- Kuryliszyn-Moskal, A.; Zarzycki, W.; Dubicki, A.; Moskal, D.; Kosztyła-Hojna, B.; Hryniewicz, A. Clinical usefulness of videocapillaroscopy and selected endothelial cell activation markers in people with Type 1 diabetes mellitus complicated by microangiopathy. Adv. Med. Sci. 2017, 62, 368–373. [Google Scholar] [CrossRef]
- Bigda, J.; Beletsky, I.; Brakebusch, C.; Varfolomeev, Y.; Engelmann, H.; Bigda, J.; Holtmann, H.; Wallach, D. Dual role of the p75 tumor necrosis factor (TNF) receptor in TNF cytotoxicity. J. Exp. Med. 1994, 180, 445–460. [Google Scholar] [CrossRef]
- Zorena, K.; Myśliwiec, M.; Hak, Ł.; Rybarczyk, K.; Malinowska, E.; Raczyńska−Woźniak, D.; Raczyńska, K.; Balcerska, A.; Myśliwska, J. Proangiogenic effects of tumor necrosis factor-alpha (TNF-α) in diabetes mellitus children. Fam. Med. Prim. Care Rev. 2008, 10, 745–748. [Google Scholar]
- Zorena, K.; Myśliwska, J.; Myśliwiec, M.; Balcerska, A.; Hak, Ł.; Lipowski, P.; Raczyńska, K. Serum TNF-alpha level predicts nonproliferative diabetic retinopathy in children. Mediat. Inflamm. 2007, 2007, 92196. [Google Scholar] [CrossRef] [PubMed]
- Myśliwiec, M.; Balcerska, A.; Zorena, K.; Myśliwska, J.; Lipowski, P.; Raczyńska, K. The assessment of the correlation between vascular endothelial growth factor (VEGF), tumor necrosis factor (TNF-alpha), interleukin 6 (IL-6), glycaemic control (HbA1c) and the development of the diabetic retinopathy in children with diabetes mellitus type 1. Klin. Ocz. 2007, 109, 150–154. [Google Scholar]
- Gustavsson, C.; Agardh, E.; Bengtsson, B.; Agardh, C.D. TNF-alpha is an independent serum marker for proliferative retinopathy in type 1 diabetic patients. J. Diabetes Complicat. 2008, 22, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Koleva-Georgieva, D.N.; Sivkova, N.P.; Terzieva, D. Serum inflammatory cytokines IL-1beta, IL-6, TNF-alpha and VEGF have influence on the development of diabetic retinopathy. Folia Medica 2011, 53, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Jörneskog, G.; Brismar, K.; Fagrell, B. Skin capillary circulation severely impaired in toes of patients with IDDM, with and without late diabetic complications. Diabetologia 1995, 38, 474–480. [Google Scholar] [CrossRef]
- Tibiriçá, E.; Rodrigues, E.; Cobas, R.A.; Gomes, M.B. Endothelial function in patients with type 1 diabetes evaluated by skin capillary recruitment. Microvasc. Res. 2007, 73, 107–112. [Google Scholar] [CrossRef]
- Abdelmaksoud, A.A.; Daifallah, S.M.; Salah, N.Y.; Saber, A.S. Nail fold microangiopathy in adolescents with type 1 diabetes: Relation to diabetic vascular complications. Microcirculation 2022, 29, e12771. [Google Scholar] [CrossRef]
- American Diabetes Association. Microvascular complications and foot care, Sec. 9. Diabetes Care 2016, 39 (Suppl. S1), 12. [Google Scholar]
- Jorneskog, G.; Brismar, K.; Fagrell, B. Skin capillary circulation is more impaired in the toes of diabetic than non-diabetic patients with peripheral vascular disease. Diabet. Med. 1995, 12, 36–41. [Google Scholar] [CrossRef]
- Junqueira, C.L.C.; Magalhães, M.E.C.; Brandão, A.A.; Ferreira, E.; Cyrino, F.Z.G.A.; Maranhão, P.A.; Souza, M.D.G.C.; Bottino, D.A.; Bouskela, E. Microcirculation and biomarkers in patients with resistant or mild-to-moderate hypertension: A cross-sectional study. Hypertens. Res. 2018, 41, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Schoina, M.; Loutradis, C.; Theodorakopoulou, M.; Dimitroulas, T.; Triantafillidou, E.; Doumas, M.; Karagiannis, A.; Garyfallos, A.; Papagianni, A.; Sarafidis, P. The presence of diabetes mellitus further impairs structural and functional capillary density in patients with chronic kidney disease. Microcirculation 2021, 28, e12665. [Google Scholar] [CrossRef] [PubMed]
- Neubauer-Geryk, J.; Kozera, G.M.; Wolnik, B.; Szczyrba, S.; Nyka, W.M.; Bieniaszewski, L. Decreased reactivity of skin microcirculation in response to L -arginine in later -onset type 1 diabetes. Diabetes Care 2013, 36, 950–956. [Google Scholar] [CrossRef]
- Gasser, P.; Berger, W. Nailfold videomicroscopy and local cold test in type 1 diabetics. Angiology 1992, 43, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Kuryliszyn-Moskal, A.; Dubicki, A.; Zarzycki, W.; Zonnenberg, A.; Górska, M. Microvascular abnormalities in capillaroscopy correlate with higher serum IL-18 and sE-selectin levels in patients with type 1 diabetes complicated by microangiopathy. Folia Histochem. Cytobiol. 2011, 49, 104–110. [Google Scholar] [CrossRef]
- Hoffmann, M.; Neubauer-Geryk, J.; Wielicka, M.; Kowaleczko, M.; Myśliwiec, M.; Bieniaszewski, L. The impact of autoimmune thyroiditis on skin microcirculation in children with non-complicated type 1 diabetes mellitus. Microvasc. Res. 2019, 123, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Tooke, J.E.; Lins, P.E.; Ostergren, J.; Adamson, U.; Fagrell, B. The effects of intravenous insulin infusion on skin microcirculatory flow in Type 1 diabetes. Int. J. Microcirc. Clin. Exp. 1985, 4, 69–83. [Google Scholar]
- Costa, G.; Shushanof, M.; Bouskela, E.; Bottino, D. Oral L-Arginine (5 g/day) for 14 Days Improves Microcirculatory Function in Healthy Young Women and Healthy and Type 2 Diabetes Mellitus Elderly Women. J. Vasc. Res. 2022, 59, 24–33. [Google Scholar] [CrossRef]
- Buss, C.; Maranhão, P.A.; de Souza, M.D.G.C.; Bouskela, E.; Kraemer-Aguiar, L.G. Obesity blunts cephalic-phase microvascular responses to food. Physiol. Behav. 2020, 225, 113087. [Google Scholar] [CrossRef]
- Bastos da Cunha, C.; Sicuro, F.; Maranhão, P.A.; Borges, M.A.; Cyrino, F.Z.; Gazolla, F.M.; Madeira, I.R.; Bordallo, M.A.N.; Bouskela, E.; Kraemer-Aguiar, L.G. Microcirculation, Adiposity, and Traditional and Emerging Cardiovascular Risk Factors in Prepubertal Children. J. Endocr. Soc. 2017, 1, 908–917. [Google Scholar] [CrossRef]
- Maranhão, P.A.; de Souza, M.d.; Kraemer-Aguiar, L.G.; Bouskela, E. Dynamic nailfold videocapillaroscopy may be used for early detection of microvascular dysfunction in obesity. Microvasc. Res. 2016, 106, 31–35. [Google Scholar] [CrossRef]
- Lopes, K.G.; das Graças Coelho de Souza, M.; da Costa Tavares Bezerra, M.; Bessa, L.M.; Farinatti, P.; Bouskela, E.; Madeira, M.; Kraemer-Aguiar, L.G. Effects of physical training on physical and functional fitness, physical activity level, endothelial function, hemodynamic variables, bone metabolism, and quality of life of post-bariatric patients: Study protocol for a randomized controlled trial. Trials 2022, 23, 733. [Google Scholar] [CrossRef]
- Barcelos, A.; Tibirica, E.; Lamas, C. Evaluation of microvascular endothelial function and capillary density in patients with infective endocarditis using laser speckle contrast imaging and video-capillaroscopy. Microvasc. Res. 2018, 118, 61–68. [Google Scholar] [CrossRef]
- Junqueira, C.L.C.; Ferreira, E.; Junqueira, A.S.M.; Cyrino, F.Z.G.A.; Maranhão, P.A.; Kraemer-Aguiar, L.G.; Bottino, D.A.; de Souza, M.D.G.C.; Bouskela, E. Peripheral microvascular dysfunction is also present in patients with ischemia and no obstructive coronary artery disease (INOCA). Clin. Hemorheol. Microcirc. 2021, 79, 381–393. [Google Scholar] [CrossRef]
- Shore, A.C. Capillaroscopy and the measurement of capillary pressure. Br. J. Clin. Pharmacol. 2000, 50, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Wong, B.J.; Wilkins, B.W.; Holowatz, L.A.; Minson, C.T. Nitric oxide synthase inhibition does not alter the reactive hyperemic response in the cutaneous circulation. J. Appl. Physiol. 2003, 95, 504–1049. [Google Scholar] [CrossRef]
- Cracowski, J.L.; Minson, C.T.; Salvat-Melis, M.; Halliwill, J.R. Methodological issues in the assessment of skin microvascular endothelial function in humans. Trends Pharmacol. Sci. 2006, 27, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, S.; Minson, C.T. Human cutaneous reactive hyperaemia: Role of BKCa channels and sensory nerves. J. Physiol. 2007, 585, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Larkin, S.W.; Williams, T.J. Evidence for sensory nerve involvement in cutaneous reactive hyperemia in humans. Circ. Res. 1993, 73, 147–154. [Google Scholar] [CrossRef]
- Patterson, G.C. The role of intravascular pressure in the causation of reactive hyperaemia in the human forearm. Clin. Sci. 1956, 15, 17–25. [Google Scholar] [PubMed]
- Kontos, H.A.; Mauck, J.; Patterson, J. Mechanism of reactive hyperemia in limbs of anesthetized dogs. Am. J. Physiol. 1965, 209, 1106–1114. [Google Scholar] [CrossRef]
- Riccieri, V.; Germano, V.; Alessandri, C.; Vasile, M.; Ceccarelli, F.; Sciarra, I.; Di Franco, M.; Spadaro, A.; Valesini, G. More severe nailfold capillaroscopy findings and anti-endothelial cell antibodies. Are they useful tools for prognostic use in systemic sclerosis? Clin. Exp. Rheumatol. 2008, 26, 992–997. [Google Scholar]
- Ingegnoli, F.; Boracchi, P.; Gualtierotti, R.; Biganzoli, E.M.; Zeni, S.; Lubatti, C.; Fantini, F. Improving outcome prediction of systemic sclerosis from isolated Raynaud’s phenomenon: Role of autoantibodies and nail-fold capillaroscopy. Rheumatology 2010, 49, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Movasat, A.; Shahram, F.; Carreira, P.E.; Nadji, A.; Akhlaghi, M.; Naderi, N.; Davatchi, F. Nailfold capillaroscopy in Behçet’s disease, analysis of 128 patients. Clin. Rheumatol. 2009, 28, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Tektonidou, M.; Kaskani, E.; Skopouli, F.N.; Moutsopoulos, H.M. Microvascular abnormalities in Sjogren’s syndrome: Nailfold capillaroscopy. Rheumatology 1999, 38, 826–830. [Google Scholar] [CrossRef] [PubMed]
- Ruaro, B.; Sulli, A.; Smith, V.; Pizzorni, C.; Paolino, S.; Alessandri, E.; Cutolo, M. Microvascular damage evaluation in systemic sclerosis: The role of nailfold videocapillaroscopy and laser techniques. Reumatismo 2017, 69, 147–155. [Google Scholar] [CrossRef]
- Sulli, A.; Soldano, S.; Pizzorni, C.; Montagna, P.; Secchi, M.E.; Villaggio, B.; Seriolo, B.; Brizzolara, R.; Cutolo, M. Raynaud’s phenomenon and plasma endothelin: Correlations with capillaroscopic patterns in systemic sclerosis. J. Rheumatol. 2009, 36, 1235–1239. [Google Scholar] [CrossRef] [PubMed]
- Harper, F.E.; Maricq, H.R.; Turner, R.E. A prospective study of Raynaud phenomenon and early connective tissue disease. A five-year report. Am. J. Med. 1982, 72, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Singer, D.E.; Nathan, D.M.; Fogel, H.A.; Schachat, A.P. Screening guidelines for diabetic retinopathy. American College of Physicians. American Diabetes Association and American Academy of Ophthalmology. Ann. Intern. Med. 1992, 116, 683–685. [Google Scholar]
- Dyck, P.J. Detection, characterization, and staging of polyneuropathy: Assessed in diabetics. Muscle Nerve 1988, 11, 21–32. [Google Scholar] [CrossRef]
- Neubauer-Geryk, J.; Hoffmann, M.; Wielicka, M.; Piec, K.; Kozera, G.; Brzeziński, M.; Bieniaszewski, L. Current methods for the assessment of skin microcirculation: Part 1. Postep. Dermatol. Alergol. 2019, 36, 247–254. [Google Scholar] [CrossRef]
- Kernick, D.P.; Tooke, J.E.; Shore, A.C. The biological zero signal in laser Doppler fluximetry—Origins and practical implications. Pflug. Arch. 1999, 437, 624–631. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef]
- Smith, V.; Pizzorni, C.; De Keyser, F.; Decuman, S.; Van Praet, J.T.; Deschepper, E.; Sulli, A.; Cutolo, M. Reliability of the qualitative and semiquantitative nailfold videocapillaroscopy assessment in a systemic sclerosis cohort: A two-centre study. Ann. Rheum. Dis. 2010, 69, 1092–1096. [Google Scholar] [CrossRef] [PubMed]
- Paiva, H.; Laakso, J.; Kahonen, M.; Turjanmaa, V.; Koobi, T.; Majahalme, S.; Lehtimaki, T.; Ruokonen, I.; Laaksonen, R. Asymmetric dimethylarginine and hemodynamic regulation in middle-aged men. Metabolism 2006, 55, 771–777. [Google Scholar] [CrossRef]
- IJzerman, R.G.; de Jongh, R.T.; Beijk, M.A.; van Weissenbruch, M.M.; de Waal, H.A.D.; Serné, E.H.; Stehouwer, C.D. Individuals at increased coronary heart disease risk are characterized by an impaired microvascular function in skin. Eur. J. Clin. Investig. 2003, 33, 536–542. [Google Scholar] [CrossRef]
- Zorena, K.; Myśliwska, J.; Myśliwiec, M.; Balcerska, A.; Lipowski, P.; Raczyńska, K. Interleukin-12 and tumour necrosis factor-alpha equilibrium is a prerequisite for clinical course free from late complications in children with type 1 diabetes mellitus. Scand. J. Immunol. 2008, 67, 204–208. [Google Scholar] [CrossRef]
- Yokoi, M.; Yamagishi, S.I.; Takeuchi, M.; Ohgami, K.; Okamoto, T.; Saito, W.; Muramatsu, M.; Imaizumi, T.; Ohno, S. Elevations of AGE and vascular endothelial growth factor with decreased total antioxidant status in the vitreous fluid of diabetic patients with retinopathy. Br. J. Ophthalmol. 2005, 89, 673–675. [Google Scholar] [CrossRef] [PubMed]
- Santilli, F.; Spagnoli, A.; Mohn, A.; Tumini, S.; Verrotti, A.; Cipollone, F.; Mezzetti, A.; Chiarelli, F. Increased vascular endothelial growth factor serum concentrations may help to identify patients with onset of type 1 diabetes during childhood at risk for developing persistent microalbuminuria. J. Clin. Endocrinol. Metab. 2001, 86, 3871–3876. [Google Scholar] [CrossRef] [PubMed]
- Chiarelli, F.; Spagnoli, A.; Basciani, F.; Tumini, S.; Mezzetti, A.; Cipollone, F.; Cuccurullo, F.; Morgese, G.; Verrotti, A. Vascular endothelial growth factor (VEGF) in children, adolescents and young adults with Type 1 diabetes mellitus: Relation to glycaemic control and microvascular complications. Diabet. Med. 2000, 17, 650–656. [Google Scholar] [CrossRef]
- Espes, D.; Singh, K.; Sandler, S.; Carlsson, P.O. Increased Interleukin-35 Levels in Patients With Type 1 Diabetes With Remaining C-Peptide. Diabetes Care 2017, 40, 1090–1095. [Google Scholar] [CrossRef] [PubMed]
- Collison, L.W.; Delgoffe, G.M.; Guy, C.S.; Vignali, K.M.; Chaturvedi, V.; Fairweather, D.; Satoskar, A.R.; Garcia, K.C.; Hunter, C.A.; Drake, C.G.; et al. The composition and signaling of the IL-35 receptor are unconventional. Nat. Immunol. 2012, 13, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; Yano, H.; Workman, C.J.; Vignali, D.A. Interleukin-35: Structure, Function and Its Impact on Immune-Related Diseases. J. Interferon Cytokine Res. 2021, 41, 391–406. [Google Scholar] [CrossRef]
- Reza Lahimchi, M.; Eslami, M.; Yousefi, B. Interleukin-35 and Interleukin-37 anti-inflammatory effect on inflammatory bowel disease: Application of non-coding RNAs in IBD therapy. Int. Immunopharmacol. 2023, 117, 109932. [Google Scholar] [CrossRef] [PubMed]
- Kamal, A.; Hosny, M.; Abd Elwahab, A.; Kamal, Y.S.; Shehata, H.S.; Hassan, A. FOXP3rs3761548 gene variant and interleukin-35 serum levels as biomarkers in patients with multiple sclerosis. Rev. Neurol. 2021, 177, 647–654. [Google Scholar] [CrossRef]
- Luo, Z.; Lundin, S.; Mejia-Cordova, M.; Hassani, I.; Blixt, M.; Hjelmqvist, D.; Lau, J.; Espes, D.; Carlsson, P.O.; Sandler, S.; et al. Interleukin-35 Prevents Development of Autoimmune Diabetes Possibly by Maintaining the Phenotype of Regulatory B Cells. Int. J. Mol. Sci. 2021, 30, 12988. [Google Scholar] [CrossRef]
- Nordwall, M.; Abrahamsson, M.; Dhir, M.; Fredrikson, M.; Ludvigsson, J.; Arnqvist, H.J. Impact of HbA1c, followed from onset of type 1 diabetes, on the development of severe retinopathy and nephropathy: The VISS Study (Vascular Diabetic Complications in Southeast Sweden). Diabetes Care 2015, 38, 308–315. [Google Scholar] [CrossRef]
- Giordano, C.; Amato, M.C.; Ciresi, A.; Citarrella, R.; Mantione, L.; Accidenti, M.; Pantò, F.; Guarnotta, V.; Allotta, M.L.; Criscimanna, A.; et al. Predictors of microvascular complications in type 1 diabetic patients at onset: The role of metabolic memory. Eur. J. Intern. Med. 2011, 22, 266–274. [Google Scholar] [CrossRef]
- Neubauer-Geryk, J.; Wielicka, M.; Kozera, G.M.; Brandt-Varma, A.; Wołoszyn-Durkiewicz, A.; Myśliwiec, M.; Bieniaszewski, L. Skin oxygenation impairment is associated with increased total cholesterol level in children with short-lasting type 1 diabetes mellitus. Postep. Dermatol. I Alergol. 2021, 38, 615–621. [Google Scholar] [CrossRef]
- Antonios, T.F.T.; Kaski, J.C.; Hasan, K.M.; Brown, S.J.; Singer, D.R.J. Rarefaction of skin capillaries in patients with anginal chest pain and normal coronary arteriograms. Eur. Heart J. 2001, 22, 1144–1148. [Google Scholar] [CrossRef]
- Abi-Chahin, T.C.; Hausen, M.D.A.; Mansano-Marques, C.M.; Halfoun, V.L. Microvascular reactivity in type 1 diabetics. Arq. Bras. De Endocrinol. E Metabol. 2009, 53, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Schalkwijk, C.G.; Stehouwer, C.D. Vascular complications in diabetes mellitus: The role of endothelial dysfunction. Clin. Sci. 2005, 109, 143–159. [Google Scholar] [CrossRef]
- Kuryliszyn-Moskal, A.; Ciołkiewicz, M.; Dubicki, A. Morphological alterations in nailfold capillaroscopy and the clinical picture of vascular involvement in autoimmune diseases: Systemic lupus erythematosus and type 1 diabetes. Ann. Acad. Med. Stetin. 2010, 56 (Suppl. S1), 73–79. [Google Scholar] [PubMed]
- Tooke, J.E.; Lins, P.E.; Ostergren, J.; Fagrell, B. Skin microvascular autoregulatory responses in type i diabetes: The influence of duration and control. Int. J. Microcirc. Clin. Exp. 1985, 4, 249–256. [Google Scholar] [PubMed]
- Tibirica, E.; Rodrigues, E.; Cobas, R.A.; Gomes, M.B. Increased functional and structural skin capillary density in type 1 diabetes patients with vascular complications. Diabetol. Metab. Syndr. 2009, 1, 24. [Google Scholar] [CrossRef]
- Tehrani, S.; Bergen, K.; Azizi, L.; Jörneskog, G. Skin microvascular reactivity correlates to clinical microangiopathy in type 1 diabetes: A pilot study. Diabetes Vasc. Dis. Res. 2020, 17, 1479164120928303. [Google Scholar] [CrossRef]




| Characteristics | Diabetic Patients n = 46 | Diabetic Patients Subgroups According to Median Coveragebase | p for between Subgroups Comparison | |
|---|---|---|---|---|
| Subgroup A, n = 23 | subgroup B, n = 23 | |||
| Mean ± SD/ Median (Range) | Mean ± SD/ Median (Range) | Mean ± SD/ Median (Range) | ||
| Males, n (%) | 22 (47.8) | 12 (50) | 11 (47.8) | 1 |
| Age [years] | 15.6 ± 2.4/ 15.6 (8.4–18) | 15.0 ± 2/ 15.4 (11.1–18) | 15.2 ± 2.8/ 15.7 (8.4–18) | 0.59 |
| Onset of diabetes [age] | 7.3 ± 3.7/ 8.2 (1.2–13.8) | 7. 2 ± 3.7/ 8.4 (1.2–11.8) | 7.4 ± 4/ 7.6 (1.8–13.8) | 0.82 |
| Diabetes duration [years] | 7.0 ± 3.9/ 7.0 (1.7–15.9) | 7.8 ± 4/ 7.6 (1.8–14.6) | 7.7 ± 4 / 7.00 (1.7–15.9) | 0.85 |
| Autoimmune thyroiditis | 18 (39.1) | 8 (34.8) | 10 (43.5) | 0.54 |
| Treatment with statins, n (%) | 3 (6.5) | 0 | 3 (13) | 0.23 |
| BMI [kg/m²] | 20.4 (15–26.8) | 20.2 (15.7–24.8) | 20.4 (15–26.8) | 0.88 |
| HBA1c at onset of DM [%] | 9.9 (6.5–16.4) | 11.8 (6.6–16.4) | 9.1 (6.5–14.7) | 0.02 |
| HBA1c 1st year of DM [%] | 8.2 (6.2–12.7) | 9.3 (6.5–12.7) | 7.90 (6.2–10.5) | 0.04 |
| HBA1c 2nd year of DM [%] | 7.35 (5.5–10.4) | 7.8 (5.5–10) | 7.00 (5.7–10.4) | 0.04 |
| HBA1c at initiation of study [%] | 8.1 (5.9–13.4) | 8.1 (5.9–13.4) | 8.0 (6.3–11.8) | 0.44 |
| HBA1c following one year [%] | 8.5 (5.9–13.9) | 8.6 (5.9–13.9) | 8.0 (6.3–12.2) | 0.11 |
| Insulin dose units/24 h | 46 (20–100) | 46 (20–90) | 45 (21–100) | 0.88 |
| Treatment with pump [%] | 28 (60.2) | 12 (52.2) | 16 (69.6) | 0.22 |
| Time of pump treatment in ratio to DM duration [%] | 64 (0–100) | 19 (0 -95) | 75.6 (0–100) | 0.18 |
| Episodes of mild hypoglicaemia [N/last month] | 10 (3–30) | 10 (3.0–20) | 10 (3–30) | 0.80 |
| Episodes of severe hypoglicaemia [N/last year] | 0 (0–1) | 0 (0–1) | 0 (0–1) | 0.57 |
| Characteristics | Diabetic Patients | Diabetic Patients Subgroups According to Median CoverageBASE | p for between subgroups Comparison | |
|---|---|---|---|---|
| n = 46 | Subgroup A, n = 23 | Subgroup B, n = 23 | ||
| Median (Range) | Median (Range) | Median (Range) | ||
| Total cholesterol [mg/dL] | ||||
| baseline | 181 (125–288) | 184 (135–288) | 177 (125–285) | 0.44 |
| after one year | 180 (120–319) | 177 (148–315) | 184 (120–319) | 0.59 |
| p for one year comparison | 0.66 | 0.05 | 0.15 | |
| Cholesterol LDL [mg/dL] | ||||
| baseline | 108 (61–192) | 111 (61–188) | 105 (61–192) | 0.46 |
| after one year | 101 (48–200) | 99 (48–140) | 109 (70–200) | 0.29 |
| p for one year comparison | 0.19 | 0.005 | 0.46 | |
| Cholesterol HDL [mg/dL] | ||||
| baseline | 55 (38–90) | 59 (42–90) | 54 (38–83) | 0.77 |
| after one year | 54 (31–82) | 53 (31–81) | 54 (36–82) | 0.72 |
| p for one year comparison | 0.68 | 0.16 | 0.52 | |
| Triglycerides [mg/dL] | ||||
| baseline | 72 (34–274) | 78 (38–249) | 65 (34–274) | 0.10 |
| after one year | 78 (34–583) | 91 (34–408) | 70 (50–583) | 0.22 |
| p for one year comparison | 0.06 | 0.60 | 0.02 | |
| Serum creatinine [mg/dL] | ||||
| baseline | 0.68 (0.45–0.95) | 0.69 (0.5–0.95) | 0.65 (0.45–0.95) | 0.98 |
| after one year | 0.67 (0.44–0.93) | 0.67 (0.44–0.93) | 0.62 (0.45–0.91) | 0.75 |
| p for one year comparison | 0.03 | 0.01 | 0.53 | |
| Albuminuria [mg/dL] | ||||
| baseline | 6.9 (2.5–28) | 8.2 (2.5–24.4) | 6 (2.5–28) | 0.60 |
| after one year | 9.79 (5–27.74) | 10 (5–25) | 9 (5–27.74) | 0.81 |
| p for one year comparison | 0.01 | 0.14 | 0.05 | |
| TSH [mIU/L] | ||||
| baseline | 1.82 (0.57–5.1) | 1.6 (0.96–3.9) | 2.1 (0.57–5.1) | 0.15 |
| after one year | 1.77 (0.11–5.2) | 1.58 (0.1–3.4) | 2 (1.1–5.2) | 0.02 |
| p for one year comparison | 0.44 | 0.20 | 0.98 | |
| fT4 [pmol/L] | ||||
| baseline | 12.5 (9.0–15) | 12.5 (9–14.6) | 12.5 (9.33–15) | 0.52 |
| after one year | 13 (9.0–16) | 13 (9–16) | 13 (10.8–15.3) | 0.68 |
| p for one year comparison | 0.03 | 0.32 | 0.08 | |
| C-reactive protein [mg/L] | 0.41 (0.1–4.8) | 0.37 (0.1–4.8) | 0.44 (0.1–4.3) | 0.64 |
| TNF-α [pg/mL] | 2.4 (0–6.78) | 3.4 (0–6.8) | 1.25 (0–4.3) | 0.001 |
| IL-35 [ng/mL] | 4.85 (1.2–22.67) | 3.9 (1.2–22.4) | 6.8 (1.8–22.7) | 0.08 |
| ratio TNF-α/IL-35 | 0.44 (0–3.44) | 0.89 (0–3.4) | 0.18 (0–1.1) | 0.001 |
| VEGF [pg/mL] | 211.75 (38.8–533.73) | 232.2 (120.4–533.7) | 176.8 (38.81–357.4) | 0.03 |
| Characteristics | Diabetic Patients | Diabetic Patients Subgroups According to CoverageBASE Median | p for between | |
|---|---|---|---|---|
| n = 46 | Subgroup A n = 23 | Subgroup B n = 23 | Subgroups Comparison | |
| Median (Range) | Median (Range) | Median (range) | ||
| coverage before PORH test [ %] (coverageBASE) | ||||
| baseline | 17.1 (12.7–24.8) | 15.4 (12.7–17) | 17.8 (17.1–24.8) | --- |
| after one year | 18 (12–23) | 17(12–22) | 18 (15–23) | 0.33 |
| p for one year comparison | 0.19 | 0.03 | 0.54 | |
| difference between ΔcoverageBASE_12 and coverageBASE [%] (ΔcoveregaBASE) | ||||
| 0.7 ((−8.8)7.6) | 2 ((−3.6)–7.6) | −0.1 ((−9.4)–7.5) | 0.03 | |
| coverage after PORH test [%] (coveragePORH) | ||||
| baseline | 16 (10.4–24.4) | 15 (10.4–18.4) | 17.2 (11.5–24.4) | <0.001 |
| after one year | 16 (11–24) | 17 (11–24) | 16 (11–20) | 0.56 |
| p for one year comparison | 0.88 | 0.07 | 0.02 | |
| difference between ΔcoveragePORH_12 and coveragePORH [%] (ΔcoveregaPORH) | ||||
| −0.3 ((−9.4)–12.6) | 2.3 ((−4.5)–12.6)) | −1.2 ((−9.4)–7.5) | 0.005 | |
| capillary reactivity [%] Δcoverage between coverage after and before PORH test [%] (ΔcoverageBP) | ||||
| baseline | −0.7 ((−8.1)–4.6) | −0.3 ((−4.1)–4.2) | −1.1 ((−8.1)–4.6) | 0.57 |
| after one year | −1.0 ((−1.0)–5.0) | −1 ((−6)–5) | −1 ((−10)–2) | 0.10 |
| p for one year comparison | 0.18 | 0.72 | 0.11 | |
| (the ratio of Δcoverage between coverage after and before PORH test, and coverage before PORH test) (R_coverage) | ||||
| baseline | −3.9 ((−41.3)–31.3) | −1.9 ((−26.5)–31.3) | −5.8 ((−41.3)–26.3) | 0.63 |
| after one year | −4.5 ((−43)–44) | −1.0 ((−28)–44) | −7.0 ((−43)–16) | 0.16 |
| p for one year comparison | 0.30 | 0.97 | 0.12 | |
| ratio between capillary reactivity after one year to capillary reactivity at baseline | ||||
| 0 ((−19)–18) | 0.5 ((−18)–19) | 0 ((−12)–4) | 0.44 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neubauer-Geryk, J.; Wielicka, M.; Myśliwiec, M.; Zorena, K.; Bieniaszewski, L. The Relationship between TNF-a, IL-35, VEGF and Cutaneous Microvascular Dysfunction in Young Patients with Uncomplicated Type 1 Diabetes. Biomedicines 2023, 11, 2857. https://doi.org/10.3390/biomedicines11102857
Neubauer-Geryk J, Wielicka M, Myśliwiec M, Zorena K, Bieniaszewski L. The Relationship between TNF-a, IL-35, VEGF and Cutaneous Microvascular Dysfunction in Young Patients with Uncomplicated Type 1 Diabetes. Biomedicines. 2023; 11(10):2857. https://doi.org/10.3390/biomedicines11102857
Chicago/Turabian StyleNeubauer-Geryk, Jolanta, Melanie Wielicka, Małgorzata Myśliwiec, Katarzyna Zorena, and Leszek Bieniaszewski. 2023. "The Relationship between TNF-a, IL-35, VEGF and Cutaneous Microvascular Dysfunction in Young Patients with Uncomplicated Type 1 Diabetes" Biomedicines 11, no. 10: 2857. https://doi.org/10.3390/biomedicines11102857
APA StyleNeubauer-Geryk, J., Wielicka, M., Myśliwiec, M., Zorena, K., & Bieniaszewski, L. (2023). The Relationship between TNF-a, IL-35, VEGF and Cutaneous Microvascular Dysfunction in Young Patients with Uncomplicated Type 1 Diabetes. Biomedicines, 11(10), 2857. https://doi.org/10.3390/biomedicines11102857