Evaluation of the Autologous Genetically Enriched Leucoconcentrate on the Lumbar Spinal Cord Morpho-Functional Recovery in a Mini Pig with Thoracic Spine Contusion Injury
Abstract
:1. Introduction
2. Materials and Methods
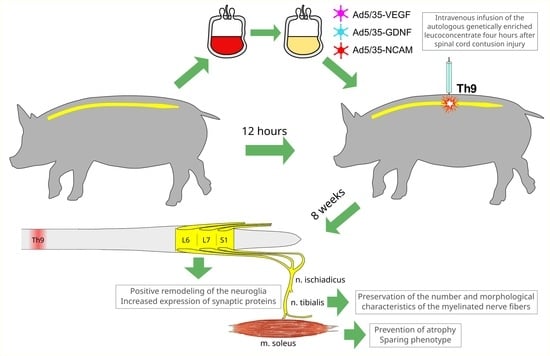
2.1. Preparation of the Autologous Genetically Enriched Leucoconcentrate
2.2. Animals Treatment
2.3. Behavioral Assessment
2.4. Electrophysiological Study
2.5. Sample Collection
2.6. Immunofluorescence Study of Lumbar Spinal Cord
2.7. Morphometric Analysis of the Sciatic Nerve Myelinated Fibers
2.8. Hind Limb Skeletal Muscle Study
2.9. Statistics
3. Results
3.1. Hind Limb Skeletal Muscle Recovery
3.1.1. Motor Activity
3.1.2. Electrophysiology
3.1.3. Histology
3.2. Lumbar Spinal Cord and Tibial Nerve Plasticity
3.2.1. Motor Neurons
3.2.2. Neuroglial Cells
3.2.3. Tibial Nerve Myelinated Fibers
4. Discussion
4.1. Lumbar Spinal Cord
4.2. Peripheral Nerves
4.3. Hind Limb Skeletal Muscle
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O’Shea, T.M.; Burda, J.E.; Sofroniew, M.V. Cell Biology of Spinal Cord Injury and Repair. J. Clin. Investig. 2017, 127, 3259–3270. [Google Scholar] [CrossRef]
- Wang, D.; Fawcett, J. The Perineuronal Net and the Control of CNS Plasticity. Cell Tissue Res. 2012, 349, 147–160. [Google Scholar] [CrossRef]
- Chai, R.J.; Vukovic, J.; Dunlop, S.; Grounds, M.D.; Shavlakadze, T. Striking Denervation of Neuromuscular Junctions without Lumbar Motoneuron Loss in Geriatric Mouse Muscle. PLoS ONE 2011, 6, e28090. [Google Scholar] [CrossRef] [PubMed]
- Eidelberg, E.; Nguyen, L.H.; Polich, R.; Walden, J.G. Transsynaptic Degeneration of Motoneurones Caudal to Spinal Cord Lesions. Brain Res. Bull. 1989, 22, 39–45. [Google Scholar] [CrossRef]
- McBride, R.L.; Feringa, E.R. Ventral Horn Motoneurons 10, 20 and 52 Weeks after T-9 Spinal Cord Transection. Brain Res. Bull. 1992, 28, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Yokota, K.; Kubota, K.; Kobayakawa, K.; Saito, T.; Hara, M.; Kijima, K.; Maeda, T.; Katoh, H.; Ohkawa, Y.; Nakashima, Y.; et al. Pathological Changes of Distal Motor Neurons after Complete Spinal Cord Injury. Mol. Brain 2019, 12, 4. [Google Scholar] [CrossRef]
- Wang, F.; Yuan, Y.; Li, J. Reconstruction of Lower Extremity Function of Complete Spinal Cord Injury Rats by First Neuron Connection. Chin. J. Reparative Reconstr. Surg. 2015, 29, 1528–1533. [Google Scholar]
- Detloff, M.R.; Fisher, L.C.; McGaughy, V.; Longbrake, E.E.; Popovich, P.G.; Basso, D.M. Remote Activation of Microglia and Pro-Inflammatory Cytokines Predict the Onset and Severity of below-Level Neuropathic Pain after Spinal Cord Injury in Rats. Exp. Neurol. 2008, 212, 337–347. [Google Scholar] [CrossRef]
- McKay, S.M.; Brooks, D.J.; Hu, P.; McLachlan, E.M. Distinct Types of Microglial Activation in White and Grey Matter of Rat Lumbosacral Cord after Mid-Thoracic Spinal Transection. J. Neuropathol. Exp. Neurol. 2007, 66, 698–710. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, H.; Honjoh, K.; Watanabe, S.; Kubota, A.; Matsumine, A. Distribution and Polarization of Microglia and Macrophages at Injured Sites and the Lumbar Enlargement after Spinal Cord Injury. Neurosci. Lett. 2020, 737, 135152. [Google Scholar] [CrossRef] [PubMed]
- Honjoh, K.; Nakajima, H.; Hirai, T.; Watanabe, S.; Matsumine, A. Relationship of Inflammatory Cytokines From M1-Type Microglia/Macrophages at the Injured Site and Lumbar Enlargement with Neuropathic Pain after Spinal Cord Injury in the CCL21 Knockout (Plt) Mouse. Front. Cell. Neurosci. 2019, 13, 525. [Google Scholar] [CrossRef]
- Gwak, Y.S.; Kang, J.; Unabia, G.C.; Hulsebosch, C.E. Spatial and Temporal Activation of Spinal Glial Cells: Role of Gliopathy in Central Neuropathic Pain Following Spinal Cord Injury in Rats. Exp. Neurol. 2012, 234, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Pallottie, A.; Ratnayake, A.; Ni, L.; Acioglu, C.; Li, L.; Mirabelli, E.; Heary, R.F.; Elkabes, S. A Toll-like Receptor 9 Antagonist Restores below-Level Glial Glutamate Transporter Expression in the Dorsal Horn Following Spinal Cord Injury. Sci. Rep. 2018, 8, 8723. [Google Scholar] [CrossRef]
- Min, K.-J.; Jeong, H.-K.; Kim, B.; Hwang, D.H.; Shin, H.Y.; Nguyen, A.T.; Kim, J.-H.; Jou, I.; Kim, B.G.; Joe, E.-H. Spatial and Temporal Correlation in Progressive Degeneration of Neurons and Astrocytes in Contusion-Induced Spinal Cord Injury. J. Neuroinflammation 2012, 9, 100. [Google Scholar] [CrossRef] [PubMed]
- Bisicchia, E.; Sasso, V.; Catanzaro, G.; Leuti, A.; Besharat, Z.M.; Chiacchiarini, M.; Molinari, M.; Ferretti, E.; Viscomi, M.T.; Chiurchiù, V. Resolvin D1 Halts Remote Neuroinflammation and Improves Functional Recovery after Focal Brain Damage via ALX/FPR2 Receptor-Regulated MicroRNAs. Mol. Neurobiol. 2018, 55, 6894–6905. [Google Scholar] [CrossRef]
- Courtine, G.; Sofroniew, M.V. Spinal Cord Repair: Advances in Biology and Technology. Nat. Med. 2019, 25, 898–908. [Google Scholar] [CrossRef]
- Cofano, F.; Boido, M.; Monticelli, M.; Zenga, F.; Ducati, A.; Vercelli, A.; Garbossa, D. Mesenchymal Stem Cells for Spinal Cord Injury: Current Options, Limitations, and Future of Cell Therapy. Int. J. Mol. Sci. 2019, 20, 2698. [Google Scholar] [CrossRef] [PubMed]
- Anna, Z.; Katarzyna, J.-W.; Joanna, C.; Barczewska, M.; Joanna, W.; Wojciech, M. Therapeutic Potential of Olfactory Ensheathing Cells and Mesenchymal Stem Cells in Spinal Cord Injuries. Stem Cells Int. 2017, 2017, 3978595. [Google Scholar] [CrossRef]
- Kanno, H.; Pearse, D.D.; Ozawa, H.; Itoi, E.; Bunge, M.B. Schwann Cell Transplantation for Spinal Cord Injury Repair: Its Significant Therapeutic Potential and Prospectus. Rev. Neurosci. 2015, 26, 121–128. [Google Scholar] [CrossRef]
- Zörner, B.; Schwab, M.E. Anti-Nogo on the Go: From Animal Models to a Clinical Trial. Ann. N. Y. Acad. Sci. 2010, 1198, E22–E34. [Google Scholar] [CrossRef]
- Fehlings, M.G.; Kim, K.D.; Aarabi, B.; Rizzo, M.; Bond, L.M.; McKerracher, L.; Vaccaro, A.R.; Okonkwo, D.O. Rho Inhibitor VX-210 in Acute Traumatic Subaxial Cervical Spinal Cord Injury: Design of the SPinal Cord Injury Rho INhibition InvestiGation (SPRING). Clin. Trial. J. Neurotrauma 2018, 35, 1049–1056. [Google Scholar] [CrossRef]
- Xiao, Z.; Tang, F.; Zhao, Y.; Han, G.; Yin, N.; Li, X.; Chen, B.; Han, S.; Jiang, X.; Yun, C.; et al. Significant Improvement of Acute Complete Spinal Cord Injury Patients Diagnosed by a Combined Criteria Implanted with NeuroRegen Scaffolds and Mesenchymal Stem Cells. Cell Transplant. 2018, 27, 907–915. [Google Scholar] [CrossRef]
- Siddiqui, A.M.; Islam, R.; Cuellar, C.A.; Silvernail, J.L.; Knudsen, B.; Curley, D.E.; Strickland, T.; Manske, E.; Suwan, P.T.; Latypov, T.; et al. Newly Regenerated Axons via Scaffolds Promote Sub-Lesional Reorganization and Motor Recovery with Epidural Electrical Stimulation. NPJ Regen. Med. 2021, 6, 66. [Google Scholar] [CrossRef]
- Yi, H.; Wang, Y. A Meta-Analysis of Exosome in the Treatment of Spinal Cord Injury. Open Med. 2021, 16, 1043–1060. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Chu, X.; Yuan, H.; Qiu, J.; Zhao, C.; Xin, D.; Li, T.; Ma, W.; Wang, H.; Wang, Z.; et al. Mesenchymal Stem Cell Derived EVs Mediate Neuroprotection after Spinal Cord Injury in Rats via the MicroRNA-21-5p/FasL Gene Axis. Biomed. Pharmacother. 2019, 115, 108818. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Lin, E.-Y.; Chiou, T.-W.; Harn, H.-J. Exosomes in Clinical Trial and Their Production in Compliance with Good Manufacturing Practice. Tzu-Chi Med. J. 2020, 32, 113–120. [Google Scholar] [CrossRef]
- Fehlings, M.; Zavvarian, M.M.; Toossi, A.; Khazaei, M.; Hong, J. Novel Innovations in Cell and Gene Therapies for Spinal Cord Injury. F1000Research 2020, 9. [Google Scholar] [CrossRef]
- Lavrov, I.; Islamov, R. Implementing Principles of Neuroontogenesis and Neuroplasticity for Spinal Cord Injury Therapy. Front. Biosci. 2022, 27, 163. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, C.J.; Viskontas, M.; Janowicz, K.; Sani, Y.; Håkansson, M.E.; Heidari, A.; Huang, W.; Bo, X. The Potential of Gene Therapies for Spinal Cord Injury Repair: A Systematic Review and Meta-Analysis of Pre-Clinical Studies. Neural Regen. Res. 2023, 18, 299–305. [Google Scholar] [CrossRef]
- Hanna, E.; Rémuzat, C.; Auquier, P.; Toumi, M. Gene Therapies Development: Slow Progress and Promising Prospect. J. Mark. Access Health Policy 2017, 5, 1265293. [Google Scholar] [CrossRef] [PubMed]
- Nardone, R.; Florea, C.; Höller, Y.; Brigo, F.; Versace, V.; Lochner, P.; Golaszewski, S.; Trinka, E. Rodent, Large Animal and Non-Human Primate Models of Spinal Cord Injury. Zoology 2017, 123, 101–114. [Google Scholar] [CrossRef]
- Sharif-Alhoseini, M.; Khormali, M.; Rezaei, M.; Safdarian, M.; Hajighadery, A.; Khalatbari, M.M.; Safdarian, M.; Meknatkhah, S.; Rezvan, M.; Chalangari, M.; et al. Animal Models of Spinal Cord Injury: A Systematic Review. Spinal Cord 2017, 55, 714–721. [Google Scholar] [CrossRef]
- Davleeva, M.A.; Garifulin, R.R.; Bashirov, F.V.; Izmailov, A.A.; Nurullin, L.F.; Salafutdinov, I.I.; Gatina, D.Z.; Shcherbinin, D.N.; Lysenko, A.A.; Tutykhina, I.L.; et al. Molecular and Cellular Changes in the Post-Traumatic Spinal Cord Remodeling after Autoinfusion of a Genetically-Enriched Leucoconcentrate in a Mini-Pig Model. Neural Regen. Res. 2023, 18, 1505–1511. [Google Scholar] [CrossRef]
- Islamov, R.; Bashirov, F.; Fadeev, F.; Shevchenko, R.; Izmailov, A.; Markosyan, V.; Sokolov, M.; Kuznetsov, M.; Davleeva, M.; Garifulin, R.; et al. Epidural Stimulation Combined with Triple Gene Therapy for Spinal Cord Injury Treatment. Int. J. Mol. Sci. 2020, 21, 8896. [Google Scholar] [CrossRef]
- Islamov, R.R.; Izmailov, A.A.; Sokolov, M.E.; Fadeev, F.O.; Bashirov, F.V.; Eremeev, A.A.; Shmarov, M.M.; Naroditskiy, B.S.; Chelyshev, Y.A.A.; Lavrov, I.A.; et al. Evaluation of Direct and Cell-Mediated Triple-Gene Therapy in Spinal Cord Injury in Rats. Brain Res. Bull. 2017, 132, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Islamov, R.R.; Bashirov, F.V.; Sokolov, M.E.; Izmailov, A.A.; Fadeev, F.O.; Markosyan, V.A.; Davleeva, M.A.; Zubkova, O.V.; Smarov, M.M.; Logunov, D.Y.; et al. Gene-Modified Leucoconcentrate for Personalized Ex Vivo Gene Therapy in a Mini Pig Model of Moderate Spinal Cord Injury. Neural Regen. Res. 2021, 16, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Islamov, R.; Bashirov, F.; Izmailov, A.; Fadeev, F.; Markosyan, V.; Sokolov, M.; Shmarov, M.; Logunov, D.; Naroditsky, B.; Lavrov, I. New Therapy for Spinal Cord Injury: Autologous Genetically-Enriched Leucoconcentrate Integrated with Epidural Electrical Stimulation. Cells 2022, 11, 144. [Google Scholar] [CrossRef]
- Izmailov, A.A.; Povysheva, T.V.; Bashirov, F.V.; Sokolov, M.E.; Fadeev, F.O.; Garifulin, R.R.; Naroditsky, B.S.; Logunov, D.Y.; Salafutdinov, I.I.; Chelyshev, Y.A.; et al. Spinal Cord Molecular and Cellular Changes Induced by Adenoviral Vector- and Cell-Mediated Triple Gene Therapy after Severe Contusion. Front. Pharmacol. 2017, 8, 813. [Google Scholar] [CrossRef]
- Lee, J.H.T.; Jones, C.F.; Okon, E.B.; Anderson, L.; Tigchelaar, S.; Kooner, P.; Godbey, T.; Chua, B.; Gray, G.; Hildebrandt, R.; et al. A Novel Porcine Model of Traumatic Thoracic Spinal Cord Injury. J. Neurotrauma 2013, 30, 142–159. [Google Scholar] [CrossRef] [PubMed]
- Islamov, R.R.; Sokolov, M.E.; Bashirov, F.V.; Fadeev, F.O.; Shmarov, M.M.; Naroditskiy, B.S.; Povysheva, T.V.; Shaymardanova, G.F.; Yakupov, R.A.; Chelyshev, Y.A.; et al. A Pilot Study of Cell-Mediated Gene Therapy for Spinal Cord Injury in Mini Pigs. Neurosci. Lett. 2017, 644, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, A.; Dyck, S.M.; Karimi-Abdolrezaee, S. Traumatic Spinal Cord Injury: An Overview of Pathophysiology, Models and Acute Injury Mechanisms. Front. Neurol. 2019, 10, 282. [Google Scholar] [CrossRef]
- Chelyshev, Y. More Attention on Segments Remote from the Primary Spinal Cord Lesion Site. Front. Biosci. 2022, 27, 235. [Google Scholar] [CrossRef]
- Kabdesh, I.M.; Mukhamedshina, Y.O.; Arkhipova, S.S.; Sabirov, D.K.; Kuznecov, M.S.; Vyshtakalyuk, A.B.; Rizvanov, A.A.; James, V.; Chelyshev, Y.A. Cellular and Molecular Gradients in the Ventral Horns with Increasing Distance from the Injury Site after Spinal Cord Contusion. Front. Cell. Neurosci. 2022, 16, 817752. [Google Scholar] [CrossRef]
- Calvo, P.M.; Hernández, R.G.; de la Cruz, R.R.; Pastor, A.M. Role of Vascular Endothelial Growth Factor as a Critical Neurotrophic Factor for the Survival and Physiology of Motoneurons. Neural Regen. Res. 2023, 18, 1691–1696. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.J.; Xu, X.M. History of Glial Cell Line-Derived Neurotrophic Factor (GDNF) and Its Use for Spinal Cord Injury Repair. Brain Sci. 2018, 8, 109. [Google Scholar] [CrossRef]
- Nicoletti, V.G.; Pajer, K.; Calcagno, D.; Pajenda, G.; Nógrádi, A. The Role of Metals in the Neuroregenerative Action of BDNF, GDNF, NGF and Other Neurotrophic Factors. Biomolecules 2022, 12, 1015. [Google Scholar] [CrossRef] [PubMed]
- Schmid, R.S.; Maness, P.F. L1 and NCAM Adhesion Molecules as Signaling Coreceptors in Neuronal Migration and Process Outgrowth. Curr. Opin. Neurobiol. 2008, 18, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Saini, V.; Loers, G.; Kaur, G.; Schachner, M.; Jakovcevski, I. Impact of Neural Cell Adhesion Molecule Deletion on Regeneration after Mouse Spinal Cord Injury. Eur. J. Neurosci. 2016, 44, 1734–1746. [Google Scholar] [CrossRef] [PubMed]
- Sytnyk, V.; Leshchyns’ka, I.; Schachner, M. Neural Cell Adhesion Molecules of the Immunoglobulin Superfamily Regulate Synapse Formation, Maintenance, and Function. Trends Neurosci. 2017, 40, 295–308. [Google Scholar] [CrossRef]
- De Almodovar, C.R.; Lambrechts, D.; Mazzone, M.; Carmeliet, P. Role and Therapeutic Potential of VEGF in the Nervous System. Physiol. Rev. 2009, 89, 607–648. [Google Scholar] [CrossRef]
- Ogunshola, O.O.; Antic, A.; Donoghue, M.J.; Fan, S.-Y.; Kim, H.; Stewart, W.B.; Madri, J.A.; Ment, L.R. Paracrine and Autocrine Functions of Neuronal Vascular Endothelial Growth Factor (VEGF) in the Central Nervous System. J. Biol. Chem. 2002, 277, 11410–11415. [Google Scholar] [CrossRef] [PubMed]
- Zachary, I. Neuroprotective Role of Vascular Endothelial Growth Factor: Signalling Mechanisms, Biological Function, and Therapeutic Potential. Neurosignals 2005, 14, 207–221. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Xu, W.; Luo, C.; Gozal, D.; Liu, R. VEGF-Induced Activation of the PI3-K/Akt Pathway Reduces Mutant SOD1-Mediated Motor Neuron Cell Death. Mol. Brain Res. 2003, 111, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez, C.F.; Andressoo, J.O. Biology of GDNF and Its Receptors—Relevance for Disorders of the Central Nervous System. Neurobiol. Dis. 2017, 97, 80–89. [Google Scholar] [CrossRef]
- Nicole, O.; Ali, C.; Docagne, F.; Plawinski, L.; MacKenzie, E.T.; Vivien, D.; Buisson, A. Neuroprotection Mediated by Glial Cell Line-Derived Neurotrophic Factor: Involvement of a Reduction of NMDA-Induced Calcium Influx by the Mitogen-Activated Protein Kinase Pathway. J. Neurosci. 2001, 21, 3024–3033. [Google Scholar] [CrossRef]
- Cintrón-Colón, A.F.; Almeida-Alves, G.; Boynton, A.M.; Spitsbergen, J.M. GDNF Synthesis, Signaling, and Retrograde Transport in Motor Neurons. Cell Tissue Res. 2020, 382, 47–56. [Google Scholar] [CrossRef]
- Paratcha, G.; Ledda, F.; Ibáñez, C.F. The Neural Cell Adhesion Molecule NCAM Is an Alternative Signaling Receptor for GDNF Family Ligands. Cell 2003, 113, 867–879. [Google Scholar] [CrossRef]
- Crystal, R.G. Adenovirus: The First Effective In Vivo Gene Delivery Vector. Hum. Gene Ther. 2014, 25, 3–11. [Google Scholar] [CrossRef]
- Nasser, M.; Bejjani, F.; Raad, M.; Abou-El-Hassan, H.; Mantash, S.; Nokkari, A.; Ramadan, N.; Kassem, N.; Mondello, S.; Hamade, E.; et al. Traumatic Brain Injury and Blood-Brain Barrier Cross-Talk. CNS Neurol. Disord. Drug Targets 2016, 15, 1030–1044. [Google Scholar] [CrossRef]
- Islamov, R.R.; Rizvanov, A.A.; Fedotova, V.Y.; Izmailov, A.A.; Safiullov, Z.Z.; Garanina, E.E.; Salafutdinov, I.I.; Sokolov, M.E.; Mukhamedyarov, M.A.; Palotás, A. Tandem Delivery of Multiple Therapeutic Genes Using Umbilical Cord Blood Cells Improves Symptomatic Outcomes in ALS. Mol. Neurobiol. 2017, 54, 4756–4763. [Google Scholar] [CrossRef]
- Galea, M.P.; van Zyl, N.; Messina, A. Peripheral Nerve Dysfunction after Spinal Cord Injury. OBM Neurobiol. 2020, 4, 17. [Google Scholar] [CrossRef]
- Messina, A.; van Zyl, N.; Weymouth, M.; Flood, S.; Nunn, A.; Cooper, C.; Hahn, J.; Galea, M.P. Morphology of Donor and Recipient Nerves Utilised in Nerve Transfers to Restore Upper Limb Function in Cervical Spinal Cord Injury. Brain Sci. 2016, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Krakora, D.; Mulcrone, P.; Meyer, M.; Lewis, C.; Bernau, K.; Gowing, G.; Zimprich, C.; Aebischer, P.; Svendsen, C.N.; Suzuki, M. Synergistic Effects of GDNF and VEGF on Lifespan and Disease Progression in a Familial ALS Rat Model. Mol. Ther. 2013, 21, 1602–1610. [Google Scholar] [CrossRef] [PubMed]
- Ismailov, S.M.; Barykova, I.A.; Shmarov, M.M.; Tarantul, V.Z.; Barskov, I.V.; Kucherianu, V.G.; Brylev, L.V.; Logunov, D.I.; Tutykhina, I.L.; Bocharov, E.V.; et al. Experimental Approach to the Gene Therapy of Motor Neuron Disease with the Use of Genes Hypoxia-Inducible Factors. Genetika 2014, 50, 591–601. [Google Scholar] [CrossRef]
- Lisyukov, A.N.; Kuznetsov, M.S.; Saitov, V.R.; Salnikova, M.M.; Bikmullina, I.A.; Koshpaeva, E.S.; Tyapkina, O.V.; Valiullin, V.V.; Islamov, R.R. Morphological Changes in Myelinated Fibers of the Spinal Cord and the Sciatic Nerve in Mice after Modeling of the Hypogravity and the Approach of Their Correction by Preventive Gene Therapy. Genes Cells 2021, 16, 75–80. [Google Scholar] [CrossRef]






| Antibody against: | Host | Dilution |
|---|---|---|
| Choline Acetyltransferase (ChAT) | Rabbit | 1:100 |
| Glial fibrillary acidic protein (GFAP) | Mouse | 1:200 |
| Ionized calcium binding adaptor molecule 1 (Iba1) | Rabbit | 1:150 |
| The K+–Cl− cotransporter isoform 2 (KCC2) | Rabbit | 1:100 |
| Oligodendrocyte transcription factor 2 (Olig2) | Rabbit | 1:100 |
| Postsynaptic density protein 95 kDa (PSD95) | Rabbit | 1:200 |
| Slow Skeletal Myosin Heavy chain | Rabbit | 1:100 |
| Synaptophysin | Rabbit | 1:100 |
| Mouse IgG conjugated with Alexa 488 | Donkey | 1:200 |
| Rabbit IgG conjugated with Alexa 488 | Donkey | 1:200 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garifulin, R.; Davleeva, M.; Izmailov, A.; Fadeev, F.; Markosyan, V.; Shevchenko, R.; Minyazeva, I.; Minekayev, T.; Lavrov, I.; Islamov, R. Evaluation of the Autologous Genetically Enriched Leucoconcentrate on the Lumbar Spinal Cord Morpho-Functional Recovery in a Mini Pig with Thoracic Spine Contusion Injury. Biomedicines 2023, 11, 1331. https://doi.org/10.3390/biomedicines11051331
Garifulin R, Davleeva M, Izmailov A, Fadeev F, Markosyan V, Shevchenko R, Minyazeva I, Minekayev T, Lavrov I, Islamov R. Evaluation of the Autologous Genetically Enriched Leucoconcentrate on the Lumbar Spinal Cord Morpho-Functional Recovery in a Mini Pig with Thoracic Spine Contusion Injury. Biomedicines. 2023; 11(5):1331. https://doi.org/10.3390/biomedicines11051331
Chicago/Turabian StyleGarifulin, Ravil, Maria Davleeva, Andrei Izmailov, Filip Fadeev, Vage Markosyan, Roman Shevchenko, Irina Minyazeva, Tagir Minekayev, Igor Lavrov, and Rustem Islamov. 2023. "Evaluation of the Autologous Genetically Enriched Leucoconcentrate on the Lumbar Spinal Cord Morpho-Functional Recovery in a Mini Pig with Thoracic Spine Contusion Injury" Biomedicines 11, no. 5: 1331. https://doi.org/10.3390/biomedicines11051331
APA StyleGarifulin, R., Davleeva, M., Izmailov, A., Fadeev, F., Markosyan, V., Shevchenko, R., Minyazeva, I., Minekayev, T., Lavrov, I., & Islamov, R. (2023). Evaluation of the Autologous Genetically Enriched Leucoconcentrate on the Lumbar Spinal Cord Morpho-Functional Recovery in a Mini Pig with Thoracic Spine Contusion Injury. Biomedicines, 11(5), 1331. https://doi.org/10.3390/biomedicines11051331