Drug-Loaded Silver Nanoparticles—A Tool for Delivery of a Mebeverine Precursor in Inflammatory Bowel Diseases Treatment
Abstract
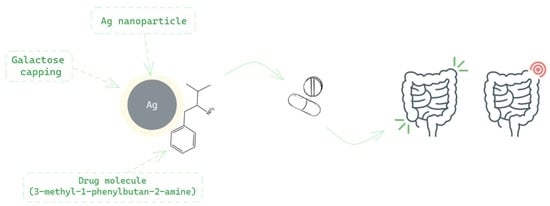
:1. Introduction
2. Materials and Methods
2.1. Synthetic Protocol
2.1.1. Synthesis of MP [65]
2.1.2. Synthesis of Galactose-Assisted Ag NPs
2.1.3. Synthesis of Galactose-Assisted MP-Loaded Ag NPs
2.2. Characterization of the Ag NPs. Analytical Techniques
2.2.1. UV-Vis Spectra
2.2.2. FTIR Spectra
2.2.3. spICP-MS
2.2.4. TEM
2.2.5. DLS and Zeta Potential
2.2.6. X-ray Diffraction (XRD)
2.3. In Vitro Drug Release
3. Results and Discussion
3.1. Characteristics of Galactose-Assisted Ag NPs Compared to Galactose-Assisted Drug-Loaded Ag NPs
3.2. Parametric Drug-Release Optimization
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Talaei, F.; Atyabi, F.; Azhdarzadeh, M.; Dinarvand, R.; Saadatzadeh, A. Overcoming therapeutic obstacles in inflammatory bowel diseases: A comprehensive review on novel drug delivery strategies. Eur. J. Pharm. Sci. 2013, 49, 712–722. [Google Scholar] [CrossRef]
- Friend, D.R. New oral delivery systems for treatment of inflammatory bowel disease. Adv. Drug Deliv. Rev. 2005, 57, 247–265. [Google Scholar] [CrossRef]
- Vong, L.; Mo, J.; Abrahamsson, B.; Nagasaki, Y. Specific accumulation of orally administered redox nanotherapeutics in the inflamed colon reducing inflammation with dose–response efficacy. J. Control. Release 2015, 210, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.; Verma, A.; Kumar Panda, P.; Saraf, S.; Jain, A.; Jain, S. Stimuli-responsive polysaccharides for colon-targeted drug delivery. In Biomaterials, Stimuli Responsive Polymeric Nanocarriers for Drug Delivery Applications; Makhlouf, A.S.H., Abu-Thabit, N.Y., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 547–566. [Google Scholar] [CrossRef]
- Wang, C.-P.; Ji Byun, M.; Kim, S.-N.; Park, W.; Park, H.; Kim, T.-H.; Lee, J.; Park, C. Biomaterials as therapeutic drug carriers for inflammatory bowel disease treatment. J. Control. Release 2022, 345, 1–19. [Google Scholar] [CrossRef]
- Collnot, E.-M.; Ali, H.; Lehr, C.-M. Nano- and microparticulate drug carriers for targeting of the inflamed intestinal mucosa. J. Control. Release 2012, 161, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Nidhi; Rashid, M.; Kaur, V.; Hallan, S.S.; Sharma, S.; Mishra, N. Microparticles as controlled drug delivery carrier for the treatment of ulcerative colitis: A brief review. Saudi Pharm. J. 2016, 24, 458–472. [Google Scholar] [CrossRef] [PubMed]
- Katas, H.; Moden, N.Z.; Lim, C.S.; Celesistinus, T.; Chan, J.Y.; Ganasan, P.; Abdalla, S.S.I. Biosynthesis and potential applications of silver and gold nanoparticles and their chitosan-based nanocomposites in nanomedicine. J. Nanotechnol. 2018, 2018, 4290705. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.; Dufresne, A.; Danquah, M. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050. [Google Scholar] [CrossRef] [PubMed]
- Ordikhani, F.; Erdem Arslan, M.; Marcelo, R.; Sahin, I.; Grigsby, P.; Schwarz, J.K.; Azab, A.K. Drug Delivery Approaches for the Treatment of Cervical Cancer. Pharmaceutics 2016, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Kodiha, M.; Wang, Y.; Hutter, E.; Maysinger, D.; Stochaj, U. Off to the Organelles—Killing Cancer Cells with Targeted Gold Nanoparticles. Theranostics 2015, 5, 357–370. [Google Scholar] [CrossRef]
- Petros, R.; DeSimone, J. Strategies in the design of nanoparticles for therapeutic applications. Nat. Rev. Drug Discov. 2010, 9, 615–627. [Google Scholar] [CrossRef]
- Rai, A.; Prabhune, A.; Perry, C. Antibiotic mediated synthesis of gold nanoparticles with potent antimicrobial activity and their application in antimicrobial coatings. J. Mater. Chem. 2010, 20, 6789. [Google Scholar] [CrossRef]
- Peng, G.; Tisch, U.; Adams, O.; Hakim, M.; Shehada, N.; Broza, Y.; Billan, S.; Abdah-Bortnyak, R.; Kuten, A.; Haick, H. Diagnosing lung cancer in exhaled breath using gold nanoparticles. Nat. Nanotech. 2009, 4, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, M.; Asmalash, T.; Gebrekidan, A.; Gebreegziabiher, D.; Araya, T.; Hilekiros, H.; Barabadi, H.; Ramanathan, K. Nano-Medicine as a Newly Emerging Approach to Combat Human Immunodeficiency Virus (HIV). Pharm. Nanotechnol. 2018, 6, 17. [Google Scholar] [CrossRef] [PubMed]
- Barabadi, H.; Alizadeh, Z.; Rahimi, M.T.; Barac, A.; Maraolo, A.E.; Robertson, L.J.; Masjedi, A.; Shahrivar, F.; Ahmadpour, E. Nanobiotechnology as an Emerging Approach to Combat Malaria: A Systematic Review. Nanomedicine 2019, 18, 221–233. [Google Scholar] [CrossRef]
- Virmani, I.; Sasi, C.; Priyadarshini, E.; Kumar, R.; Kumar Sharma, S.; Pratap Singh, G.; Babu Pachwarya, R.; Paulraj, R.; Barabadi, H.; Saravanan, M.; et al. Comparative Anticancer Potential of Biologically and Chemically Synthesized Gold Nanoparticles. J. Clust. Sci. 2020, 31, 867–876. [Google Scholar] [CrossRef]
- Mostafavi, E.; Zarepour, A.; Barabadi, H.; Zarrabi, A.; Truong, L.; Medina-Cruz, D. Antineoplastic activity of biogenic silver and gold nanoparticles to combat leukemia: Beginning a new era in cancer theragnostic. Biotechnol. Rep. 2022, 34, e00714. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, M.; Vahidi, H.; Cruz, D.M.; Vernet-Crua, A.; Mostafavi, E.; Stelmach, R.; Webster, T.J.; Mahjoub, M.A.; Rashedi, M.; Barabadi, H. Emerging antineoplastic biogenic gold nanomaterials for breast cancer therapeutics: A systematic review. Int. J. Nanomed. 2020, 15, 3577–3595. [Google Scholar] [CrossRef] [PubMed]
- Barabadi, H.; Webster, T.J.; Vahidi, H.; Sabori, H.; Damavandi Kamali, K.; Jazayeri Shoushtari, F.; Mahjoub, M.A.; Rashedi, M.; Mostafavi, E.; Medina Cruz, D.; et al. Green nanotechnology-based gold nanomaterials for hepatic cancer therapeutics: A systematic review. Iran. J. Pharm. Res. 2020, 3, 19. [Google Scholar] [CrossRef]
- Jain, A.; Pawar, P.; Sarkar, A.; Junnuthula, V.; Dyawanapelly, S. Bionanofactories for Green Synthesis of Silver Nanoparticles: Toward Antimicrobial Applications. Int. J. Mol. Sci. 2021, 22, 11993. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Lu, J.; Xu, H.; Patel, A.; Chen, Z.-S.; Chen, G. Silver nanoparticles: Synthesis, properties, and therapeutic applications. Drug Discov. Today 2015, 20, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Khorrami, S.; Abdollahi, Z.; Eshaghi, G.; Khosravi, A.; Bidram, E.; Zarrabi, A. An improved method for fabrication of Ag-GO nanocomposite with controlled anticancer and anti-bacterial behavior; a comparative study. Sci. Rep. 2019, 9, 9167. [Google Scholar] [CrossRef]
- Yadi, M.; Mostafavi, E.; Saleh, B.; Davaran, S.; Aliyeva, I.; Khalilov, R.; Nikzamir, M.; Nikzamir, N.; Akbarzadeh, A.; Panahi, Y.; et al. Current developments in green synthesis of metallic nanoparticles using plant extracts: A review. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. S3), S336–S343. [Google Scholar] [CrossRef]
- Kalantari, K.; Mostafavi, E.; Afifi, A.; Izadiyan, Z.; Jahangirian, H.; Rafiee Moghaddam, R.; Webster, T. Wound dressings functionalized with silver nanoparticles: Promises and pitfalls. Nanoscale 2020, 4, 12. [Google Scholar] [CrossRef]
- Lotfollahzadeh, R.; Yari, M.; Sedaghat, S.; Delbari, A. Biosynthesis and characterization of silver nanoparticles for the removal of amoxicillin from aqueous solutions using Oenothera biennis water extract. J. Nanostructure Chem. 2021, 4, 693–706. [Google Scholar] [CrossRef]
- Abbas, Q.; Saleem, M.; Phull, A.; Rafiq, M.; Hassan, M.; Lee, K.-H.; Seo, S.-Y. Green synthesis of silver nanoparticles using Bidens frondosa extract and their tyrosinase activity. Iran. J. Pharm. Res. 2017, 16, 760. [Google Scholar]
- Karimi, N.; Chardoli, A.; Fattahi, A. Biosynthesis, characterization, antimicrobial and cytotoxic effects of silver nanoparticles using Nigellaarvensis seed extract. Iran. J. Pharm. Res. 2017, 16, 1167. [Google Scholar]
- Amin, Z.; Khashyarmanesh, Z.; Bazzaz, B.; Noghabi, Z. Does biosynthetic silver nanoparticles are more stable with lower toxicity than their synthetic counterparts? Iran. J. Pharm. Res. 2019, 18, 210–221. [Google Scholar]
- Salari, S.; Bahabadi, S.; Samzadeh-Kermani, A.; Yosefzaei, F. In-vitro evaluation of antioxidant and antibacterial potential of greensynthesized silver nanoparticles using prosopis farcta fruit extract. Iran. J. Pharm. Res. 2019, 18, 430–455. [Google Scholar] [PubMed]
- Yaqoob, A.A.; Ahmad, H.; Parveen, T.; Ahmad, A.; Oves, M.; Ismail, I.M.I.; Qari, H.A.; Umar, K.; Ibrahim, M.N.M. Recent advances in metal decorated nanomaterials and their various biological applications: A review. Front. Chem. 2020, 8, 341. [Google Scholar] [CrossRef]
- Barabadi, H.; Kobarfard, F.; Vahidi, H. Biosynthesis and Characterization of Biogenic Tellurium Nanoparticles by Using Penicillium chrysogenum PTCC 5031: A Novel Approach in Gold Biotechnology. Iran. J. Pharm. Res. 2018, 17, 87–97. [Google Scholar]
- Barabadi, H.; Honary, S.; Ebrahimi, P.; Alizadeh, A.; Naghibi, F.; Saravanan, M. Optimization of myco-synthesized silver nanoparticles by response surface methodology employing Box-Behnken design. Inorg. Nano Met. Chem. 2019, 49, 33–43. [Google Scholar] [CrossRef]
- De Matteis, V.; Rizzello, L.; Cascione, M.; Liatsi-Douvitsa, E.; Apriceno, A.; Rinaldi, R. Green Plasmonic Nanoparticles and Bio-Inspired Stimuli-Responsive Vesicles in Cancer Therapy Application. Nanomaterials 2020, 10, 1083. [Google Scholar] [CrossRef] [PubMed]
- Chairam, S.; Poolperm, C.; Somsook, E. Starch vermicelli template-assisted synthesis of size/shape-controlled nanoparticles. Carbohydr. Polym. 2009, 75, 694–704. [Google Scholar] [CrossRef]
- Kassaee, M.Z.; Akhavan, A.; Sheikh, N.; Beteshobabrud, R. γ-Ray synthesis of starch-stabilized silver nanoparticles with antibacterial activities. Radiat. Phys. Chem. 2008, 77, 1074–1078. [Google Scholar] [CrossRef]
- Vigneshwaran, N.; Nachane, R.P.; Balasubramanya, R.H.; Varadarajan, P.V. A novel one-pot green synthesis of stable silver nanoparticles using soluble starch. Carbohydr. Res. 2006, 341, 2012–2018. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.; Rai, A.; Ahmad, A.; Sastry, M. Rapid synthesis of Au, Ag, and bimetallic Au core-Ag shell nanoparticles using Neem (Azadirachta indica) leaf broth. J. Colloid Interface Sci. 2004, 275, 496–502. [Google Scholar] [CrossRef]
- Philip, D. Biosynthesis of Au, Ag and Au-Ag nanoparticles using edible mushroom extract. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2009, 73, 374–381. [Google Scholar] [CrossRef]
- Sharma, V.K.; Yngard, R.A.; Lin, Y. Silver nanoparticles: Green synthesis and their antimicrobial activities. Adv. Colloid Interface Sci. 2009, 145, 83–96. [Google Scholar] [CrossRef]
- Panacek, A.; Kvítek, L.; Prucek, R.; Kolar, M.; Vecerova, R.; Pizúrova, N.; Sharma, V.; Nevecna, T.; Zboril, R. Silver colloid nanoparticles: Synthesis, characterization, and their antibacterial activity. J Phys. Chem. B 2006, 110, 16248–16253. [Google Scholar] [CrossRef]
- Sabbagh, F.; Kiarostami, K.; Khatir, N.; Rezania, S.; Muhamad, I.; Hosseini, F. Effect of zinc content on structural, functional, morphological, and thermal properties of kappa-carrageenan/NaCMC nanocomposites. Polym. Test. 2021, 93, 106922. [Google Scholar] [CrossRef]
- Kamble, S.; Bhosale, K.; Mohite, M.; Navale, S. Methods of Preparation of Nanoparticles. Int. Adv. Res. Sci. Commun. Technol. 2022, 2, 2581–9429. [Google Scholar] [CrossRef]
- Filippo, E.; Manno, D.; Serra, A. Self assembly and branching of sucrose stabilized silver nanoparticles by microwave assisted synthesis: From nanoparticles to branched nanowires structures. Colloids Surf. A Physicochem. Eng. Asp. 2009, 348, 205–211. [Google Scholar] [CrossRef]
- Filippo, E.; Serra, A.; Buccolieri, A.; Manno, D. Green synthesis of silver nanoparticles with sucrose and maltose: Morphological and structural characterization. J. Non-Cryst. Solids 2010, 356, 344–350. [Google Scholar] [CrossRef]
- Shervani, Z.; Yamamoto, Y. Carbohydrate-directed synthesis of silver and gold nanoparticles: Effect of the structure of carbohydrates and reducing agents on the size and morphology of the composites. Carbohydr. Res. 2011, 346, 651–658. [Google Scholar] [CrossRef]
- Ghiyasiyan-Arani, M.; Salavati-Niasari, M.; Masjedi-Arani, M.; Mazloom, F. An Easy Sonochemical Route for Synthesis, Characterization and Photocatalytic Performance of Nanosized FeVO 4 in the Presence of Aminoacids as green Capping Agents. J. Mater. Sci. Mater. Elect. 2018, 29, 474–485. [Google Scholar] [CrossRef]
- Caschera, D.; Toro, R.G.; Federici, F.; Montanari, R.; de Caro, T.; Al-Shemy, M.T.; Adel, A.M. Green Approach for the Fabrication of Silver-Oxidized Cellulose Nano-composite with Antibacterial Properties. Cellulose 2020, 27, 8059–8073. [Google Scholar] [CrossRef]
- Garza-Cervantes, J.A.; Mendiola-Garza, G.; de Melo, E.M.; Dugmore, T.I.; Matharu, A.S.; Morones-Ramirez, J.R. Antimicrobial Activity of a Silver-Microfibrillated Cellulose Biocomposite against Susceptible and Resistant Bacteria. Sci. Rep. 2020, 10, 7281. [Google Scholar] [CrossRef]
- Rather, R.A.; Sarwara, R.K.; Das, N.; Pal, B. Impact of Reducing and Capping Agents on Carbohydrates for the Growth of Ag and Cu Nanostructures and Their Antibacterial Activities. Particuology 2019, 43, 219–226. [Google Scholar] [CrossRef]
- Shanmuganathan, R.; Edison, T.N.J.I.; LewisOscar, F.; Kumar, P.; Shanmugam, S.; Pugazhendhi, A. Chitosan Nanopolymers: An Overview of Drug Delivery against Cancer. Int. J. Biol. Macromol. 2019, 130, 727–736. [Google Scholar] [CrossRef]
- Maiti, P.K.; Ghosh, A.; Parveen, R.; Saha, A.; Choudhury, M.G. Preparation of Carboxy-Methyl Cellulose-Capped Nanosilver Particles and Their Antimicrobial Evaluation by an Automated Device. Appl. Nanosci. 2019, 9, 105–111. [Google Scholar] [CrossRef]
- Javed, R.; Zia, M.; Naz, S.; Aisida, S.O.; Ain, N.U.; Ao, Q. Role of Capping Agents in the Application of Nanoparticles in Biomedicine and Environmental Remediation: Recent Trends and Future Prospects. J. Nanobiotechnology 2020, 18, 172. [Google Scholar] [CrossRef]
- Kumar, A.; Das, N.; Satija, N.K.; Mandrah, K.; Roy, S.K.; Rayavarapu, R.G. A Novel Approach towards Synthesis and Characterization of Non-cytotoxic Gold Nanoparticles Using Taurine as Capping Agent. Nanomaterials 2020, 10, 45. [Google Scholar] [CrossRef]
- Priya, N.; Kaur, K.; Sidhu, A.K. Green Synthesis: An Eco-Friendly Route for the Synthesis of Iron Oxide Nanoparticles. Front. Nanotechnol. 2021, 3, 47. [Google Scholar] [CrossRef]
- Duan, H.; Wang, D.; Li, Y. Green chemistry for nanoparticle synthesis. Chem. Soc. Rev. 2015, 44, 5778–5792. [Google Scholar] [CrossRef]
- Zeng, Q.; Shao, D.; Ji, W.; Li, J.; Chen, L.; Song, J.T. The nanotoxicity investigation of optical nanoparticles to cultured cells in vitro. Toxicol. Rep. 2014, 2, 137–144. [Google Scholar] [CrossRef]
- Maribel Guzman, M.A.; Dille, J.; Godet, S.; Rousse, C. Effect of the Concentration of NaBH4 and N2H4 as Reductant Agent on the Synthesis of Copper Oxide Nanoparticles and its Potential Antimicrobial Applications. Nano Biomed. Eng. 2018, 10, 392–405. [Google Scholar] [CrossRef]
- Ranoszek-Soliwoda, K.; Tomaszewska, E.; Socha, E.; Krzyczmonik, P.; Ignaczak, A.; Orlowski, P.; Krzyzowska, M.; Celichowski, G.; Grobelny, J. The role of tannic acid and sodium citrate in the synthesis of silver nanoparticles. J. Nanopart Res. 2017, 19, 273. [Google Scholar] [CrossRef]
- Buchman, A.L. Side effects of corticosteroid therapy. J. Clin. Gastroenterol. 2001, 33, 289–294. [Google Scholar] [CrossRef]
- Ransford, R.; Langman, M. Sulphasalazine and mesalazine: Serious adverse reactions re-evaluated on the basis of suspected adverse reaction reports to the Committee on Safety of Medicines. Gut 2002, 51, 536–539. [Google Scholar] [CrossRef]
- Rutgeerts, P.; Sandborn, W.J.; Feagan, B.G.; Reinisch, W.; Olson, A.; Johanns, J.; Travers, S.; Rachmilewitz, D.; Hanauer, S.B.; Lichtenstein, G.R.; et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 2005, 353, 2462–2476. [Google Scholar] [CrossRef]
- Papadakis, K.A.; Shaye, O.A.; Vasiliauskas, E.A.; Ippoliti, A.; Dubinsky, M.C.; Birt, J.; Paavola, J.; Lee, S.K.; Price, J.; Targan, S.R.; et al. Safety and efficacy of adalimumab (D2E7) in Crohn’s disease patients with an attenuated response to infliximab. Am. J. Gastroenterol. 2005, 100, 75–79. [Google Scholar] [CrossRef]
- Sowmya, C.; Reddy, C.S.; Priya, N.V.; Sandhya, R.; Keerthi, K. Colon specific drug delivery systems: A review on pharmaceutical approaches with current trends. Int. Res. J. Pharm. 2012, 3, 45–55. [Google Scholar]
- Milusheva, M.; Gledacheva, V.; Stefanova, I.; Pencheva, M.; Mihaylova, R.; Tumbarski, Y.; Nedialkov, P.; Cherneva, E.; Todorova, M.; Nikolova, S. In Silico, In Vitro, and Ex Vivo Biological Activity of Some Novel Mebeverine Precursors. Biomedicines 2023, 11, 605. [Google Scholar] [CrossRef] [PubMed]
- Pace, H.; Rogers, N.; Jarolimek, C.; Coleman, V.; Higgins, C.; Ranville, J. Determining Transport Efficiency for the Purpose of Counting and Sizing Nanoparticles via Single Particle Inductively Coupled Plasma Mass Spectrometry. Anal. Chem. 2011, 83, 9361–9369. [Google Scholar] [CrossRef]
- Sukirtha, R.; Priyanka, K.; Antony, J.; Kamalakkannan, S.; Thangam, R.; Gunasekaran, P.; Krishnan, M.; Achiraman, S. Cytotoxic effect of green synthesized silver nanoparticles using melia azedarach against in vitro hela cell lines and lymphoma mice model. Process Biochem. 2012, 47, 273–279. [Google Scholar] [CrossRef]
- Yesilot, S.; Aydin, C. Silver nanoparticles: A new hope in cancer therapy? Eastern J. Med. 2019, 24, 111–116. [Google Scholar] [CrossRef]
- Khorrami, S.; Zarepour, A.; Zarrabi, A. Green synthesis of silver nanoparticles at low temperature in a fast pace with unique DPPH radical scavenging and selective cytotoxicity against MCF-7 and BT-20 tumor cell lines. Biotechnol. Rep. 2019, 24, e00393. [Google Scholar] [CrossRef]
- Séguy, L.; Groo, A.-C.; Malzert-Fréon, A. How nano-engineered delivery systems can help marketed and repurposed drugs in Alzheimer’s disease treatment? Drug Discov. Today 2022, 27, 1575–1589. [Google Scholar] [CrossRef]
- Muntimadugu, E.; Dhommati, R.; Jain, A.; Challa, V.G.S.; Shaheen, M.; Khan, W. Intranasal delivery of nanoparticle encapsulated tarenflurbil: A potential brain targeting strategy for Alzheimer’s disease. Eur. J. Pharm. Sci. 2016, 92, 224–234. [Google Scholar] [CrossRef]
- Jojo, G.; Kuppusamy, G. Scope of new formulation approaches in the repurposing of pioglitazone for the management of Alzheimer’s disease. J. Clin. Pharm. Ther. 2019, 44, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Jojo, G.; Kuppusamy, G.; De, A.; Karri, V.V.S.N.R. Formulation and optimization of intranasal nanolipid carriers of pioglitazone for the repurposing in Alzheimer’s disease using Box-Behnken design. Drug. Dev. Ind. Pharm. 2019, 45, 1061–1072. [Google Scholar] [CrossRef] [PubMed]
- Angelopoulou, E.; Piperi, C. DPP-4 inhibitors: A promising therapeutic approach against Alzheimer’s disease. Ann. Transl. Med. 2018, 6, 255. [Google Scholar] [CrossRef]
- Ibrahim, A.; Abbas, I.; Hussein, M.; Muter, S.; Abdulalah, M.; Morteta, A.; Suhayla, S.; Peshawa, A.; Nabaz, A.; Mahmood, A. Effect of nano silver on gastroprotective activity against ethanol-induced stomach ulcer in rats. Biomed. Pharmacother. 2022, 154, 113550. [Google Scholar] [CrossRef]
- Kennedy, D.; Orts-Gil, G.; Lai, C.-H.; Müller, L.; Haase, A.; Luch, A.; Seeberger, P. Carbohydrate functionalization of silver nanoparticles modulates cytotoxicity and cellular uptake. J. Nanobiotechnol. 2014, 12, 59. [Google Scholar] [CrossRef]
- Pryshchepa, O.; Pomastowski, P.; Buszewski, B. Silver nanoparticles: Synthesis, investigation techniques, and properties. Adv. Colloid Interface Sci. 2020, 284, 102246. [Google Scholar] [CrossRef]
- Xavier, J.; Vincent, S.; Meder, F.; Vollmer, F. Advances in Optoplasmonic Sensors—Combining Optical Nano/Microcavities and Photonic Crystals with Plasmonic Nanostructures and Nanoparticles. Nanophotonics 2018, 7, 1–38. [Google Scholar] [CrossRef]
- Martinsson, E.; Otte, M.A.; Shahjamali, M.M.; Sepulveda, B.; Aili, D. Substrate Effect on the Refractive Index Sensitivity of Silver Nanoparticles. J. Phys. Chem. C 2014, 118, 24680–24687. [Google Scholar] [CrossRef]
- Arcas, A.S.; Jaramillo, L.; Costa, N.S.; Allil, R.C.S.B.; Werneck, M.M. Localized Surface Plasmon Resonance-Based Biosensor on Gold Nanoparticles for Taenia Solium Detection. Appl. Opt. 2021, 60, 8137–8144. [Google Scholar] [CrossRef]
- Raiche-Marcoux, G.; Loiseau, A.; Maranda, C.; Poliquin, A.; Boisselier, E. Parametric Drug Release Optimization of Anti-Inflammatory Drugs by Gold Nanoparticles for Topically Applied Ocular Therapy. Int. J. Mol. Sci. 2022, 23, 16191. [Google Scholar] [CrossRef]
- Zaheer, Z. Biogenic synthesis, optical, catalytic, and in vitro antimicrobial potential of Ag-nanoparticles prepared using Palm date fruit extract. J. Photochem. Photobiol. B Biol. 2018, 178, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Zhou, J.J.; Li, R.; Gao, Y.; Sun, T.L.; Zhao, X.W.; Xiang, Y.J.; Xie, S.S. PVP-capped twinned gold plates from nanometer to micrometer. J. Nanoparticle Res. 2006, 8, 927. [Google Scholar] [CrossRef]
- Singh, S.; Bharti, A.; Meena, V.K. Green synthesis of multi-shaped silver nanoparticles: Optical, morphological and antibacterial properties. J. Mater. Sci Mater. Electron. 2015, 26, 3638–3648. [Google Scholar] [CrossRef]
- Huang, H.; Yang, X. Synthesis of polysaccharide-stabilized gold and silver nanoparticles: A green method. Carbohydr. Res. 2004, 339, 2627–2631. [Google Scholar] [CrossRef] [PubMed]
- Wells, H.; Atalla, R. An investigation of the vibrational spectra of glucose, galactose and mannose. J. Mol. Struct. 1990, 224, 385–424. [Google Scholar] [CrossRef]
- Sivakesava, S.; Irudayaraj, J. Prediction of inverted cane sugar adulteration of honey by Fourier transform infrared spectroscopy. J. Food Sci. 2001, 66, 972–978. [Google Scholar] [CrossRef]
- David, M.; Hategan, A.; Berghian-Grosan, C.; Magdas, D. The Development of Honey Recognition Models Based on the Association between ATR-IR Spectroscopy and Advanced Statistical Tools. Int. J. Mol. Sci. 2022, 23, 9977. [Google Scholar] [CrossRef]
- AbuDalo, M.; Al-Mheidat, I.; Al-Shurafat, A.; Grinham, C.; Oyanedel-Craver, V. Synthesis of silver nanoparticles using a modified Tollens’ method in conjunction with phytochemicals and assessment of their antimicrobial activity. Peer J. 2019, 7, e6413. [Google Scholar] [CrossRef]
- Ahmed, K.B.A.; Mohammed, A.S.; Anbazhagan, V. Interaction of sugar stabilized silver nanoparticles with the T-antigen specific lectin, jacalin from Artocarpus Integrifolia. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 145, 110–116. [Google Scholar] [CrossRef]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.; Zolfaghari, B. Synthesis of silver nanoparticles: Chemical, physical and biological methods. Res. Pharm. Sci. 2014, 9, 385–406. [Google Scholar]
- Islam, N. Green synthesis and biological activities of gold nanoparticles functionalized with Salix Alba Arab. J. Chem. 2019, 12, 2914–2925. [Google Scholar] [CrossRef]
- Tripathi, D. Nanomaterials in Plants, Algae, and Microorganisms: Concepts and Controversies; Academic Press: Cambridge, MA, USA, 2018; Volume 1. [Google Scholar]
- Younas, M.; Ahmad, M.A.; Jannat, F.T.; Ashfaq, T.; Ahmad, A. 18—Role of silver nanoparticles in multifunctional drug delivery. In Micro and Nano Technologies, Nanomedicine Manufacturing and Applications; Verpoort, F., Ahmad, I., Ahmad, A., Khan, A., Chee, C.Y., Eds.; Elsevier: Orlando, FL, USA, 2021; pp. 297–319. [Google Scholar] [CrossRef]
- Ivask, A. Toxicity mechanisms in Escherichia coli vary for silver nanoparticles and differ from ionic silver. ACS Nano 2014, 8, 374–386. [Google Scholar] [CrossRef]
- Stoehr, L.; Gonzalez, E.; Stampfl, A.; Casals, E.; Duschl, A.; Puntes, V.; Oostingh, G. Shape matters: Effects of silver nanospheres and wires on human alveolar epithelial cells. Part Fibre Toxicol. 2011, 8, 36. [Google Scholar] [CrossRef]
- Huk, A.; Izak-Nau, E.; Reidy, B.; Boyles, M.; Duschl, A.; Lynch, I.; Dušinska, M. Is the toxic potential of nanosilver dependent on its size? Part Fibre Toxicol. 2014, 11, 65. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wang, L.; Chen, Q.; Chen, C. Cytotoxic potential of silver nanoparticles. Yonsei Med. J. 2014, 55, 283–291. [Google Scholar] [CrossRef]
- León-Silva, S.; Fernández-Luqueño, F.; López-Valdez, F. Silver Nanoparticles (AgNP) in the Environment: A Review of Potential Risks on Human and Environmental Health. Water Air Soil Pollut. 2016, 227, 306. [Google Scholar] [CrossRef]
- Mulvaney, P.; Liz-Marzan, L.; Giersig, M.; Ung, T.J. Silica encapsulation of quantum dots and metal clusters. Mater. Chem. 2000, 10, 1259. [Google Scholar] [CrossRef]
- Prakash, P.; Gnanaprakasam, P.; Emmanuel, R.; Arokiyaraj, M.; Saravanan, M. Green synthesis of silver nanoparticles from leaf extract of Mimusops elengi, Linn for enhanced antibacterial activity against multi drug resistant clinical isolates. Colloids Surf. B Biointerfaces 2013, 108, 255–259. [Google Scholar] [CrossRef]
- Solomon, D.; Gupta, N.; Mulla, N.S.; Shukla, S.; Guerrero, Y.A.; Gupta, V. Role of In Vitro Release Methods in Liposomal Formulation Development: Challenges and Regulatory Perspective. AAPSJ 2017, 19, 1669–1681. [Google Scholar] [CrossRef]
- Yu, M.; Yuan, W.; Li, D.; Schwendeman, A.; Schwendeman, S.P. Predicting Drug Release Kinetics from Nanocarriers inside Dialysis Bags. J. Control. Release 2019, 315, 23–30. [Google Scholar] [CrossRef]
- Kumar, B.; Jalodia, K.; Kumar, P.; Gautam, H.K. Recent Advances in Nanoparticle-Mediated Drug Delivery. J. Drug Deliv. Sci. Technol. 2017, 41, 260–268. [Google Scholar] [CrossRef]
- Shetab Boushehri, M.A.; Lamprecht, A. Nanoparticles as Drug Carriers: Current Issues with in Vitro Testing. Nanomedicine 2015, 10, 3213–3230. [Google Scholar] [CrossRef] [PubMed]
- Chambin, O.; Dupuis, G.; Champion, D.; Voilley, A.; Pourcelot, Y. Colonspecific drug delivery: Influence of solution reticulation properties upon pectin beads performance. Int. J. Pharm. 2006, 321, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Zhang, M.; Liu, K. Colon-targeted drug delivery of polysaccharide-based nanocarriers for synergistic treatment of inflammatory bowel disease: A review. Carbohydr. Polym. 2021, 272, 118530. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Todorova, M.; Milusheva, M.; Kaynarova, L.; Georgieva, D.; Delchev, V.; Simeonova, S.; Pilicheva, B.; Nikolova, S. Drug-Loaded Silver Nanoparticles—A Tool for Delivery of a Mebeverine Precursor in Inflammatory Bowel Diseases Treatment. Biomedicines 2023, 11, 1593. https://doi.org/10.3390/biomedicines11061593
Todorova M, Milusheva M, Kaynarova L, Georgieva D, Delchev V, Simeonova S, Pilicheva B, Nikolova S. Drug-Loaded Silver Nanoparticles—A Tool for Delivery of a Mebeverine Precursor in Inflammatory Bowel Diseases Treatment. Biomedicines. 2023; 11(6):1593. https://doi.org/10.3390/biomedicines11061593
Chicago/Turabian StyleTodorova, Mina, Miglena Milusheva, Lidia Kaynarova, Deyana Georgieva, Vassil Delchev, Stanislava Simeonova, Bissera Pilicheva, and Stoyanka Nikolova. 2023. "Drug-Loaded Silver Nanoparticles—A Tool for Delivery of a Mebeverine Precursor in Inflammatory Bowel Diseases Treatment" Biomedicines 11, no. 6: 1593. https://doi.org/10.3390/biomedicines11061593
APA StyleTodorova, M., Milusheva, M., Kaynarova, L., Georgieva, D., Delchev, V., Simeonova, S., Pilicheva, B., & Nikolova, S. (2023). Drug-Loaded Silver Nanoparticles—A Tool for Delivery of a Mebeverine Precursor in Inflammatory Bowel Diseases Treatment. Biomedicines, 11(6), 1593. https://doi.org/10.3390/biomedicines11061593