Reshaping the Concept of Riedel’s Thyroiditis into the Larger Frame of IgG4-Related Disease (Spectrum of IgG4-Related Thyroid Disease)
Abstract
:1. Introduction
1.1. Riedel’s Thyroiditis: Classical Approach
1.2. Immunoglobulin G4-Related Disease
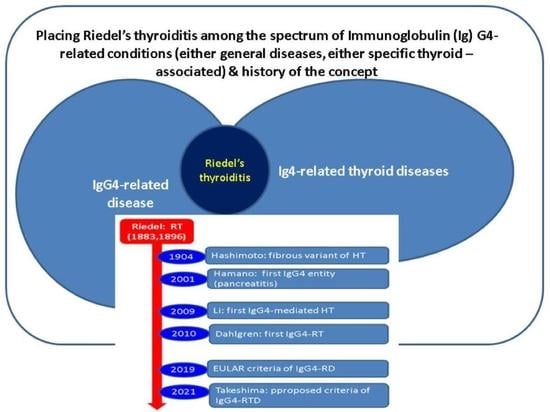
1.3. Aim
2. Materials and Methods
3. Results
3.1. Riedel’s Thyroiditis: Presentation
3.2. Riedel’s Thyroiditis: Management and Outcome
3.3. Patients with IgG4-Related Disease and Potential Thyroid Findings
4. Discussion
4.1. Practical Points on Riedel’s Thyroiditis
4.2. Non-RT Entities among the Spectrum of IgG4-Related Thyroid Disease
4.3. IgG4-RD: The Level of Thyroid Awareness
4.4. New Roads for Riedel’s Thyroiditis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACR | American College of Rheumatology |
| CRP | C-reactive protein |
| ESR | Erythrocyte sedimentation rate |
| EULAR | European League Against Rheumatism |
| HPF | High-power field |
| Ig | Immunoglobulin |
| IgG4-RD | Immunoglobulin IG4-related disease |
| IgG4-RTD | IgG4-related thyroid disease |
| RT | Riedel’s thyroiditis |
| TGF-β | Transforming growth factor beta |
| Tc | Technetium |
References
- Takeshima, K.; Li, Y.; Kakudo, K.; Hirokawa, M.; Nishihara, E.; Shimatsu, A.; Takahashi, Y.; Akamizu, T. Proposal of diagnostic criteria for IgG4-related thyroid disease. Endocr. J. 2021, 68, 1–6. [Google Scholar] [CrossRef]
- Zala, A.; Berhane, T.; Juhlin, C.C.; Calissendorff, J.; Falhammar, H. Riedel thyroiditis. J. Clin. Endocrinol. Metab. 2020, 105, dgaa468. [Google Scholar] [CrossRef]
- Inaba, H.; Ariyasu, H.; Takeshima, K.; Iwakura, H.; Akamizu, T. Comprehensive research on thyroid diseases associated with autoimmunity: Autoimmune thyroid diseases, thyroid diseases during immune-checkpoint inhibitors therapy, and immunoglobulin-G4-associated thyroid diseases. Endocr. J. 2019, 66, 843–852. [Google Scholar] [CrossRef] [Green Version]
- Hennessey, J.V. Riedel’s thyroiditis: A clinical review. J. Clin. Endocrinol. Metab. 2011, 96, 3031–3041. [Google Scholar] [CrossRef] [Green Version]
- Fatourechi, M.M.; Hay, I.D.; McIver, B.; Sebo, T.J.; Fatourechi, V. Invasive fibrous thyroiditis (Riedel’s thyroiditis): The Mayo Clinic Experience 1976–2008. Thyroid 2011, 21, 765–772. [Google Scholar] [CrossRef]
- Pandev, R.; Khan, M.; Ratheesh, V. Riedel’s Thyroiditis: Pitfalls in Diagnosis and Subsequent Complications. Case Rep. Endocrinol. 2023, 2023, 9989953. [Google Scholar] [CrossRef]
- Chong Xi, R.; Hong Qiao, W.; Yan, L. Severe trachea compression caused by Riedel’s thyroiditis: A case report and review of the literature. Ann. Med. Surg. 2016, 12, 18–20. [Google Scholar] [CrossRef]
- Slman, R.; Monpeyssen, H.; Desarnaud, S.; Haroche, J.; Du Pasquier Fediaevsky, L.; Fabrice, M.; Seret-Begue, D.; Aurengo, A.; Leenhardt, L. Ultrasound, Elastography, and Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography Imaging in Riedel’s Thyroiditis: Report of Two Cases. Thyroid 2011, 21, 799–804. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Hou, G.; Cheng, W. Isolated involvement of thyroid gland by IgG4-related disease revealed by 18F-FDG PET/CT. Eur. J. Nucl. Med. Imaging 2020, 47, 736–737. [Google Scholar] [CrossRef] [PubMed]
- Arowolo, O.A.; Ige, F.S.; Odujoko, O.; Agbakwuru, E.A. Riedel’s thyroiditis in a black African: A case report and review of literature. Niger. J. Clin. Pract. 2016, 19, 549–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Funada, M.; Nakano, K.; Miyata, H.; Nawata, A.; Tanaka, Y. IgG4-type Multiple Myeloma with Diffuse Enlargement of the Thyroid Requiring Differentiation from IgG4-related Disease. Intern. Med. 2020, 59, 711–714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.; Nam, Y.; Rim, Y.A.; Ju, J.H. Anti-fibrotic effect of a selective estrogen receptor modulator in systemic sclerosis. Stem. Cell Res. Ther. 2022, 13, 303. [Google Scholar] [CrossRef]
- Best, K.T.; Studentsova, V.; Ackerman, J.E.; Nichols, A.E.C.; Myers, M.; Cobb, J.; Knapp, E.; Awad, H.A.; Loiselle, A.E. Effects of tamoxifen on tendon homeostasis and healing: Considerations for the use of tamoxifen-inducible mouse models. J. Orthop. Res. 2021, 39, 1572–1580. [Google Scholar] [CrossRef] [PubMed]
- Levy, J.M.; Hasney, C.P.; Friedlander, P.L.; Kandil, E.; Occhipinti, E.A.; Kahn, M.J. Combined mycophenolate mofetil and prednisone therapy in tamoxifen- and prednisone-resistant Riedel’s thyroiditis. Thyroid 2010, 20, 105–107. [Google Scholar] [CrossRef] [PubMed]
- Lawless, A.; Papachristos, A.; Robinson, B.; Sidhu, S.; Eade, T. Refractory Riedel’s thyroiditis managed with low dose radiotherapy. Rep. Pract. Oncol. Radiother. 2022, 27, 591–592. [Google Scholar] [CrossRef]
- Stefanou, C.K.; Papathanakos, G.; Stefanou, S.K.; Tepelenis, K.; Kitsouli, A.; Barbouti, A.; Tsoumanis, P.; Kanavaros, P.; Kitsoulis, P. Surgical tips and techniques to avoid complications of thyroid surgery. Innov. Surg. Sci. 2022, 7, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Cannizzaro, M.A.; Lo Bianco, S.; Picardo, M.C.; Provenzano, D.; Buffone, A. How to avoid and to manage post-operative complications in thyroid surgery. Updates Surg. 2017, 69, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Cernea, C.R.; Brandão, L.G.; Hojaij, F.C.; De Carlucci, D.; Montenegro, F.L.; Plopper, C.; Vanderlei, F.; Gotoda, R.; Dias, F.L.; Lima, R.A. How to minimize complications in thyroid surgery? Auris Nasus Larynx 2010, 37, 1–5. [Google Scholar] [CrossRef]
- Young, K.S.; Cunniffe, H.A.; Ali, Z.; Nassif, R. Classical Hodgkin’s lymphoma masquerading as Riedel’s thyroiditis. BMJ Case Rep. 2022, 15, e247097. [Google Scholar] [CrossRef]
- Danish, M.H.; Wasif, M.; Ud Din, N.; Awan, M.S. Malignant peripheral nerve sheath tumour of thyroid: A diagnostic dilemma. BMJ Case Rep. 2020, 13, e234374. [Google Scholar] [CrossRef]
- Rotondi, M.; Carbone, A.; Coperchini, F.; Fonte, R.; Chiovato, L. Diagnosis of endocrine disease: IgG4-related thyroid autoimmune disease. Eur. J. Endocrinol. 2019, 180, R175–R183. [Google Scholar] [CrossRef] [Green Version]
- Osuorji, C.; Master, K.; Osuorji, I. IgG4-Related Disease With Renal and Pulmonary Involvement. Cureus 2021, 13, e17071. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.M.; Shi, Z.S.; Ma, C.L. Multifocal autoimmune pancreatitis: A retrospective study in a single tertiary center of 26 patients with a 20-year literature review. World J. Gastroenterol. 2021, 27, 4429–4440. [Google Scholar] [CrossRef] [PubMed]
- Mabood Khalil, M.A.; Rajput, A.S.; Ghani, R.; Rahmat Ullah, S.M.; Thet, M.K.; Daiwajna, R.G.; Telisinghe, P.U.; Chong, V.H.; Tan, J. Isolated Renal Involvement by IG4-Related Disorder Mimicking Multiple Myeloma, a Diagnosis Not to Miss. Saudi J. Kidney Dis. Transpl. 2021, 32, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Breville, G.; Zamberg, I.; Sadallah, S.; Stephan, C.; Ponte, B.; Seebach, J.D. Case Report: Severe Complement-Mediated Thrombotic Microangiopathy in IgG4-Related Disease Secondary to Anti-Factor H IgG4 Autoantibodies. Front. Immunol. 2021, 11, 604759. [Google Scholar] [CrossRef]
- Yadav, A.; Godasu, G.; Buxi, T.B.S. Sheth S Multiple Artery Aneurysms: Unusual Presentation of IgG4 Vasculopathy. J. Clin. Imaging Sci. 2021, 11, 17. [Google Scholar] [CrossRef] [PubMed]
- Adam, Z.; Adamová, Z.; Řehák, Z.; Koukalová, R. IgG4-releated disease. Klin. Onkol. 2021, 34, 92–102. [Google Scholar] [CrossRef]
- Nasrullah, A.; Javed, A.; Alvi, Z.; Raja, A.; Ashraf, O.; Malik, K.; Balaan, M. IgG4 related lung disease- a rare and novel mimic of malignancy and infections-a case series of three patients with a brief review of updated literature. Respir. Med. Case Rep. 2021, 33, 101452. [Google Scholar] [CrossRef]
- Mahajan, M.S.; Maitra, S.; Singh, N.; Pereira, M. IgG4-Related disease simulating paraneoplastic syndrome: Role of 18FDG PET/CT imaging. Indian J. Radiol. Imaging 2017, 27, 249–253. [Google Scholar] [CrossRef]
- Hamano, H.; Kawa, S.; Horiuchi, A.; Unno, H.; Furuya, N.; Akamatsu, T.; Fukushima, M.; Nikaido, T.; Nakayama, K.; Usuda, N.; et al. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N. Engl. J. Med. 2001, 344, 732–738. [Google Scholar] [CrossRef]
- Kamisawa, T.; Funata, N.; Hayashi, Y.; Eishi, Y.; Koike, M.; Tsuruta, K.; Okamoto, A.; Egawa, N.; Nakajima, H. A new clinicopathological entity of IgG4-related autoimmune disease. J. Gastroenterol. 2003, 38, 982–984. [Google Scholar] [CrossRef]
- Wallace, Z.S.; Naden, R.P.; Chari, S.; Choi, H.K.; Della-Torre, E.; Dicaire, J.F.; Hart, P.A.; Inoue, D.; Kawano, M.; Khosroshahi, A.; et al. The 2019 American College of Rheumatology/European League Against Rheumatism classification criteria for IgG4-related disease. Ann. Rheum. Dis. 2020, 79, 77–87. [Google Scholar] [CrossRef]
- Baker, M.C.; Cook, C.; Fu, X.; Perugino, C.A.; Stone, J.H.; Wallace, Z.S. The Positive Predictive Value of a Very High Serum IgG4 Concentration for the Diagnosis of IgG4-Related Disease. J Rheumatol. 2023, 50, 408–412. [Google Scholar] [CrossRef]
- Della-Torre, E.; Lanzillotta, M.; Germanò, T.; Mancuso, G.; Ramirez, G.A.; Capurso, G.; Falconi, M.; Dagna, L. Utility of the “2019 ACR/EULAR classification criteria” for the management of patients with IgG4-related disease. Semin. Arthritis Rheum. 2021, 51, 761–765. [Google Scholar] [CrossRef]
- Nakamura, T.; Goryo, Y.; Isojima, T.; Kawata, H. Immunoglobulin G4-related masses surrounding coronary arteries: A case report. Eur. Heart J. Case Rep. 2021, 5, ytab055. [Google Scholar] [CrossRef]
- Onda, K.; Fukuhara, T.; Matsuda, E.; Donishi, R.; Hirooka, Y.; Takeuchi, H.; Kato, M. Impact of Screening for Salivary Gland by Ultrasonography. Yonago Acta Med. 2020, 63, 42–46. [Google Scholar] [CrossRef] [Green Version]
- Huynh, K.N.; Kong, M.J.; Nguyen, B.D. Anatomic and Functional Imaging of Immunoglobulin G4-related Disease and Its Mimics. Radiographics 2023, 43, e220097. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, I.; Yamano, T.; Kawahara, H.; Hibino, S.; Nishioka, R.; Zoshima, T.; Hara, S.; Ito, K.; Fujii, H.; Nomura, H.; et al. Positive disease-specific autoantibodies have limited clinical significance in diagnosing IgG4-related disease in daily clinical practice. Rheumatology 2021, 60, 3317–3325. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, F.; Chi, X.; Zhang, Y.; Fu, J.; Bian, W.; Shen, D.; Li, Z. Needle biopsy compared with surgical biopsy: Pitfalls of small biopsy in histologial diagnosis of IgG4-related disease. Arthritis Res. Ther. 2021, 23, 54. [Google Scholar] [CrossRef]
- Adam, Z.; Chovancová, Z.; Nová, M.; Fabian, P.; Řehák, Z.; Koukalová, R.; Slávik, M.; Pour, L.; Krejčí, M.; Čermák, A.; et al. Remission of the disease associated/related with immunoglobulin IgG4 accompanied by multiple lymphadenopathy after treatment with rituximab and dexamethasone: A case report. Vnitr. Lek. 2018, 64, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Shakir, A.; Wheeler, Y.; Krishnaswamy, G. The enigmatic immunoglobulin G4-related disease and its varied cardiovascular manifestations. Heart 2021, 107, 790–798. [Google Scholar] [CrossRef] [PubMed]
- Katz, G.; Stone, J.H. Clinical Perspectives on IgG4-Related Disease and Its Classification. Annu. Rev. Med. 2022, 73, 545–562. [Google Scholar] [CrossRef] [PubMed]
- Paratz, E.D.; Ross, L.; Zentner, D.; Morgan, N.; Bouwer, H.; Lynch, M.; Parsons, S.; La Gerche, A. Intracoronary IgG4-related disease as an unusual cause of sudden cardiac arrest: A case series. Eur. Heart J. Case Rep. 2022, 6, ytac050. [Google Scholar] [CrossRef]
- Salhi, S.; Oueslati, I.; Ayari, S.; Kamoun, E.; Yazidi, M.; Chihaoui, M. A case of reversible hypoparathyroidism in a patient with Riedel’s thyroiditis treated with glucocorticoids. Clin. Case Rep. 2023, 11, e7085. [Google Scholar] [CrossRef] [PubMed]
- Er-Rahali, Y.; Massine El Hammoumi, M.; Issouani, J.; Nfad, C.A.; El Moussaoui, S.; Kabiri, E.H.; Guerboub, A.A. Reidel’s Thyroiditis, a Diagnostic and Management Challenge: A Case Report and Review of the Literature. Case Rep. Endocrinol. 2021, 2021, 5185259. [Google Scholar] [CrossRef] [PubMed]
- Góralska, M.; Podlewska, M.; Żach, M. Riedel’s thyroiditis—Difficulties in differentiating from thyroid cancer. Endokrynol. Pol. 2021, 72, 418–419. [Google Scholar] [CrossRef] [PubMed]
- Pacella, J.C.; Niwattisaiwong, S.; Newman, D. IgG4-Related Retroperitoneal Fibrosis: A Rare Association With Riedel’s Thyroiditis. Cureus 2021, 13, e13997. [Google Scholar] [CrossRef]
- Navarro-Sánchez, V.; Marín-Castañeda, L.A.; Gallegos, C.A.; Quiroz, O.; Ahumada-Ayala, M. IgG4-Related Fibrous Thyroiditis (Riedel’s Thyroiditis): A Case Report. Am. J. Case Rep. 2020, 21, e928046. [Google Scholar] [CrossRef]
- Shafi, A.A.; Saad, N.B.; AlHarthi, B. Riedel’s thyroiditis as a diagnostic dilemma—A case report and review of the literature. Ann. Med. Surg. 2020, 52, 5–9. [Google Scholar] [CrossRef]
- Mammen, S.V.; Gordon, M.B. Successful use of rituximab in a case of riedel thyroiditis resistant to treatment with prednisone and tamoxifen. AACE Clin. Case Rep. 2019, 5, e218–e221. [Google Scholar] [CrossRef] [Green Version]
- Kumar, N.; Gupta, R.; Sayed, S.; Moloo, Z.; Vinayak, S.; Ahmed, M. Difficulties in diagnosis of Riedel’s thyroiditis on aspiration cytology: A case report and brief review of the literature. Diagn. Cytopathol. 2019, 47, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Gökçay Canpolat, A.; Cinel, M.; Dizbay Sak, S.; Taşkaldıran, I.; Korkmaz, H.; Demir, Ö.; Ersoy, R.; Dağdelen, S.; Berker, D.; Dalva, K.; et al. Long-Term Outcomes of Tamoxifen Citrate Therapy and Histo- and Immunopathological Properties in Riedel Thyroiditis. Eur. Thyroid. J. 2021, 10, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Kim, B.; Jang, A.; Jeon, M.J.; Choi, Y.J.; Lee, Y.M.; Song, D.E.; Kim, W.G. Immunoglobulin G4-Related Thyroid Disease: A Single-Center Experience and Literature Review. Endocrinol. Metab. 2022, 37, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Sadacharan, D.; Ahmed, A.; Smitha, S.; Mahadevan, S.; Vimala, R.; Prasad, H. Our Uncommon Experience with 6 Cases of Riedel’s Thyroiditis (Woody Thyroiditis). Indian J. Otolaryngol. Head Neck Surg. 2022, 74 (Suppl. S2), 1757–1762. [Google Scholar] [CrossRef] [PubMed]
- Soh, S.; Pham, A.; O’Hehir, R.E.; Cherk, M.; Topliss, D.J. Novel use of rituximab in a case of Riedel’s thyroiditis refractory to glucocorticoids and tamoxifen. J. Clin. Endocrinol. Metab. 2013, 98, 3543–3549. [Google Scholar] [CrossRef] [Green Version]
- Hunt, L.; Harrison, B.; Bull, M.; Stephenson, T.; Allahabadia, A. Rituximab: A novel treatment for refractory Riedel’s thyroiditis. Endocrinol. Diabetes Metab. Case Rep. 2018, 2018, 17-0132. [Google Scholar] [CrossRef]
- Blanco, V.M.; Páez, C.A.; Victoria, A.M.; Arango, L.G.; Arrunategui, A.M.; Escobar, J.; Martínez, V.; Guzmán, G.E. Riedel’s Thyroiditis: Report of Two Cases and Literature Review. Case Rep. Endocrinol. 2019, 2019, 5130106. [Google Scholar] [CrossRef]
- Zhao, Z.; Lee, Y.J.; Zheng, S.; Khor, L.Y.; Lim, K.H. IgG4-Related Disease of the Thyroid Gland Requiring Emergent Total Thyroidectomy: A Case Report. Head Neck Pathol. 2019, 13, 523–527. [Google Scholar] [CrossRef]
- Dumitru, N.; Ghemigian, A.; Carsote, M.; Albu, S.E.; Terzea, D.; Valea, A. Thyroid nodules after initial evaluation by primary health care practitioners: An ultrasound pictorial essay. Arch. Balk. Med. Union 2016, 51, 434–438. [Google Scholar]
- Yu, Y.; Liu, J.; Yu, N.; Zhang, Y.; Zhang, S.; Li, T.; Gao, Y.; Lu, G.; Zhang, J.; Guo, X. IgG4 immunohistochemistry in Riedel’s thyroiditis and the recommended criteria for diagnosis: A case series and literature review. Clin. Endocrinol. 2021, 94, 851–857. [Google Scholar] [CrossRef]
- Nandi, D.; Ojha, V.; Singh, R.; Kumar, S. Pictorial review of computed tomography and magnetic resonance imaging findings of cardiovascular manifestations of IgG4-related disease. Pol. J. Radiol. 2023, 88, e165–e176. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Liu, Z.; Lu, H.; Peng, Y.; Li, J.; Nie, Y.; Li, J.; Peng, L.; Zhou, J.; Fei, Y.; et al. Potential impact of autoimmune diseases family history in IgG4-related disease: A retrospective cohort study. RMD Open 2023, 9, e002865. [Google Scholar] [CrossRef] [PubMed]
- Azizi, M.; Zajjari, Y.; Rafik, H.; El Kabbaj, D. Retroperitoneal fibrosis in the military hospital of Morocco. Saudi J. Kidney Dis. Transpl. 2020, 31, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Nistor, C.E.; Pantile, D.; Stanciu-Gavan, C.; Ciuche, A.; Moldovan, H. Diagnostic and Therapeutic Characteristics in Patients with Pneumotorax Associated with COVID-19 versus Non-COVID-19 Pneumotorax. Medicina 2022, 58, 1242. [Google Scholar] [CrossRef]
- Nistor, C.E.; Gavan, C.S.; Pantile, D.; Tanase, N.V.; Ciuche, A. Cervico-Thoracic Air Collections in COVID-19 Pneumonia Patients—Our Experience and Brief Review. Chirurgia 2022, 117, 317–327. [Google Scholar] [CrossRef]
- Riedel, B.M.C.L. Die chronische, zur Bildung eisenharter Tumoren führende Entzündung der Schilddrüse. Verh. Dtsch. Ges. Chir. 1896, 25, 101–105. [Google Scholar]
- Zimmermann-Belsing, T.; Rønn, A.M.; Feldt-Rasmussen, U.F.; Kirkegaard, J. Invasive fibrous thyroiditis—Riedel’s goiter. A review of the literature and a case report. Ugeskr. Laeger 1993, 155, 1121–1125. [Google Scholar]
- Zimmermann-Belsing, T.; Feldt-Rasmussen, U. Riedel’s thyroiditis: An autoimmune or primary fibrotic disease? J. Intern. Med. 1994, 235, 271–274. [Google Scholar] [CrossRef]
- Li, Y.; Bai, Y.; Liu, Z.; Ozaki, T.; Taniguchi, E.; Mori, I.; Nagayama, K.; Nakamura, H.; Kakudo, K. Immunohistochemistry of IgG4 can help subclassify Hashimoto’s autoimmune thyroiditis. Pathol. Int. 2009, 59, 636–641. [Google Scholar] [CrossRef]
- Dahlgren, M.; Khosroshahi, A.; Nielsen, G.P.; Deshpande, V.; Stone, J.H. Riedel’s thyroiditis and multifocal fibrosclerosis are part of the IgG4-related systemic disease spectrum. Arthritis Care Res. 2010, 62, 1312–1318. [Google Scholar] [CrossRef]
- Deshpande, V.; Zen, Y.; Chan, J.K.; Yi, E.E.; Sato, Y.; Yoshino, T.; Klöppel, G.; Heathcote, J.G.; Khosroshahi, A.; Ferry, J.A.; et al. Consensus statement on the pathology of IgG4-related disease. Mod. Pathol. 2012, 25, 1181–1192. [Google Scholar] [CrossRef] [Green Version]
- Sharma Khatiwada, A.; Choudhury, N. IgG4-positive Hashimoto thyroiditis and its association with IgG4-related sclerosing disease. BMJ Case Rep. 2022, 15, e249181. [Google Scholar] [CrossRef]
- Li, Y.; Inomata, K.; Nishihara, E.; Kakudo, K. IgG4 thyroiditis in the Asian population. Gland Surg. 2020, 9, 1838–1846. [Google Scholar] [CrossRef]
- Adams, S.H.; Gitto, L.; Serinelli, S.; Curtiss, C. Review of IgG4-related Hashimoto Thyroiditis With Best Practice Recommendations for Diagnosis and Reporting. Adv. Anat. Pathol. 2022, 29, 97–107. [Google Scholar] [CrossRef]
- Liu, L.; Yu, Y.; Chen, L.; Zhang, Y.; Lu, G.; Gao, Y.; Zhang, J. Clinical differences between IgG4 Hashimoto’s thyroiditis and primary thyroid lymphoma. Eur. Thyroid. J. 2022, 11, e210144. [Google Scholar] [CrossRef] [PubMed]
- Lintusaari, J.; Vesaniemi, E.; Kalfert, D.; Ilvesaro, J.; Ludvíková, M.; Kholová, I. IgG4-positive plasma cells in Hashimoto thyroiditis: IgG4-related disease or inflammation-related IgG4-positivity? Apmis 2020, 128, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Gvianishvili, T.; Gogiashvili, L.; Chkhobadze, M. Molecular-biological thyroid profile during autoimmune disease—Hashimoto and Riedel’s thyroiditis. Georgian Med. News 2019, 290, 116–120. [Google Scholar]
- Egashira, S.; Yoshimoto, T.; Tanaka, K.; Kamogawa, N.; Shiozawa, M.; Koge, J.; Toyoda, K.; Koga, M. Cerebral venous sinus thrombosis presenting transient ischemic attack after recovery from COVID-19 with Graves’ disease and IgG4-related ophthalmic disease: A case report. Rinsho Shinkeigaku 2022, 62, 928–934. [Google Scholar] [CrossRef]
- Strainiene, S.; Sarlauskas, L.; Savlan, I.; Liakina, V.; Stundiene, I.; Valantinas, J. Multi-organ IgG4-related disease continues to mislead clinicians: A case report and literature review. World J. Clin. Cases 2020, 8, 3267–3279. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Su, Q.; Li, J.; Zhou, H.; Li, H. Association Between Immunoglobulin G4-Related Ophthalmic Disease and Nonlymphoid Malignancy Case Series and Comprehensive Review of the Literature. J. Neuroophthalmol. 2023, 43, 102–109. [Google Scholar] [CrossRef]
- Olejarz, M.; Szczepanek-Parulska, E.; Ostałowska-Klockiewicz, A.; Antosik, P.; Sawicka-Gutaj, N.; Helak-Łapaj, C.; Stopa, M.; Ruchala, M. High IgG4 serum concentration is associated with active Graves orbitopathy. Front. Endocrinol. 2023, 14, 1083321. [Google Scholar] [CrossRef]
- Olejarz, M.; Szczepanek-Parulska, E.; Dadej, D.; Sawicka-Gutaj, N.; Domin, R.; Ruchała, M. IgG4 as a Biomarker in Graves’ Orbitopathy. Mediat. Inflamm. 2021, 2021, 5590471. [Google Scholar] [CrossRef]
- Men, C.J.; Kossler, A.L.; Wester, S.T. Updates on the understanding and management of thyroid eye disease. Ther. Adv. Ophthalmol. 2021, 13, 25158414211027760. [Google Scholar] [CrossRef]
- Ye, H.; Xiao, W.; Chen, R.; Zhang, P.; Tang, L.; Chen, J.; Zhang, T.; Ji, X.; Shi, L.; Yang, H. Elevated Immunoglobulin G4 Levels in Patients with Thyroid Eye Disease and Their Clinical Implications. Investig. Ophthalmol. Vis. Sci. 2020, 61, 57. [Google Scholar] [CrossRef]
- Hiratsuka, I.; Yamada, H.; Itoh, M.; Shibata, M.; Takayanagi, T.; Makino, M.; Sugimura, Y.; Hayakawa, N.; Hashimoto, S.; Suzuki, A. Changes in Serum Immunoglobulin G4 Levels in Patients with Newly Diagnosed Graves’ Disease. Exp. Clin. Endocrinol Diabetes 2020, 128, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Luo, B.; Zhang, J.; Zhou, X.; Shao, S.; Xu, W.; Yang, Y.; Yuan, G. Clinical relevance of serum immunoglobulin G4 in glucocorticoid therapy of Graves’ ophthalmopathy. Clin. Endocrinol. 2021, 95, 657–667. [Google Scholar] [CrossRef]
- Luo, B.; Yuan, X.; Wang, W.; Zhang, J.; Liu, R.; Hu, W.; Qi, X.; Xiang, N.; Chen, L. Ocular Manifestations and Clinical Implications of Serum Immunoglobulin G4 Levels in Graves’ Ophthalmopathy Patients. Ocul. Immunol. Inflamm. 2022, 30, 580–587. [Google Scholar] [CrossRef]
- Benítez Valderrama, P.; Castro Calvo, A.; Rodrigañez Riesco, L.; Regojo Zapata, R.; Parra Ramírez, P. Fibrous variant of Hashimoto’s thyroiditis as a sign of IgG4-related disease, mimicking thyroid lymphoma: Case report. Endocrinol. Diabetes Nutr. 2022, 70, 60–62. [Google Scholar] [CrossRef]
- Matos, T.; Almeida, M.M.; Batista, L.; do Vale, S. IgG4-related disease of the thyroid gland. BMJ Case Rep. 2021, 14, e238177. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, X.; Liu, Z.; Ma, J.; Lin, X.; Qin, Y.; Nishihara, E.; Miyauchi, A.; Kakudo, K. Hashimoto’s Thyroiditis with Increased IgG4-Positive Plasma Cells: Using Thyroid-Specific Diagnostic Criteria May Identify Early Phase IgG4 Thyroiditis. Thyroid 2020, 30, 251–261. [Google Scholar] [CrossRef]
- Cocolos, A.; Ghemigian, M.; Dumitru, N.; Valea, A.; Petrova, E.; Carsote, M.; Ghemigian, A. Riedel’s thyroiditis: A rare diagnosis that rarely requires thyroid surgery. J. Surg. Sci. 2018, 5, 38–41. [Google Scholar]
- Elshaer, R.K.; Halawa, M.R.; Ahmed, I.Z.; Aboelezz, N.F.; Mohamed, N.R.; Bahaa Eldin, A.M. Serum IgG4 level for malignancy prediction in indeterminate thyroid nodules among patients with or without autoimmune thyroid disease. Egypt. J. Immunol. 2022, 29, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Zita, C.; Petr, F.; Simona, R.; Iva, Z.S.; Tetiana, S.; Klára, N.; Jiří, L. IgG4 immunoglobulin subclass and related pathological conditions or how to effectively imitate cancer disease. Klin. Onkol. 2022, 35, 20–31. [Google Scholar] [PubMed]
- Deshpande, V. Igg4 related disease of the head and neck. Head Neck Pathol. 2015, 9, 24–31. [Google Scholar] [CrossRef] [Green Version]
- Bangolo, A.I.; Gupta, K.; Atoot, A. IgG4-Related Disease Retroperitoneal Fibrosis: An Unusual Cause of Low Back Pain. Cureus 2021, 13, e13608. [Google Scholar] [CrossRef]
- Raglianti, V.; Rossi, G.M.; Vaglio, A. Idiopathic retroperitoneal fibrosis: An update for nephrologists. Nephrol. Dial. Transpl. 2021, 36, 1773–1781. [Google Scholar] [CrossRef]
- Adhikari, R.; Banga, A.; Koritala, T.; Dasari, N.; Pattan, V. A Rare Co-association of Autoimmune Thyroiditis and Idiopathic Retroperitoneal Fibrosis. Cureus 2022, 14, e30980. [Google Scholar] [CrossRef]
- Gómez Rivas, J.; Quintana, L.M.; Álvarez-Maestro, M.; Aguilera, A.; Martinez Piñeiro, L.; Sarikaya, S. Retroperitoneal fibrosis: A literature review. Arch. Esp. Urol. 2020, 73, 60–67. [Google Scholar]
- Kawano, M.; Saeki, T.; Nakashima, H. IgG4-related kidney disease and retroperitoneal fibrosis: An update. Mod. Rheumatol. 2019, 29, 231–239. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, T.; Maruyama, M.; Ito, T.; Fujinaga, Y.; Ozaki, Y.; Maruyama, M.; Kodama, R.; Muraki, T.; Hamano, H.; Arakura, N.; et al. Clinical features of a new disease concept, IgG4-related thyroiditis. Scand. J. Rheumatol. 2013, 42, 325–330. [Google Scholar] [CrossRef] [Green Version]
- Langlois, F.; Varlamov, E.V.; Fleseriu, M. Hypophysitis, the Growing Spectrum of a Rare Pituitary Disease. J. Clin. Endocrinol. Metab. 2022, 107, 10–28. [Google Scholar] [CrossRef]
- Erdei, A. Immunoglobulin G4-related endocrine diseases. Orv. Hetil. 2022, 163, 1175–1180. [Google Scholar] [CrossRef]
- Rzepecka, A.; Babińska, A.; Sworczak, K. IgG4-related disease in endocrine practice. Arch. Med. Sci. 2019, 15, 55–64. [Google Scholar] [CrossRef] [Green Version]
- Rumyantsev, P.O.; Kozlov, I.G.; Kolpakova, E.A.; Chukhacheva, O.S.; Korenev, S.V.; Goncharov, A.G.; Ulanova, E.U. IGG4-related diseases in endocrinology. Probl. Endokrinol. 2020, 66, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Im, J.Y.; Sunwoo, M. Immunoglobulin G4-Related Disease Involving Various Head and Neck Regions: A Case Report. J. Korean Soc. Radiol. 2022, 83, 910–917. [Google Scholar] [CrossRef] [PubMed]
- Bong, J.B.; Lee, D.K.; Lee, M.A.; Hwang, B.W.; Kang, H.G. Immunoglobulin G4-related disease presenting with peripheral neuropathy: A case report. BMC Neurol. 2021, 21, 41. [Google Scholar] [CrossRef] [PubMed]



| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
|
|
|
|
|
|
| |
|
| First Author Year of Publication Reference Number | Clinical Aspects Thyroid Profile | Management and Outcome |
|---|---|---|
| Pandev 2023 [6] |
|
|
| Salhi 2023 [44] |
|
|
| Er-Rahali 2021 [45] |
|
|
| Góralska 2021 [46] |
|
|
| Pacella 2021 [47] |
|
|
| Navarro-Sánchez 2020 [48] |
|
|
| Shafi 2020 [49] |
|
|
| Mammen 2019 [50] |
|
|
| Kumar 2019 [51] |
|
|
| First Author Year of Publication Reference Number | Clinical Aspects Thyroid Profile | Management and Outcome |
|---|---|---|
| Sadacharan 2023 [54] |
|
|
| Gökçay Canpolat 2021 [52] |
|
|
| Yu 2021 [60] |
|
|
| Blanco 2019 [57] |
IHC report:
|
|
| First Author Publication Year Reference Number | Study Design Studied Population | IgG4-Related Thyroid Findings |
|---|---|---|
| Nandi 2023 [61] |
|
|
| Sun 2023 [62] |
|
|
| Azizi 2020 [63] |
|
|
| First Author Year of Publication Reference Number | Clinical Aspects Thyroid Profile | Management and Outcome |
|---|---|---|
| Jin 2022 [53] |
|
Post-operatory IHC:
|
| Gvianishvili 2019 [77] |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carsote, M.; Nistor, C. Reshaping the Concept of Riedel’s Thyroiditis into the Larger Frame of IgG4-Related Disease (Spectrum of IgG4-Related Thyroid Disease). Biomedicines 2023, 11, 1691. https://doi.org/10.3390/biomedicines11061691
Carsote M, Nistor C. Reshaping the Concept of Riedel’s Thyroiditis into the Larger Frame of IgG4-Related Disease (Spectrum of IgG4-Related Thyroid Disease). Biomedicines. 2023; 11(6):1691. https://doi.org/10.3390/biomedicines11061691
Chicago/Turabian StyleCarsote, Mara, and Claudiu Nistor. 2023. "Reshaping the Concept of Riedel’s Thyroiditis into the Larger Frame of IgG4-Related Disease (Spectrum of IgG4-Related Thyroid Disease)" Biomedicines 11, no. 6: 1691. https://doi.org/10.3390/biomedicines11061691
APA StyleCarsote, M., & Nistor, C. (2023). Reshaping the Concept of Riedel’s Thyroiditis into the Larger Frame of IgG4-Related Disease (Spectrum of IgG4-Related Thyroid Disease). Biomedicines, 11(6), 1691. https://doi.org/10.3390/biomedicines11061691