A Pictorial Essay Describing the CT Imaging Features of COVID-19 Cases throughout the Pandemic with a Special Focus on Lung Manifestations and Extrapulmonary Vascular Abdominal Complications
Abstract
:1. Introduction
2. SARS-CoV-2 Pathogenesis
The Important Role of the Immune System and of Gut–Lung Axis in Both Pulmonary and Extrapulmonary Manifestations
3. Lung Complications
3.1. COVID-19 Pneumonia: Role of Chest CT and Chest Features from the Wild-Type to the Omicron Variant
3.2. COVID-19 Pneumonia after Breakthrough Infections
3.3. Secondary Lung Complications
3.3.1. Acute Respiratory Distress Syndrome
3.3.2. Pneumomediastinum and Pneumothorax
3.3.3. Pulmonary Fibrosis
3.3.4. Pulmonary Thromboembolism
4. Vascular Abdominal Extrapulmonary Complications
4.1. Small Bowel Ischemia and Ischemic Colitis
4.2. Splenic and Renal Infarction
4.3. Hemorrhagic Abdominal Complications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lai, A.; Tambuzzi, S.; Bergna, A.; Battistini, A.; Della Ventura, C.; Galli, M.; Zoja, R.; Zehender, G.; Cattaneo, C. Evidence of SARS-CoV-2 Antibodies and RNA on Autopsy Cases in the Pre-Pandemic Period in Milan (Italy). Front. Microbiol. 2022, 13, 886317. [Google Scholar] [CrossRef] [PubMed]
- Carrat, F.; Figoni, J.; Henny, J.; Desenclos, J.C.; Kab, S.; de Lamballerie, X.; Zins, M. MEvidence of early circulation of SARS-CoV-2 in France: Findings from the population-based “CONSTANCES” cohort. Eur. J. Epidemiol. 2021, 36, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Gragnani, L.; Monti, M.; Santini, S.A.; Marri, S.; Madia, F.; Lorini, S.; Petraccia, L.; Stasi, C.; Basile, U.; Luti, V.; et al. SARS-CoV-2 was already circulating in Italy, in early December 2019. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 3342–3349. [Google Scholar] [PubMed]
- Zhang, T.; Wu, Q.; Zhang, Z. Probable Pangolin Origin of SARS-CoV-2 Associated with the COVID-19 Outbreak. Curr. Biol. 2020, 30, 1346–1351. [Google Scholar] [CrossRef]
- Guo, Y.R.; Cao, Q.D.; Hong, Z.S.; Tan, Y.Y.; Chen, S.D.; Jin, H.J.; Tan, K.S.; Wang, D.Y.; Yan, Y. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak—An update on the status. Mil. Med. Res. 2020, 7, 11. [Google Scholar] [CrossRef] [Green Version]
- Morens, D.M.; Breman, J.G.; Calisher, C.H.; Doherty, P.C.; Hahn, B.H.; Keusch, G.T.; Kramer, L.D.; LeDuc, J.W.; Monath, T.P.; Taubenberger, J.K. The Origin of COVID-19 and Why It Matters. Am. J. Trop. Med. Hyg. 2020, 103, 955–959. [Google Scholar] [CrossRef]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef]
- Bloom, J.D.; Chan, Y.A.; Baric, R.S.; Bjorkman, P.J.; Cobey, S.; Deverman, B.E.; Fisman, D.N.; Gupta, R.; Iwasaki, A.; Lipsitch, M.; et al. Investigate the origins of COVID-19. Science 2021, 372, 694. [Google Scholar] [CrossRef]
- Holmes, E.C.; Goldstein, S.A.; Rasmussen, A.L.; Robertson, D.L.; Crits-Christoph, A.; Wertheim, J.O.; Anthony, S.J.; Barclay, W.S.; Boni, M.F.; Doherty, P.C.; et al. The origins of SARS-CoV-2: A critical review. Cell 2021, 184, 4848–4856. [Google Scholar] [CrossRef]
- Zuin, M.; Engelen, M.M.; Bilato, C.; Vanassche, T.; Rigatelli, G.; Verhamme, P.; Vandenbriele, C.; Zuliani, G.; Roncon, L. Prevalence of Acute Pulmonary Embolism at Autopsy in Patients with COVID-19. Am. J. Cardiol. 2022, 171, 159–164. [Google Scholar] [CrossRef]
- Hao, R.; Liu, Y.; Shen, W.; Zhao, R.; Jiang, B.; Song, H.; Yan, M.; Ma, H. Surveillance of emerging infectious diseases for biosecurity. Sci. China Life Sci. 2022, 65, 1504–1516. [Google Scholar] [CrossRef]
- Poor, H.D. Pulmonary Thrombosis and Thromboembolism in COVID-19. Chest 2021, 160, 1471–1480. [Google Scholar] [CrossRef] [PubMed]
- Brogna, B.; Brogna, C.; Martino, A.; Minichiello, S.; Romeo, D.M.; Romano, P.; Bignardi, E.; Mazza, E.M.; Musto, L. SARS-CoV-2 Infection with Different Radiological Insights. Diagnostics 2020, 10, 283. [Google Scholar] [CrossRef]
- Xu, J.; Wu, Z.; Zhang, M.; Liu, S.; Zhou, L.; Yang, C.; Liu, C. The Role of the Gastrointestinal System in Neuroinvasion by SARS-CoV-2. Front. Neurosci. 2021, 15, 694446. [Google Scholar] [CrossRef] [PubMed]
- Moslehi, N.; Jahromy, M.; Ashrafi, P.; Vatani, K.; Nemati, M.H.; Moghadam, P.; Rostamian, F.; Jahromi, M. Multi-organ system involvement in coronavirus disease 2019 (COVID-19): A mega review. J. Fam. Med. Prim. Care 2022, 11, 5014–5023. [Google Scholar] [CrossRef]
- Brogna, C.; Brogna, B.; Bisaccia, D.R.; Lauritano, F.; Marino, G.; Montano, L.; Cristoni, S.; Prisco, M.; Piscopo, M. Could SARS-CoV-2 Have Bacteriophage Behavior or Induce the Activity of Other Bacteriophages? Vaccines 2022, 10, 708. [Google Scholar] [CrossRef] [PubMed]
- De, R.; Dutta, S. Role of the Microbiome in the Pathogenesis of COVID-19. Front. Cell. Infect. Microbiol. 2022, 12, 736397. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Han, X.; Jiang, N.; Cao, Y.; Alwalid, O.; Gu, J.; Fan, Y.; Zheng, C. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: A descriptive study. Lancet Infect. Dis. 2020, 20, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Gong, T.; Wang, G.; Wang, J.; Guo, X.; Cai, E.; Li, S.; Li, X.; Yu, Y.; Lin, L. Timely Diagnosis and Treatment Shortens the Time to Resolution of Coronavirus Disease (COVID-19) Pneumonia and Lowers the Highest and Last CT Scores from Sequential Chest CT. Am. J. Roentgenol. 2020, 215, 367–373. [Google Scholar] [CrossRef]
- Guillo, E.; Gomez, I.B.; Dangeard, S.; Bennani, S.; Saab, I.; Tordjman, M.; Jilet, L.; Chassagnon, G.; Revel, M.-P. COVID-19 pneumonia: Diagnostic and prognostic role of CT based on a retrospective analysis of 214 consecutive patients from Paris, France. Eur. J. Radiol. 2020, 131, 109209. [Google Scholar] [CrossRef]
- Brogna, B.; Bignardi, E.; Brogna, C.; Volpe, M.; Lombardi, G.; Rosa, A.; Gagliardi, G.; Capasso, P.F.M.; Gravino, E.; Maio, F.; et al. A Pictorial Review of the Role of Imaging in the Detection, Management, Histopathological Correlations, and Complications of COVID-19 Pneumonia. Diagnostics 2021, 11, 437. [Google Scholar] [CrossRef]
- Violi, F.; Pignatelli, P.; Vestri, A.R.; Spagnoli, A.; Cipollone, F.; Ceccarelli, G.; Oliva, A.; Amitrano, M.; Pirro, M.; Taliani, G.; et al. ADA (Age-D-Dimer-Albumin) Score to Predict Thrombosis in SARS-CoV-2. Thromb. Haemost. 2022, 122, 1567–1572. [Google Scholar] [CrossRef]
- Acharya, Y.; Alameer, A.; Calpin, G.; Alkhattab, M.; Sultan, S. A comprehensive review of vascular complications in COVID-19. J. Thromb. Thrombolysis 2021, 53, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.E.; Gong, A.J.; Gawande, R.S.; Fishman, E.K.; Vadvala, H.V. Vascular findings in CTA body and extremity of critically ill COVID-19 patients: Commonly encountered vascular complications with review of literature. Emerg. Radiol. 2022, 29, 263–279. [Google Scholar] [CrossRef] [PubMed]
- Prince, M.R.; Dev, H.; Lane, E.G.; Margolis, D.J.; DeSancho, M.T. Major hemorrhage and mortality in COVID-19 patients on therapeutic anticoagulation for venous thromboembolism. J. Thromb. Thrombolysis 2022, 54, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Brogna, B.; Romano, A.; Tibullo, L.; Montuori, M.; Nunziata, M.; Russo, G.; Musto, L.A. Rare findings of spontaneous hemothorax and small subpleural lung hematoma in a COVID-19 patient: A case report. Acta Radiol. Open 2021, 10, 1–6. [Google Scholar] [CrossRef]
- Kirca, F.; Aydoğan, S.; Gözalan, A.; Kayipmaz, A.E.; Özdemir, F.A.E.; Tekçe, Y.T.; Beşer, İ.O.; Gün, P.; Ökten, R.S.; Dinç, B. Comparison of clinical characteristics of wild-type SARS-CoV-2 and Omicron. Rev. Assoc. Med. Bras. 2022, 68, 1476–1480. [Google Scholar] [CrossRef]
- Lee, J.E.; Hwang, M.; Kim, Y.H.; Chung, M.J.; Sim, B.H.; Jeong, W.G.; Jeong, Y.J. SARS-CoV-2 Variants Infection in Relationship to Imaging-based Pneumonia and Clinical Outcomes. Radiology 2023, 306, e221795. [Google Scholar] [CrossRef]
- Addo, I.Y.; Dadzie, F.A.; Okeke, S.R.; Boadi, C.; Boadu, E.F. Duration of immunity following full vaccination against SARS-CoV-2: A systematic review. Arch. Public Health 2022, 80, 200. [Google Scholar] [CrossRef]
- Gong, I.Y.; Vijenthira, A.; Powis, M.; Calzavara, A.; Patrikar, A.; Sutradhar, R.; Hicks, L.K.; Wilton, D.; Singh, S.; Krzyzanowska, M.K.; et al. Association of COVID-19 Vaccination with Breakthrough Infections and Complications in Patients with Cancer. JAMA Oncol. 2023, 9, 386. [Google Scholar] [CrossRef]
- Mirouse, A.; Friol, A.; Moreau, A.-S.; Jung, B.; Jullien, E.; Bureau, C.; Djibré, M.; de Prost, N.; Zafrani, L.; Argaud, L.; et al. Severe SARS-Cov2 pneumonia in vaccinated patients: A multicenter cohort study. Sci. Rep. 2023, 13, 1–9. [Google Scholar] [CrossRef]
- Tong, X.; Huang, Z.; Zhang, X.; Si, G.; Lu, H.; Zhang, W.; Xue, Y.; Xie, W. Old Age is an Independent Risk Factor for Pneumonia Development in Patients with SARS-CoV-2 Omicron Variant Infection and a History of Inactivated Vaccine Injection. Infect. Drug Resist. 2022, 15, 5567–5573. [Google Scholar] [CrossRef]
- Kim, A.H.; Sparks, J.A. Immunosuppression and SARS-CoV-2 breakthrough infections. Lancet Rheumatol. 2022, 4, e379–e380. [Google Scholar] [CrossRef]
- Brogna, B.; Bignardi, E.; Brogna, C.; Capasso, C.; Gagliardi, G.; Martino, A.; Musto, L.A. COVID-19 Pneumonia in Vaccinated Population: A Six Clinical and Radiological Case Series. Medicina 2021, 57, 891. [Google Scholar] [CrossRef]
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.-M.; Wang, W.; Song, Z.-G.; Hu, Y.; Tao, Z.-W.; Tian, J.-H.; Pei, Y.-Y.; et al. A new coronavirus associated with human respiratory disease in China. Nature 2020, 579, 265–269. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.; Yang, J.; Zhou, C.; Chen, B.; Fang, H.; Chen, S.; Zhang, X.; Wang, L.; Zhang, L. A review of SARS-CoV2: Compared with SARS-CoV and MERS-CoV. Front. Med. 2021, 8, 628370. [Google Scholar] [CrossRef]
- Jeong, Y.J.; Wi, Y.M.; Park, H.; Lee, J.E.; Kim, S.-H.; Lee, K.S. Current and Emerging Knowledge in COVID-19. Radiology 2023, 306, e222462. [Google Scholar] [CrossRef]
- Elrobaa, I.H.; New, K.J. COVID-19: Pulmonary and Extra Pulmonary Manifestations. Front. Public Health 2021, 9, 711616. [Google Scholar] [CrossRef]
- Sarkesh, A.; Sorkhabi, A.D.; Sheykhsaran, E.; Alinezhad, F.; Mohammadzadeh, N.; Hemmat, N.; Baghi, H.B. Extrapulmonary Clinical Manifestations in COVID-19 Patients. Am. J. Trop. Med. Hyg. 2020, 103, 1783–1796. [Google Scholar] [CrossRef]
- Pozdnyakova, V.; Weber, B.; Cheng, S.; Ebinger, J.E. Review of Immunologic Manifestations of COVID-19 Infection and Vaccination. Cardiol. Clin. 2022, 40, 301–308. [Google Scholar] [CrossRef]
- Davitt, E.; Davitt, C.; Mazer, M.B.; Areti, S.S.; Hotchkiss, R.S.; Remy, K.E. COVID-19 disease and immune dysregulation. Best Pract. Res. Clin. Haematol. 2022, 35, 101401. [Google Scholar] [CrossRef] [PubMed]
- Arish, M.; Qian, W.; Narasimhan, H.; Sun, J. COVID-19 immunopathology: From acute diseases to chronic sequelae. J. Med. Virol. 2022, 95, e28122. [Google Scholar] [CrossRef] [PubMed]
- Klavina, P.A.; Leon, G.; Curtis, A.M.; Preston, R.J. Dysregulated haemostasis in thrombo-inflammatory disease. Clin. Sci. 2022, 136, 1809–1829. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Madhavan, M.V.; Sehgal, K.; Nair, N.; Mahajan, S.; Sehrawat, T.S.; Bikdeli, B.; Ahluwalia, N.; Ausiello, J.C.; Wan, E.Y.; et al. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020, 26, 1017–1032. [Google Scholar] [CrossRef]
- Zhou, D.; Wang, Q.; Liu, H. Coronavirus disease 2019 and the gut–lung axis. Int. J. Infect. Dis. 2021, 113, 300–307. [Google Scholar] [CrossRef]
- Allali, I.; Bakri, Y.; Amzazi, S.; Ghazal, H. Gut-Lung Axis in COVID-19. Interdiscip. Perspect. Infect. Dis. 2021, 2021, 1–6. [Google Scholar] [CrossRef]
- de Oliveira, G.L.V.; Oliveira, C.N.S.; Pinzan, C.F.; de Salis, L.V.V.; Cardoso, C.R.D.B. Microbiota Modulation of the Gut-Lung Axis in COVID-19. Front. Immunol. 2021, 12, 635471. [Google Scholar] [CrossRef]
- Natarajan, A.; Zlitni, S.; Brooks, E.F.; Vance, S.E.; Dahlen, A.; Hedlin, H.; Park, R.M.; Han, A.; Schmidtke, D.T.; Verma, R.; et al. Gastrointestinal symptoms and fecal shedding of SARS-CoV-2 RNA suggest prolonged gastrointestinal infection. Med 2022, 3, 371–387. [Google Scholar] [CrossRef]
- Brogna, B.; Brogna, C.; Petrillo, M.; Conte, A.M.; Benincasa, G.; Montano, L.; Piscopo, M. SARS-CoV-2 detection in fecal sample from a patient with typical findings of COVID-19 pneumonia on CT but negative to multiple SARS-CoV-2 RT-PCR tests on oropharyngeal and nasopharyngeal swab samples. Medicina 2021, 57, 290. [Google Scholar] [CrossRef]
- Petrillo, M.; Brogna, C.; Cristoni, S.; Querci, M.; Piazza, O.; Van den Eede, G. Increase of SARS-CoV-2 RNA load in faecal samples prompts for rethinking of SARS-CoV-2 biology and COVID-19 epidemiology. F1000Research 2021, 10, 370. [Google Scholar] [CrossRef]
- Brogna, C.; Cristoni, S.; Brogna, B.; Bisaccia, D.R.; Marino, G.; Viduto, V.; Montano, L.; Piscopo, M. Toxin-like Peptides from the Bacterial Cultures Derived from Gut Microbiome Infected by SARS-CoV-2—New Data for a Possible Role in the Long COVID Pattern. Biomedicines 2022, 11, 87. [Google Scholar] [CrossRef] [PubMed]
- Brogna, C.; Cristoni, S.; Petrillo, M.; Querci, M.; Piazza, O.; Van den Eede, G. Toxin-like peptides in plasma, urine and faecal samples from COVID-19 patients. F1000Research 2021, 10, 550. [Google Scholar] [CrossRef] [PubMed]
- Pistollato, F.; Petrillo, M.; Clerbaux, L.A.; Leoni, G.; Ponti, J.; Bogni, A.; Brogna, C.; Cristoni, S.; Sanges, R.; Mendoza-de Gyves, E.; et al. Effects of spike protein and toxin-like peptides found in COVID-19 patients on human 3D neuronal/glial model undergoing differentiation: Possible implications for SARS-CoV-2 impact on brain development. Reprod. Toxicol. 2022, 111, 34–48. [Google Scholar] [CrossRef] [PubMed]
- Ito, N.; Kitahara, Y.; Miwata, K.; Okimoto, M.; Takafuta, T. Comparison of COVID-19 pneumonia during the SARS-CoV-2 Omicron wave and the previous non-Omicron wave in a single facility. Respir. Investig. 2022, 60, 772–778. [Google Scholar] [CrossRef]
- Kwee, T.C.; Kwee, R.M. Chest CT in COVID-19: What the Radiologist Needs to Know. Radiographics 2022, 42, E32. [Google Scholar] [CrossRef]
- Kim, H. Outbreak of novel coronavirus (COVID-19): What is the role of radiologists? Eur. Radiol. 2020, 30, 3266–3267. [Google Scholar] [CrossRef] [Green Version]
- Inui, S.; Gonoi, W.; Kurokawa, R.; Nakai, Y.; Watanabe, Y.; Sakurai, K.; Ishida, M.; Fujikawa, A.; Abe, O. The role of chest imaging in the diagnosis, management, and monitoring of coronavirus disease 2019 (COVID-19). Insights Imaging 2021, 12, 1–14. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, C.; Hu, Y.; Li, C.; Ren, Q.; Zhang, X.; Shi, H.; Zhou, M. Temporal Changes of CT Findings in 90 Patients with COVID-19 Pneumonia: A Longitudinal Study. Radiology 2020, 296, E55–E64. [Google Scholar] [CrossRef] [Green Version]
- Hu, Q.; Guan, H.; Sun, Z.; Huang, L.; Chen, C.; Ai, T.; Pan, Y.; Xia, L. CT features and temporal lung changes in COVID-19 pneumonia in Wuhan, China. Eur. J. Radiol. 2020, 128, 109017. [Google Scholar] [CrossRef]
- Li, X.; Zeng, W.; Li, X.; Chen, H.; Shi, L.; Li, X.; Xiang, H.; Cao, Y.; Chen, H.; Liu, C.; et al. CT imaging changes of corona virus disease 2019(COVID-19): A multi-center study in Southwest China. J. Transl. Med. 2020, 18, 154. [Google Scholar] [CrossRef] [Green Version]
- Caruso, D.; Polidori, T.; Guido, G.; Nicolai, M.; Bracci, B.; Cremona, A.; Zerunian, M.; Polici, M.; Pucciarelli, F.; Rucci, C.; et al. Typical and atypical COVID-19 computed tomography findings. World J. Clin. Cases 2020, 8, 3177–3187. [Google Scholar] [CrossRef]
- Guarnera, A.; Podda, P.; Santini, E.; Paolantonio, P.; Laghi, A. Differential diagnoses of COVID-19 pneumonia: The current challenge for the radiologist—A pictorial essay. Insights Imaging 2021, 12, 1–11. [Google Scholar] [CrossRef]
- Jalaber, C.; Chassagnon, G.; Hani, C.; Dangeard, S.; Babin, M.; Launay, O.; Revel, M.P. Is COVID-19 pneumonia differentiable from other viral pneumonia on CT scan? Respir. Med. Res. 2021, 79, 100824. [Google Scholar]
- Duzgun, S.A.; Durhan, G.; Demirkazik, F.B.; Akpinar, M.G.; Ariyurek, O.M. COVID-19 pneumonia: The great radiological mimicker. Insights Imaging 2020, 11, 1–15. [Google Scholar] [CrossRef]
- Garrana, S.H.; Som, A.; Ndakwah, G.S.; Yeung, T.; Febbo, J.; Heeger, A.P.; Lang, M.; McDermott, S.; Mendoza, D.P.; Zhang, E.W.; et al. Comparison of Chest CT Findings of COVID-19, Influenza, and Organizing Pneumonia: A Multireader Study. Am. J. Roentgenol. 2021, 217, 1093–1102. [Google Scholar] [CrossRef]
- Zheng, F.; Li, L.; Zhang, X.; Song, Y.; Huang, Z.; Chong, Y.; Chen, Z.; Zhu, H.; Wu, J.; Chen, W.; et al. Accurately Discriminating COVID-19 from Viral and Bacterial Pneumonia According to CT Images Via Deep Learning. Interdiscip. Sci. Comput. Life Sci. 2021, 13, 273–285. [Google Scholar] [CrossRef]
- Askani, E.; Mueller-Peltzer, K.; Madrid, J.; Knoke, M.; Hasic, D.; Bamberg, F.; Schlett, C.L.; Agarwal, P. Computed Tomographic Imaging Features of COVID-19 Pneumonia Caused by the Delta (B.1.617.2) and Omicron (B.1.1.529) Variant in a German Nested Cohort Pilot Study Group. Tomography 2022, 8, 2435–2449. [Google Scholar] [CrossRef] [PubMed]
- Inui, S.; Fujikawa, A.; Gonoi, W.; Kawano, S.; Sakurai, K.; Uchida, Y.; Ishida, M.; Abe, O. Comparison of CT findings of coronavirus disease 2019 (COVID-19) pneumonia caused by different major variants. Jpn. J. Radiol. 2022, 40, 1246–1256. [Google Scholar] [CrossRef]
- Yoon, S.H.; Lee, J.H.; Kim, B.N. Chest CT Findings in Hospitalized Patients with SARS-CoV-2: Delta versus Omicron Variants. Radiology 2023, 305, E66. [Google Scholar] [CrossRef]
- Blanca, D.; Nicolosi, S.; Bandera, A.; Blasi, F.; Mantero, M.; Hu, C.; de Amicis, M.M.; Lucchi, T.; Schinco, G.; Peyvandi, F.; et al. Comparison between the first and second COVID-19 waves in Internal Medicine wards in Milan, Italy: A retrospective observational study. Intern. Emerg. Med. 2022, 17, 2219–2228. [Google Scholar] [CrossRef]
- González-Castro, A.; Fito, E.C.; Fernandez, A.; Acha, P.E.; Borregán, J.R.; Peñasco, Y. First and second wave of coronavirus-19 disease: A comparative study in patients hospitalized in an ICU of a third-level university hospital. Med. Intensiv. 2022, 46, 166–168. [Google Scholar] [CrossRef] [PubMed]
- Portacci, A.; Carpagnano, G.E.; Tummolo, M.G.; Santomasi, C.; Palma, L.; Fasano, D.; Resta, E.; Lozupone, M.; Solfrizzi, V.; Panza, F.; et al. COVID-19 clinical phenotypes and short-term outcomes: Differences between the first and the second wave of pandemic in Italy. Expert Rev. Respir. Med. 2021, 15, 1619–1625. [Google Scholar] [CrossRef]
- Bajči, M.P.; Lendak, D.F.; Ristić, M.; Drljača, M.M.; Brkić, S.; Turkulov, V.; Petrović, V. COVID-19 Breakthrough Infections among Patients Aged ≥65 Years in Serbia: Morbidity and Mortality Overview. Vaccines 2022, 10, 1818. [Google Scholar] [CrossRef]
- Abdullah, F.; Myers, J.; Basu, D.; Tintinger, G.; Ueckermann, V.; Mathebula, M.; Ramlall, R.; Spoor, S.; de Villiers, T.; Van der Walt, Z.; et al. Decreased severity of disease during the first global omicron variant covid-19 outbreak in a large hospital in tshwane, south africa. Int. J. Infect. Dis. 2022, 116, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Chen, J.; Chen, L.; Jia, X.; Fan, Y.; Zheng, Y.; Alwalid, O.; Liu, J.; Li, Y.; Li, N.; et al. Comparative Analysis of Clinical and CT Findings in Patients with SARS-CoV-2 Original Strain, Delta and Omicron Variants. Biomedicines 2023, 11, 901. [Google Scholar] [CrossRef]
- Hirotsu, Y.; Kakizaki, Y.; Saito, A.; Tsutsui, T.; Hanawa, S.; Yamaki, H.; Ide, S.; Kawaguchi, M.; Kobayashi, H.; Miyashita, Y.; et al. Lung tropism in hospitalized patients following infection with SARS-CoV-2 variants from D614G to Omicron BA. 2. Commun. Med. 2023, 3, 32. [Google Scholar] [CrossRef]
- Tsakok, M.T.; Watson, R.A.; Saujani, S.J.; Kong, M.; Xie, C.; Peschl, H.; Wing, L.; MacLeod, F.K.; Shine, B.; Talbot, N.P.; et al. Reduction in Chest CT Severity and Improved Hospital Outcomes in SARS-CoV-2 Omicron Compared with Delta Variant Infection. Radiology 2023, 306, 261–269. [Google Scholar] [CrossRef]
- Yang, N.; Wang, C.; Huang, J.; Dong, J.; Ye, J.; Fu, Y.; Huang, J.; Xu, D.; Cao, G.; Qian, G. Clinical and Pulmonary CT Characteristics of Patients Infected with the SARS-CoV-2 Omicron Variant Compared with Those of Patients Infected with the Alpha Viral Strain. Front. Public Health 2022, 10, 931480. [Google Scholar] [CrossRef]
- Li, M.; Wang, H.; Tian, L.; Pang, Z.; Yang, Q.; Huang, T.; Fan, J.; Song, L.; Tong, Y.; Fan, H. COVID-19 vaccine development: Milestones, lessons and prospects. Signal Transduct. Target. Ther. 2022, 7, 1–32. [Google Scholar] [CrossRef]
- Halim, M.; Halim, A.; Tjhin, Y. COVID-19 Vaccination Efficacy and Safety Literature Review. J. Clin. Med. Res. 2021, 3, 1–10. [Google Scholar] [CrossRef]
- León, T.M.; Dorabawila, V.; Nelson, L.; Lutterloh, E.; Bauer, U.E.; Backenson, B.; Bassett, M.T.; Henry, H.; Bregman, B.; Midgley, C.M.; et al. COVID-19 Case and Hospitalizations by COVID-19 Vaccination Status and Previous COVID-19 Diagnosis—California and New York, May–November 2021. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Hewins, B.; Rahman, M.; Bermejo-Martin, J.F.; Kelvin, A.A.; Richardson, C.D.; Rubino, S.; Kumar, A.; Ndishimye, P.; Toloue Ostadgavahi, A.; Mahmud-Al-Rafat, A.; et al. Alpha, Beta, Delta, Omicron, and SARS-CoV-2 Breakthrough Cases: Defining Immunological Mechanisms for Vaccine Waning and Vaccine-Variant Mismatch. Front. Virol. 2022, 2, 849936. [Google Scholar] [CrossRef]
- Piñana, J.L.; Vazquez, L.; Calabuig, M.; López-Corral, L.; Martin-Martin, G.; Villalon, L.; Sanz-Linares, G.; Conesa-Garcia, V.; Sanchez-Salinas, A.; Gago, B.; et al. One-year breakthrough SARS-CoV-2 infection and correlates of protection in fully vaccinated hematological patients. Blood Cancer J. 2023, 13, 8. [Google Scholar] [CrossRef] [PubMed]
- Ferdinands, J.M.; Rao, S.; Dixon, B.E.; Mitchell, P.K.; DeSilva, M.B.; Irving, S.A.; Lewis, N.; Natarajan, K.; Stenehjem, E.; Grannis, S.J.; et al. Waning 2-Dose and 3-Dose Effectiveness of mRNA Vaccines Against COVID-19-Associated Emergency Department and Urgent Care Encounters and Hospitalizations Among Adults During Periods of Delta and Omicron Variant Predominance-VISION Network, 10 States, August 2021–January MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 255–263. [Google Scholar]
- Levine-Tiefenbrun, M.; Yelin, I.; Alapi, H.; Herzel, E.; Kuint, J.; Chodick, G.; Gazit, S.; Patalon, T.; Kishony, R. Waning of SARS-CoV-2 booster viral-load reduction effectiveness. Nat. Commun. 2022, 13, 1237. [Google Scholar] [CrossRef]
- Bardosh, K.; de Figueiredo, A.; Gur-Arie, R.; Jamrozik, E.; Doidge, J.; Lemmens, T.; Keshavjee, S.; E Graham, J.; Baral, S. The unintended consequences of COVID-19 vaccine policy: Why mandates, passports and restrictions may cause more harm than good. BMJ Glob. Health 2022, 7, e008684. [Google Scholar] [CrossRef]
- Amanatidou, E.; Gkiouliava, A.; Pella, E.; Serafidi, M.; Tsilingiris, D.; Vallianou, N.G.; Karampela, I.; Dalamaga, M. Breakthrough infections after COVID-19 vaccination: Insights, perspectives and challenges. Metab. Open 2022, 14, e100180. [Google Scholar] [CrossRef]
- Li, M.; Liu, Q.; Wu, D.; Tang, L.; Wang, X.; Yan, T.; An, Z.; Yin, Z.; Gao, G.F.; Wang, F.; et al. Association of COVID-19 Vaccination and Clinical Severity of Patients Infected with Delta or Omicron Variants-China, May 21, 2021–February 28, 2022. China CDC Wkly. 2022, 4, 293–297. [Google Scholar] [CrossRef]
- Wada, N.; Li, Y.; Hino, T.; Gagne, S.; Valtchinov, V.I.; Gay, E.; Nishino, M.; Madore, B.; Guttmann, C.R.; Bond, S.; et al. COVID-19 Vaccination reduced pneumonia severity. Eur. J. Radiol. Open 2022, 9, e100456. [Google Scholar] [CrossRef]
- Lamacchia, G.; Mazzoni, A.; Spinicci, M.; Vanni, A.; Salvati, L.; Peruzzi, B.; Bencini, S.; Capone, M.; Carnasciali, A.; Farahvachi, P.; et al. Clinical and Immunological Features of SARS-CoV-2 Breakthrough Infections in Vaccinated Individuals Requiring Hospitalization. J. Clin. Immunol. 2022, 42, 1379–1391. [Google Scholar] [CrossRef]
- Ben, F.S.; Ghammem, R.; Zammit, N.; Maatouk, A.; Haddad, N.; Haddad, N.; Kachroudi, M.; Rebai, S.; Laadhari, H.; Ghodhbani, M.M.; et al. Risk factors for severe COVID-19 breakthrough infections: An observational longitudinal study. BMC Infect. Dis. 2022, 22, 894. [Google Scholar]
- Granata, V.; Fusco, R.; Villanacci, A.; Magliocchetti, S.; Urraro, F.; Tetaj, N.; Marchioni, L.; Albarello, F.; Campioni, P.; Cristofaro, M.; et al. Imaging Severity COVID-19 Assessment in Vaccinated and Unvaccinated Patients: Comparison of the Different Variants in a High Volume Italian Reference Center. J. Pers. Med. 2022, 12, 955. [Google Scholar] [CrossRef] [PubMed]
- Sakuraba, A.; Luna, A.; Micic, D. Serologic response following SARS-COV2 vaccination in patients with cancer: A systematic review and meta-analysis. J. Hematol. Oncol. 2022, 15, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, T.; Ferguson, N.M.; Nash, S.G.; Webster, H.H.; Flaxman, S.; Andrews, N.; Hinsley, W.; Bernal, J.L.; Kall, M.; Bhatt, S.; et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: A cohort study. Lancet 2022, 399, 1303–1312. [Google Scholar] [CrossRef] [PubMed]
- Rzymski, P.; Pazgan-Simon, M.; Kamerys, J.; Moniuszko-Malinowska, A.; Sikorska, K.; Wernik, J.; Zarębska-Michaluk, D.; Supronowicz, Ł.; Sobala-Szczygieł, B.; Skrzat-Klapaczyńska, A.; et al. Severe Breakthrough COVID-19 Cases during Six Months of Delta Variant (B.1.617.2) Domination in Poland. Vaccines 2022, 10, 557. [Google Scholar] [CrossRef]
- Lee, J.E.; Hwang, M.; Kim, Y.-H.; Chung, M.J.; Sim, B.H.; Chae, K.J.; Yoo, J.Y.; Jeong, Y.J. Imaging and Clinical Features of COVID-19 Breakthrough Infections: A Multicenter Study. Radiology 2022, 303, 682–692. [Google Scholar] [CrossRef]
- Verma, A.; Kumar, I.; Singh, P.K.; Ansari, M.S.; Singh, H.A.; Sonkar, S.; Prakash, A.; Ojha, R.; Shukla, R.C. Initial comparative analysis of pulmonary involvement on HRCT between vaccinated and non-vaccinated subjects of COVID-19. Eur. Radiol. 2022, 32, 4275–4283. [Google Scholar] [CrossRef]
- Hughes, T.D.; Subramanian, A.; Chakraborty, R.; Cotton, S.A.; Herrera, M.D.P.G.; Huang, Y.; Lambert, N.; Pinto, M.D.; Rahmani, A.M.; Sierra, C.J.; et al. The effect of SARS-CoV-2 variant on respiratory features and mortality. Sci. Rep. 2023, 13, 4503. [Google Scholar] [CrossRef]
- Schultz, M.J.; van Meenen, D.M.; Bos, L.D. COVID-19-related acute respiratory distress syndrome: Lessons learned during the pandemic. Lancet Respir. Med. 2022, 10, 1108–1110. [Google Scholar] [CrossRef]
- Aslan, A.; Aslan, C.; Zolbanin, N.M.; Jafari, R. Acute respiratory distress syndrome in COVID-19: Possible mechanisms and therapeutic management. Pneumonia 2021, 13, 1–15. [Google Scholar] [CrossRef]
- Lu, S.; Huang, X.; Liu, R.; Lan, Y.; Lei, Y.; Zeng, F.; Tang, X.; He, H. Comparison of COVID-19 Induced Respiratory Failure and Typical ARDS: Similarities and Differences. Front. Med. 2022, 9, 829771. [Google Scholar] [CrossRef]
- Pfortmueller, C.A.; Spinetti, T.; Urman, R.D.; Luedi, M.M.; Schefold, J.C. COVID-19-associated acute respiratory distress syndrome (CARDS): Current knowledge on pathophysiology and ICU treatment—A narrative review. Best Pract. Res. Clin. Anaesthesiol. 2020, 35, 351–368. [Google Scholar] [CrossRef]
- Gosangi, B.; Rubinowitz, A.N.; Irugu, D.; Gange, C.; Bader, A.; Cortopassi, I. COVID-19 ARDS: A review of imaging features and overview of mechanical ventilation and its complications. Emerg. Radiol. 2022, 29, 23–34, Erratum in Emerg. Radiol. 2022, 29, 225. [Google Scholar] [CrossRef]
- Zhang, B. Gross Pathology in COVID-19. Encyclopedia 2022, 2, 1790–1802. [Google Scholar] [CrossRef]
- Van Do, T.; Manabe, T.; Van Vu, G.; Nong, V.M.; Fujikura, Y.; Phan, D.; Pham, T.T.; Do, C.D.; Doan, T.T.; Nguyen, N.T.; et al. Clinical characteristics and mortality risk among critically ill patients with COVID-19 owing to the B.1.617.2 (Delta) variant in Vietnam: A retrospective observational study. PLoS ONE 2023, 18, e0279713. [Google Scholar] [CrossRef]
- Jeican, I.I.; Inișca, P.; Gheban, D.; Anton, V.; Lazăr, M.; Vică, M.L.; Mironescu, D.; Rebeleanu, C.; Crivii, C.B.; Aluaș, M.; et al. Histopathological Lung Findings in COVID-19 B.1.617.2 SARS-CoV-2 Delta Variant. J. Pers. Med. 2023, 13, 279. [Google Scholar] [CrossRef]
- Van Goethem, N.; Chung, P.Y.J.; Meurisse, M.; Vandromme, M.; De Mot, L.; Brondeel, R.; Stouten, V.; Klamer, S.; Cuypers, L.; Braeye, T.; et al. Clinical Severity of SARS-CoV-2 Omicron Variant Compared with Delta among Hospitalized COVID-19 Patients in Belgium during Autumn and Winter Season 2021–2022. Viruses 2022, 14, 1297. [Google Scholar] [CrossRef]
- Sridhar, P.; Singh, A.; Salomon, N.; Steiger, D.J. Vaccine-Induced Antibody Dependent Enhancement in COVID-19. Chest 2022, 162, A646–A647. [Google Scholar] [CrossRef]
- Yoshimura, Y.; Sasaki, H.; Miyata, N.; Miyazaki, K.; Okudela, K.; Tateishi, Y.; Hayashi, H.; Kawana-Tachikawa, A.; Iwashita, H.; Maeda, K.; et al. An autopsy case of COVID-19-like acute respiratory distress syndrome after mRNA-1273 SARS-CoV-2 vaccination. Int. J. Infect. Dis. 2022, 121, 98–101. [Google Scholar] [CrossRef]
- Melhorn, J.; Achaiah, A.; Conway, F.M.; Thompson, E.M.; Skyllberg, E.W.; Durrant, J.; Hasan, N.A.; Madani, Y.; Naran, P.; Vijayakumar, B.; et al. Pneumomediastinum in COVID-19: A phenotype of severe COVID-19 pneumonitis? The results of the United Kingdom (POETIC) survey. Eur. Respir. J. 2022, 60, 2102522. [Google Scholar] [CrossRef]
- Khaire, N.; Deshmukh, S.; Agarwal, E.; Mahale, N.; Khaladkar, S.; Desai, S.; Kulkarni, A. “Pneumomediastinum: A marker of severity in COVID-19 disease”. Heliyon 2023, 9, e12981. [Google Scholar] [CrossRef] [PubMed]
- Tetaj, N.; Garotto, G.; Albarello, F.; Mastrobattista, A.; Maritti, M.; Stazi, G.V.; Marini, M.C.; Caravella, I.; Macchione, M.; De Angelis, G.; et al. Incidence of Pneumothorax and Pneumomediastinum in 497 COVID-19 Patients with Moderate–Severe ARDS over a Year of the Pandemic: An Observational Study in an Italian Third Level COVID-19 Hospital. J. Clin. Med. 2021, 10, 5608. [Google Scholar] [CrossRef] [PubMed]
- Kecskes, G.; Szabo, A.; Sutori, D.; Maroti, P.; Marovics, G.; Molnar, T.F. Pneumothorax/pneumomediastinum and pre-existing lung pathology in ventilated COVID-19 patients: A cohort study. J. Thorac. Dis. 2022, 14, 4733–4740. [Google Scholar] [CrossRef] [PubMed]
- Dubey, R.; Sen, K.K.; Mishra, A. Barotrauma and its complications in COVID-19 patients: A retrospective study at tertiary care hospital of Eastern India. Bull. Natl. Res. Cent. 2022, 46, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Capaccione, K.M.; D’souza, B.; Leb, J.; Luk, L.; Duong, J.; Tsai, W.-Y.; Navot, B.; Dumeer, S.; Mohammed, A.; Salvatore, M.M. Pneumothorax rate in intubated patients with COVID-19. Acute Crit. Care 2021, 36, 81–84. [Google Scholar] [CrossRef]
- McGuinness, G.; Zhan, C.; Rosenberg, N.; Azour, L.; Wickstrom, M.; Mason, D.M.; Thomas, K.M.; Moore, W.H. IncreaIncidence of Barotrauma in Patients with COVID-19 on Invasive Mechanical Ventilation. Radiology 2020, 297, E252–E262. [Google Scholar] [CrossRef]
- Martinelli, A.W.; Ingle, T.; Newman, J.; Nadeem, I.; Jackson, K.; Lane, N.D.; Melhorn, J.; Davies, H.E.; Rostron, A.J.; Adeni, A.; et al. COVID-19 and pneumothorax: A multicentre retrospective case series. Eur. Respir. J. 2020, 56, 2002697. [Google Scholar] [CrossRef]
- Rodriguez-Arciniega, T.G.; Sierra-Diaz, E.; Flores-Martinez, J.A.; Alvizo-Perez, M.E.; Lopez-Leal, I.N.; Corona-Nakamura, A.L.; Castellanos-Garcia, H.E.; Bravo-Cuellar, A. Frequency and Risk Factors for Spontaneous Pneumomediastinum in COVID-19 Patients. Front. Med. 2021, 8, 662358. [Google Scholar] [CrossRef]
- Zantah, M.; Castillo, E.D.; Townsend, R.; Dikengil, F.; Criner, G.J. Pneumothorax in COVID-19 disease- incidence and clinical characteristics. Respir. Res. 2020, 21, 1–9. [Google Scholar] [CrossRef]
- Shahsavarinia, K.; Rahvar, G.; Soleimanpour, H.; Saadati, M.; Vahedi, L.; Mahmoodpoor, A. Spontaneous pneumomediastinum, pneumothorax and subcutaneous emphysema in critically ill COVID-19 patients: A systematic review. Pak. J. Med. Sci. 2022, 38, 730–735. [Google Scholar] [CrossRef]
- Hamouri, S.; AlQudah, M.; Albawaih, O.; Al-Zoubi, N.; Syaj, S. Spontaneous pneumothorax, pneumomediastinum and subcutaneous emphysema in non-ventilated COVID-19 patients. Futur. Sci. OA 2022, 8, FSO771. [Google Scholar] [CrossRef]
- Shaikh, N.; Al Ameri, G.; Shaheen, M.; Abdaljawad, W.I.; Al Wraidat, M.; Al Alawi, A.A.S.; Ali, H.S.; Mohamed, A.S.; Daeri, H.; Khatib, M.Y.; et al. Spontaneous pneumomediastinum and pneumothorax in COVID-19 patients: A tertiary care experience. Health Sci. Rep. 2021, 4, e339. [Google Scholar] [CrossRef]
- Gandolfo, C.; Bonfiglio, M.; Spinetto, G.; Ferraioli, G.; Barlascini, C.; Nicolini, A.; Solidoro, P. Pneumomediastinum associated with severe pneumonia related to COVID-19: Diagnosis and management. Minerva Medica 2022, 112, 779–785. [Google Scholar] [CrossRef]
- Espinosa, C.; Morente, L.M.; Mansour, E.H.; Yousefzadeh, M.L.; Muzaffarr, Z.M.; Salguero, D.; Vianna, S.D.; Quesada, L.D.; Poli, S.; Garcia, H. Predictors of Spontaneous Pneumomediastinum in Patients with COVID-19 and Ards on High-Flow Nasal Cannula. Chest 2022, 162, A1360–A1361. [Google Scholar] [CrossRef]
- Coppola, M.G.; Lugarà, M.; Tamburrini, S.; Madonna, P.; Panico, C.; Noschese, G.; Pone, E. Pneumomediastinum and Pneumothorax as Relevant Complications of Sub-Intensive Care of Patients with COVID-19: Description of a Case Series. Medicina 2021, 57, 919. [Google Scholar] [CrossRef]
- Palumbo, D.; Campochiaro, C.; Belletti, A.; Marinosci, A.; Dagna, L.; Zangrillo, A.; De Cobelli, F. Pneumothorax/pneumomediastinum in non-intubated COVID-19 patients: Differences between first and second Italian pandemic wave. Eur. J. Intern. Med. 2021, 88, 144–146. [Google Scholar] [CrossRef]
- Xiang, T.; Fang, J.; Cheng, T.; Li, Z.; Wu, D.; Zhang, S.; Ge, S.; Zhang, W. Case report: Severe pneumonia and pneumomediastinum in a previously robust adolescent caused by Omicron BA.5.2. Front. Med. 2023, 10, e1132630. [Google Scholar] [CrossRef]
- Kanne, J.P.; Little, B.P.; Schulte, J.J.; Haramati, A.; Haramati, L.B. Long-term Lung Abnormalities Associated with COVID-19 Pneumonia. Radiology 2023, 306, e221806. [Google Scholar] [CrossRef]
- Han, X.; Fan, Y.; Alwalid, O.; Zhang, X.; Jia, X.; Zheng, Y.; Shi, H. Fibrotic Interstitial Lung Abnormalities at 1-year Follow-up CT after Severe COVID-19. Radiology 2021, 301, E438–E440. [Google Scholar] [CrossRef]
- Watanabe, A.; So, M.; Iwagami, M.; Fukunaga, K.; Takagi, H.; Kabata, H.; Kuno, T. One-year follow-up CT findings in COVID-19 patients: A systematic review and meta-analysis. Respirology 2022, 27, 605–616. [Google Scholar] [CrossRef]
- Bocchino, M.; Lieto, R.; Romano, F.; Sica, G.; Bocchini, G.; Muto, E.; Capitelli, L.; Sequino, D.; Valente, T.; Fiorentino, G.; et al. Chest CT–based Assessment of 1-year Outcomes after Moderate COVID-19 Pneumonia. Radiology 2022, 305, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Cocconcelli, E.; Bernardinello, N.; Giraudo, C.; Castelli, G.; Giorgino, A.; Leoni, D.; Petrarulo, S.; Ferrari, A.; Saetta, M.; Cattelan, A.; et al. Characteristics and Prognostic Factors of Pulmonary Fibrosis After COVID-19 Pneumonia. Front. Med. 2022, 8, 823600. [Google Scholar] [CrossRef] [PubMed]
- Wismüller, A.; Dsouza, A.M.; Abidin, A.Z.; Vosoughi, M.A.; Gange, C.; Cortopassi, I.O.; Bozovic, G.; Bankier, A.A.; Batra, K.; Chodakiewitz, Y.; et al. Early-stage COVID-19 pandemic observations on pulmonary embolism using nationwide multi-institutional data harvesting. NPJ Digit. Med. 2022, 5, 1–9. [Google Scholar] [CrossRef]
- Mouzarou, A.; Ioannou, M.; Leonidou, E.; Chaziri, I. Pulmonary Embolism in Post-CoviD-19 Patients, a Literature Review: Red Flag for Increased Awareness? SN Compr. Clin. Med. 2022, 4, 190. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, C. Thromboembolic Findings in COVID-19 Autopsies: Pulmonary Thrombosis or Embolism? Ann. Intern. Med. 2020, 173, 394–395. [Google Scholar] [CrossRef]
- Gorog, D.A.; Storey, R.F.; Gurbel, P.A.; Tantry, U.S.; Berger, J.S.; Chan, M.Y.; Duerschmied, D.; Smyth, S.S.; Parker, W.A.; Ajjan, R.A.; et al. Current and novel biomarkers of thrombotic risk in COVID-19: A Consensus Statement from the International COVID-19 Thrombosis Biomarkers Colloquium. Nat. Rev. Cardiol. 2022, 19, 475–495. [Google Scholar] [CrossRef]
- Wichmann, D.; Sperhake, J.P.; Lütgehetmann, M.; Steurer, S.; Edler, C.; Heinemann, A.; Heinrich, F.; Mushumba, H.; Kniep, I.; Schröder, A.S.; et al. Autopsy Findings and Venous Thromboembolism in Patients with COVID-19: A Prospective Cohort Study. Ann. Intern. Med. 2020, 173, 268–277. [Google Scholar] [CrossRef]
- Lax, S.F.; Skok, K.; Zechner, P.; Kessler, H.H.; Kaufmann, N.; Koelblinger, C.; Vander, K.; Bargfrieder, U.; Trauner, M. Pulmonary Arterial Thrombosis in COVID-19 with Fatal Outcome: Results from a Prospective, Single-Center, Clinicopathologic Case Series. Ann. Intern. Med. 2020, 173, 350–361. [Google Scholar] [CrossRef]
- Sakr, Y.; Giovini, M.; Leone, M.; Pizzilli, G.; Kortgen, A.; Bauer, M.; Tonetti, T.; Duclos, G.; Zieleskiewicz, L.; Buschbeck, S.; et al. Pulmonary embolism in patients with coronavirus disease-2019 (COVID-19) pneumonia: A narrative review. Ann. Intensive Care 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Močibob, L.; Šušak, F.; Šitum, M.; Višković, K.; Papić, N.; Vince, A. COVID-19 and Pulmonary Thrombosis—An Unresolved Clinical Puzzle: A Single-Center Cohort Study. J. Clin. Med. 2022, 11, 7049. [Google Scholar] [CrossRef]
- Katsoularis, I.; Fonseca-Rodríguez, O.; Farrington, P.; Jerndal, H.; Lundevaller, E.H.; Sund, M.; Lindmark, K.; Connolly, A.M.F. Risks of deep vein thrombosis, pulmonary embolism, and bleeding after COVID-19: Nationwide self- controlled cases series and matched cohort study. BMJ 2022, 377, e069590. [Google Scholar] [CrossRef]
- Manzur-Pineda, K.; O’neil, C.F.; Bornak, A.; Lalama, M.J.; Shao, T.; Kang, N.; Kennel-Pierre, S.; Tabbara, M.; Velazquez, O.C.; Rey, J. COVID-19-related thrombotic complications experience before and during delta wave. J. Vasc. Surg. 2022, 76, 1374–1382.e1. [Google Scholar] [CrossRef]
- Law, N.; Chan, J.; Kelly, C.; Auffermann, W.F.; Dunn, D.P. Incidence of pulmonary embolism in COVID-19 infection in the ED: Ancestral, Delta, Omicron variants and vaccines. Emerg. Radiol. 2022, 29, 625–629. [Google Scholar] [CrossRef]
- Ho, F.K.; Pell, J.P. Thromboembolism and bleeding after COVID-19. BMJ 2022, 377, o817. [Google Scholar] [CrossRef]
- Murphy, M.C.; Little, B.P. Chronic Pulmonary Manifestations of COVID-19 Infection: Imaging Evaluation. Radiology 2023, 307, e222379. [Google Scholar] [CrossRef]
- Ravaglia, C.; Doglioni, C.; Chilosi, M.; Piciucchi, S.; Dubini, A.; Rossi, G.; Pedica, F.; Puglisi, S.; Donati, L.; Tomassetti, S.; et al. Clinical, radiological and pathological findings in patients with persistent lung disease following SARS-CoV-2 infection. Eur. Respir. J. 2022, 60, 2102411. [Google Scholar] [CrossRef]
- Bonaffini, P.A.; Franco, P.N.; Bonanomi, A.; Giaccherini, C.; Valle, C.; Marra, P.; Norsa, L.; Marchetti, M.; Falanga, A.; Sironi, S. Ischemic and hemorrhagic abdominal complications in COVID-19 patients: Experience from the first Italian wave. Eur. J. Med. Res. 2022, 27, 1–9. [Google Scholar] [CrossRef]
- Peshevska-Sekulovska, M.; Boeva, I.; Sekulovski, M.; Zashev, M.; Peruhova, M. Gastrointestinal Ischemia—Stumbling Stone in COVID-19 Patients. Gastroenterol. Insights 2022, 13, 206–217. [Google Scholar] [CrossRef]
- Norsa, L.; Bonaffini, P.A.; Caldato, M.; Bonifacio, C.; Sonzogni, A.; Indriolo, A.; Valle, C.; Furfaro, F.; Bonanomi, A.; Franco, P.N.; et al. Intestinal ischemic manifestations of SARS-CoV-2: Results from the ABDOCOVID multicentre study. World J. Gastroenterol. 2021, 27, 5448–5459. [Google Scholar] [CrossRef]
- Singh, B.; Kaur, P. COVID-19 and acute mesenteric ischemia: A review of literature. Hematol. Transfus. Cell Ther. 2020, 43, 112–116. [Google Scholar] [CrossRef]
- Fathy, A.; Rizk, A.; Elnekeidy, A.; Gharraf, H.S.; Abdelgawad, M.S.; Samir, A. Imaging of COVID-19 vasculopathy from head to toe: Egyptian collective experience after 2 years of the pandemic. Egypt. J. Radiol. Nucl. Med. 2022, 53, 1–21. [Google Scholar] [CrossRef]
- Evrev, D.; Sekulovski, M.; Gulinac, M.; Dobrev, H.; Velikova, T.; Hadjidekov, G. Retroperitoneal and abdominal bleeding in anticoagulated COVID-19 hospitalized patients: Case series and brief literature abdominal bleeding in anticoagulated COVID-19 hospitalized patients: Case series and brief literature review. World J. Clin. Cases 2023, 11, 1528–1548. [Google Scholar] [CrossRef] [PubMed]
- Sposato, B.; Croci, L.; Di Tomassi, M.; Puttini, C.; Olivieri, C.; Alessandri, M.; Ronchi, M.C.; Donati, E.; Garcea, A.; Brazzi, A.; et al. Spontaneous abdominal bleeding associated with SARS-CoV-2 infection: Causality or coincidence? Acta Biomed. 2021, 92, e2021199. [Google Scholar] [PubMed]
- Mahmoudabadi, H.Z.; Hadadi, A.; Fattahi, M.R.; Kafan, S.; Ashouri, M.; Allahbeigi, R.; Hajebi, R. Rectus Sheath Hematoma in COVID-19 Patients as a Mortal Complication: A Retrospective Report. Int. J. Clin. Pract. 2022, 2022, 7436827. [Google Scholar] [CrossRef]
- Maruyama, S.; Wada, D.; Oishi, T.; Saito, F.; Yoshiya, K.; Nakamori, Y.; Kuwagata, Y. A descriptive study of abdominal complications in patients with mild COVID-19 presenting to the emergency department: A single-center experience in Japan during the omicron variant phase. BMC Gastroenterol. 2023, 23, 43. [Google Scholar] [CrossRef]
- Sarkardeh, M.; Meftah, E.; Mohammadzadeh, N.; Koushki, J.; Sadrzadeh, Z. COVID-19 and Intestinal Ischemia: A Multicenter Case Series. Front. Med. 2022, 9, 879996. [Google Scholar] [CrossRef]
- Bhayana, R.; Som, A.; Li, M.D.; Carey, D.E.; Anderson, M.A.; Blake, M.A.; Catalano, O.; Gee, M.S.; Hahn, P.F.; Harisinghani, M.; et al. Abdominal Imaging Findings in COVID-19: Preliminary Observations. Radiology 2020, 297, E207–E215. [Google Scholar] [CrossRef]
- Makker, J.; Mantri, N.; Patel, H.K.; Abbas, H.; Baiomi, A.; Sun, H.; Choi, Y.; Chilimuri, S.; Nayudu, S.K. The Incidence and Mortality Impact of Gastrointestinal Bleeding in Hospitalized COVID-19 Patients. Clin. Exp. Gastroenterol. 2021, 14, 405–411. [Google Scholar] [CrossRef]
- Caruso, D.; Zerunian, M.; Pucciarelli, F.; Lucertini, E.; Bracci, B.; Polidori, T.; Guido, G.; Polici, M.; Rucci, C.; Iannicelli, E.; et al. Imaging of abdominal of abdominal complications of COVID-19 infection. BJR Open 2021, 2, 20200052. [Google Scholar] [CrossRef]
- Ojha, V.; Mani, A.; Mukherjee, A.; Kumar, S.; Jagia, P. Mesenteric ischemia in patients with COVID-19: An updated systematic review of abdominal CT findings in 75 patients. Abdom. Imaging 2021, 47, 1565–1602. [Google Scholar] [CrossRef]
- Boraschi, P.; Giugliano, L.; Mercogliano, G.; Donati, F.; Romano, S.; Neri, E. Abdominal and gastrointestinal manifestations in COVID-19 patients: Is imaging useful? World J. Gastroenterol. 2021, 27, 4143–4159. [Google Scholar] [CrossRef]
- Keshavarz, P.; Rafiee, F.; Kavandi, H.; Goudarzi, S.; Heidari, F.; Gholamrezanezhad, A. Ischemic gastrointestinal complications of COVID-19: A systematic review on imaging presentation. Clin. Imaging 2020, 73, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Sharma, O.; Srikanth, K.; Mishra, R.; Tandon, A.; Rajput, D. Review of Mesenteric Ischemia in COVID-19 Patients. Indian J. Surg. 2022, 85, 313–321. [Google Scholar] [CrossRef]
- Reginelli, A.; Genovese, E.; Cappabianca, S.; Iacobellis, F.; Berritto, D.; Fonio, P.; Coppolino, F.; Grassi, R. Intestinal Ischemia: US-CT findings correlations. Crit. Ultrasound J. 2013, 5, S7. [Google Scholar] [CrossRef] [Green Version]
- Paul, T.; Joy, A.R.; Alsoub, H.A.R.S.; Parambil, J.V. Case Report: Ischemic Colitis in Severe COVID-19 Pneumonia: An Unforeseen Gastrointestinal Complication. Am. J. Trop. Med. Hyg. 2021, 104, 63–65. [Google Scholar] [CrossRef]
- Uhlenhopp, D.J.; Ramachandran, R.; Then, E.; Parvataneni, S.; Grantham, T.; Gaduputi, V. COVID-19-Associated Ischemic Colitis: A Rare Manifestation of COVID-19 Infection—Case Report and Review. J. Investig. Med. High Impact Case Rep. 2022, 10, 1–6. [Google Scholar] [CrossRef]
- Krejčová, I.; Berková, A.; Kvasnicová, L.; Vlček, P.; Veverková, L.; Penka, I.; Zoufalý, D.; Červeňák, V. Ischemic Colitis in a Patient with Severe COVID-19 Pneumonia. Case Rep. Gastroenterol. 2022, 16, 526–534. [Google Scholar] [CrossRef]
- Alratrout, H.; Debroux, E. Acute right-sided ischemic colitis in a COVID-19 patient: A case report and review of the literature. J. Med. Case Rep. 2022, 16, 1–5. [Google Scholar] [CrossRef]
- Cui, M.-H.; Hou, X.-L.; Liu, J.-Y. Ischemic colitis after receiving the second dose of a COVID-19 inactivated vaccine: A case report. World J. Clin. Cases 2022, 10, 3866–3871. [Google Scholar] [CrossRef]
- Mönkemüller, K.; Abdullayeva, E.; Manovski, K.; Cacho-Díaz, M. Ischemic colitis after COVID-19 mRNA vaccine. Endoscopy 2022, 54, E765–E766. [Google Scholar] [CrossRef]
- Castro, G.R.A.; Collaço, I.A.; Bosco, C.L.B.D.; Corrêa, G.G.; Bosco, G.B.D.; Corrêa, G.L. Splenic infarction as a complication of covid-19 in a patient without respiratory symptoms: A case report and literature review. IDCases 2021, 24, e01062. [Google Scholar] [CrossRef] [PubMed]
- Prentice, G.; Wilson, S.; Coupland, A.; Bicknell, S. Complete splenic infarction in association with COVID-19. BMJ Case Rep. 2021, 14, e246274. [Google Scholar] [CrossRef] [PubMed]
- Al Suwaidi, S.; Alakasheh, B.J.; Al-Ozaibi, L.S. Splenic Infarction in a COVID-19 Patient without Respiratory Symptoms. Dubai Med. J. 2022, 5, 74–77. [Google Scholar] [CrossRef]
- Childers, J.; Do, T.V.C.; Smith, F.; Vangara, A.; Ganti, S.S.; Akella, R. Incidental and Asymptomatic Splenic Infarction and Infrarenal Thrombus in a COVID-19 Patient. Cureus 2022, 14, e26555. [Google Scholar] [CrossRef] [PubMed]
- Post, A.; den Deurwaarder, E.S.G.; Bakker, S.J.L.; de Haas, R.J.; van Meurs, M.; Gansevoort, R.T.; Berger, S.P. Kidney Infarction in Patients with COVID-19. Am. J. Kidney Dis. 2020, 76, 431–435. [Google Scholar] [CrossRef]
- Jana, K.; Janga, K.C.; Greenberg, S.; Kumar, K. Bilateral renal infarction with COVID-19 pneumonia: A case report. Oxf. Med. Case Rep. 2021, 2021, omab121. [Google Scholar] [CrossRef]
- Murray, N.P.; Fuentealba, C.; Reyes, E.; Salazar, A. Renal infarction associated with asymptomatic COVID-19 infection. Hematol. Transfus. Cell Ther. 2021, 43, 353–356. [Google Scholar] [CrossRef]
- Plouffe, B.; Van Hooren, T.; Barton, M.; Nashid, N.; Demirkaya, E.; Norozi, K.; Rachinsky, I.; Delport, J.; Knauer, M.; Tole, S.; et al. Renal Infarcts—A Perplexing Case in the Middle of the COVID-19 Pandemic. Front. Pediatr. 2021, 9, 669453. [Google Scholar] [CrossRef]
- Gentili, G.; Pérez, P.L.; Laplumé-Elizalde, E.; España, S. Kidney infarction in patient with covid-19: Clinical case. Am. J. Kidney Dis. 2022, 82, 788. [Google Scholar] [CrossRef]
- Al-Mashdali, A.F.; Alwarqi, A.F.; Elawad, S.M. Simultaneous renal infarction and splenic infarction as a possible initial manifestation of COVID-19: A case report. Clin. Case Rep. 2021, 9, 4819. [Google Scholar] [CrossRef]
- Brem, F.L.; Abu Al Tayef, T.; Rasras, H.; El Mahi, O.; El Ouafi, N.; Zakaria, B. Concomitant renal and splenic infarctions in a COVID-19-patient with a catastrophic thrombotic syndrome. Radiol. Case Rep. 2022, 17, 4030–4033. [Google Scholar] [CrossRef]
- Ramanathan, M.; Chueng, T.; Fernandez, E.; Gonzales-Zamora, J. Concomitant renal and splenic infarction as a complication of COVID-19: A case report and literature review. Infez. Med. 2020, 28, 611–615. [Google Scholar]
- Mavraganis, G.; Ioannou, S.; Kallianos, A.; Rentziou, G.; Trakada, G. A COVID-19 Patient with Simultaneous Renal Infarct, Splenic Infarct and Aortic Thrombosis during the Severe Disease. Healthcare 2022, 10, 150. [Google Scholar] [CrossRef]
- Dimitriou, I.; Christodoulou, N.; Chatzimargaritis, K.; Kaikis, A.; Kasti, E.; Triantos, G. Splenic Artery Infarct Requiring Surgery: A Rare Complication of COVID-19 Infection. Case Rep. Surg. 2022, 2022, e3391405. [Google Scholar] [CrossRef]
- Javaid, U.; Young, P.; Gill, G.; Bhargava, P. Acute complete splenic infarction secondary to COVID-19 infection. Radiol. Case Rep. 2022, 17, 1402–1406. [Google Scholar] [CrossRef]
- Graça, L.L.; Amaral, M.J.; Serôdio, M.; Costa, B. Extensive thrombosis after COVID-19 vaccine: Cause or coincidence? BMJ Case Rep. 2021, 14, e244878. [Google Scholar] [CrossRef]
- Anderson, A.; Seddon, M.; Shahzad, K.; Lunevicius, R. Post-COVID-19 vaccination occurrence of splenic infarction due to arterial thrombosis. BMJ Case Rep. 2021, 14, e243846. [Google Scholar] [CrossRef]
- See, I.; Lale, A.; Marquez, P.; Streiff, M.B.; Wheeler, A.P.; Tepper, N.K.; Woo, E.J.; Broder, K.R.; Edwards, K.M.; Gallego, R.; et al. Case series of thrombosis with thrombocytopenia syndrome after COVID-19 vaccination—United States, December 2020 to August 2021. Ann. Intern. Med. 2022, 175, 513–522. [Google Scholar] [CrossRef]
- Gabarre, P.; Dumas, G.; Dupont, T.; Darmon, M.; Azoulay, E.; Zafrani, L. Acute kidney injury in critically ill patients with COVID-19. Intensiv. Care Med. 2020, 46, 1339–1348. [Google Scholar] [CrossRef]
- Espinosa, W.A.V.; Argueta, A.S.; Tandazo, V.A.H.; Espinosa, C.F.V. A Case Report of a Young Female with Renal Infarction Secondary to Breakthrough COVID Infection. Cureus 2022, 14, 25527. [Google Scholar] [CrossRef]
- Mahboubi-Fooladi, Z.; Arabi, K.P.; Khazaei, M.; Nekooghadam, S.; Shadbakht, B.; Moharamzad, Y.; Taheri, M.S. Parenteral Anticoagulation and Retroperitoneal Hemorrhage in COVID-19: Case Report of Five Patients. SN ComPract. Clin. Med. 2021, 3, 2005–2010. [Google Scholar] [CrossRef] [PubMed]
- Dubovský, M.; Hajská, M.; Panyko, A.; Vician, M. Severe Retroperitoneal Hemorrhage in a COVID-19 Patient on a Therapeutic Dose of Low Molecular Weight Heparin: A Case Report. Cureus 2022, 14, 26275. [Google Scholar] [CrossRef] [PubMed]
- Yeoh, W.C.; Lee, K.T.; Zainul, N.H.; Alwi, S.B.S.; Low, L.L. Spontaneous retroperitoneal hematoma: A rare bleeding occurrence in COVID-19. Oxf. Med. Case Rep. 2021, 2021, omab081. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Colombo, J.P.; Chandna, S.; Rana, H. Life-Threatening Retroperitoneal Hematoma in a Patient with COVID-19. Case Rep. Hematol. 2021, 2021, e8774010. [Google Scholar] [CrossRef]
- Belfiore, M.P.; Russo, G.M.; Gallo, L.; Atripaldi, U.; Tamburrini, S.; Caliendo, V.; Impieri, L.; Del Canto, M.T.; Ciani, G.; Parrella, P.; et al. Secondary Complications in COVID-19 Patients: A Case Series. Tomography 2022, 8, 1836–1850. [Google Scholar] [CrossRef]



























| Typical Appearance | GGOs with a crazy-paving pattern and consolidations in a peripheral and posterior or central-peripheral distribution; multilobar involvement; vascular enlargement, the halo and reversed halo sign; subpleural and parenchymal bands; and architectural distortion. They were predominant since the Delta wave. |
| Indeterminate Appearance | GGOs and consolidations with a unilateral, central, or upper-lobe distribution. |
| Atypical Appearance | Lobar consolidation, lung nodules or masses, miliary patterns, tree-in-bud patterns, cavitation, pleural effusion, central distribution, and lymphadenopathy. Atypical appearances were predominant during the Omicron waves. |
| Chest CT Findings According to the Virus Variant | Virus Variant | Authors | Chest CT Features |
|---|---|---|---|
| Typical appearance | From the wild-type to the Delta variant. | Askani et al., 2022 [67] | The Delta variant presented more frequent typical features with more extensive lung involvement than the Omicron variant. The Omicron variant was more frequently associated with the absence of pneumonia. |
| Inui et al., 2021 [68] | Typical findings were characteristic of the wild type to the Delta variant. GGOs with consolidation and repair changes were more frequent in the Delta variant. The Delta variant also showed more rapid pneumonia progression than the wild-type and Alpha variants. | ||
| Ito et al., 2022 [54] | Peripheral GGO distributions were more frequent in the Alpha and Delta variants than the Omicron variant. | ||
| Kirka et al., 2022 [27] | Typical features were found in 40.8% of patients with the wild-type variant and 1.7% of patients with the Omicron variant. | ||
| Lee et al., 2023 [28] | Typical CT patterns were more frequent in the Delta group (76%) than in those with the Omicron variant (42%). | ||
| Yang et al., 2022 [78] | Of patients with the Alpha variant, 86.84% presented typical COVID-19 pneumonia CT features. | ||
| Yoon et al., 2023 [69] | Only 32% of patients with the Omicron variant presented typical findings, compared with 57% of the Delta variant cases. | ||
| Indeterminate appearance | Omicron variant | Ito et al., 2022 [54] | Cluster-like GGOs in the Omicron wave. |
| Atypical appearance | Omicron Variant | Hang et al., 2023 [75] | Patients infected with the Omicron variant presented a significantly higher prevalence of nodules, tree-in-bud patterns, and halo signs than patients with the original strain. |
| Ito et al., 2022 [54] | Prevalence of non-peripheral distribution with random distribution during the Omicron wave. | ||
| Lee et al., 2023 [28] | Peribroncovascular pneumonia with the Omicron variant and lower rates of severe pneumonia than the Delta variant. | ||
| Tsakok et al., 2023 [77] | Patients with an Omicron infection presented a greater frequency of bronchial wall thickening but less severe disease compared with the Delta variant. | ||
| Yang et al., 2022 [78] | Only 1.3% of patients infected with the Omicron variant had foci of pneumonia, and the GGOs were unilateral and centrilobular. | ||
| Yoon et al., 2023 [69] | Peribroncovascular GGOs or centrolobular foci during the Omicron wave with less extensive pneumonia. |
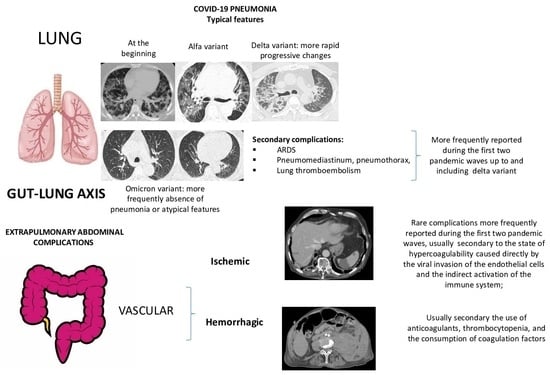
| Lung Complications | Extrapulmonary Vascular Abdominal Complications | ||
|---|---|---|---|
| COVID-19 Pneumonia | Secondary Lung Complications | Ischemic | Hemorrhagic |
| Typical findings of interstitial pneumonia with peripheral or peripheral central distribution from the wild/type variant since Delta variant |
| Intestinal ischemia, ischemic colitis; parenchymal organ ischemia (Spleen and renal infarcts); all of these complications were rare and reported mainly during the first two waves of the pandemic | Spontaneous hematoma were more frequently located in the retroperitoneum and muscular abdominal wall; rare complications and more frequently reported during the first two pandemic waves as a consequence of anticoagulant treatment and consumption of coagulations factors |
| Absence of pneumonia or atypical findings during the Omicron wave | |||
| Possible severe forms of COVID-19 pneumonia in breakthrough infections in the elderly and in patients with an immunosuppressive state | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brogna, B.; Bignardi, E.; Megliola, A.; Laporta, A.; La Rocca, A.; Volpe, M.; Musto, L.A. A Pictorial Essay Describing the CT Imaging Features of COVID-19 Cases throughout the Pandemic with a Special Focus on Lung Manifestations and Extrapulmonary Vascular Abdominal Complications. Biomedicines 2023, 11, 2113. https://doi.org/10.3390/biomedicines11082113
Brogna B, Bignardi E, Megliola A, Laporta A, La Rocca A, Volpe M, Musto LA. A Pictorial Essay Describing the CT Imaging Features of COVID-19 Cases throughout the Pandemic with a Special Focus on Lung Manifestations and Extrapulmonary Vascular Abdominal Complications. Biomedicines. 2023; 11(8):2113. https://doi.org/10.3390/biomedicines11082113
Chicago/Turabian StyleBrogna, Barbara, Elio Bignardi, Antonia Megliola, Antonietta Laporta, Andrea La Rocca, Mena Volpe, and Lanfranco Aquilino Musto. 2023. "A Pictorial Essay Describing the CT Imaging Features of COVID-19 Cases throughout the Pandemic with a Special Focus on Lung Manifestations and Extrapulmonary Vascular Abdominal Complications" Biomedicines 11, no. 8: 2113. https://doi.org/10.3390/biomedicines11082113
APA StyleBrogna, B., Bignardi, E., Megliola, A., Laporta, A., La Rocca, A., Volpe, M., & Musto, L. A. (2023). A Pictorial Essay Describing the CT Imaging Features of COVID-19 Cases throughout the Pandemic with a Special Focus on Lung Manifestations and Extrapulmonary Vascular Abdominal Complications. Biomedicines, 11(8), 2113. https://doi.org/10.3390/biomedicines11082113