Immunomorphogenesis in Degenerative Disc Disease: The Role of Proinflammatory Cytokines and Angiogenesis Factors
Abstract
:1. Introduction
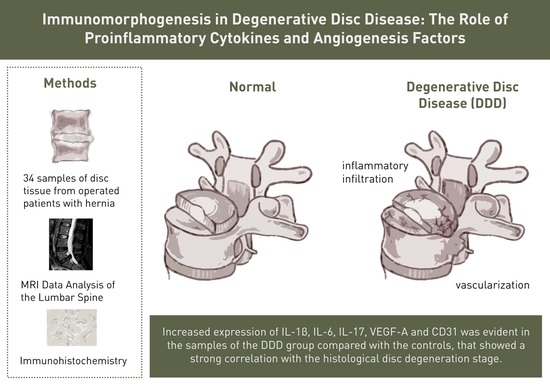
2. Materials and Methods
2.1. Patients
2.2. Tissue Samples
2.3. Histological Examination
2.4. Immunohistochemistry
2.5. Morphometric Analysis
2.6. MRI Data Analysis of the Lumbar Spine
2.7. Statistical Analysis
3. Results
3.1. Clinical Characteristics of Patients and MRI Data
3.2. Results of MRI of the Lumbar Spine
3.3. Histological and Morphometric Data
3.4. Immunohistochemical Analysis
4. Discussion
5. Limitation of Our Study
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ochsmann, E.B.; Escobar Pinzón, C.L.; Letzel, S.; Kraus, T.; Michaelis, M.; Muenster, E. Prevalence of Diagnosis and Direct Treatment Costs of Back Disorders in 644,773 Children and Youths in Germany. BMC Musculoskelet. Disord. 2010, 11, 193. [Google Scholar] [CrossRef] [Green Version]
- Karademir, M.; Eser, O.; Karavelioglu, E. Adolescent Lumbar Disc Herniation: Impact, Diagnosis, and Treatment. J. Back Musculoskelet. Rehabil. 2017, 30, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Diamond, S.; Borenstein, D. Chronic Low Back Pain in a Working-Age Adult. Best Pract. Res. Clin. Rheumatol. 2006, 20, 707–720. [Google Scholar] [CrossRef]
- Meucci, R.D.; Fassa, A.G.; Paniz, V.M.; Silva, M.C.; Wegman, D.H. Increase of Chronic Low Back Pain Prevalence in a Medium-Sized City of Southern Brazil. BMC Musculoskelet. Disord. 2013, 14, 155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersson, G.B. Epidemiological Features of Chronic Low-Back Pain. Lancet 1999, 354, 581–585. [Google Scholar] [CrossRef]
- Cheng, C.; Xu, Z.; Yang, C.; Wu, X. Lactylation driven by lactate metabolism in the disc accelerates intervertebral disc degeneration: A hypothesis. Med. Hypotheses 2022, 159, 110758. [Google Scholar] [CrossRef]
- Boos, N.; Weissbach, S.; Rohrbach, H.; Weiler, C.; Spratt, K.F.; Nerlich, A.G. Classification of age-related changes in lumbar intervertebral discs: 2002 Volvo Award in basic science. Spine 2002, 27, 2631–2644. [Google Scholar] [CrossRef]
- Sadowska, A.; Touli, E.; Hitzl, W.; Greutert, H.; Ferguson, S.J.; Wuertz-Kozak, K.; Hausmann, O.N. Inflammaging in cervical and lumbar degenerated intervertebral discs: Analysis of proinflammatory cytokine and TRP channel expression. Eur. Spine J. 2018, 27, 564–577. [Google Scholar] [CrossRef] [Green Version]
- Shnayder, N.A.; Ashhotov, A.V.; Trefilova, V.V.; Nurgaliev, Z.A.; Novitsky, M.A.; Vaiman, E.E.; Petrova, M.M.; Nasyrova, R.F. Cytokine Imbalance as a Biomarker of Intervertebral Disk Degeneration. Int. J. Mol. Sci. 2023, 24, 2360. [Google Scholar] [CrossRef]
- Schnake, K.J.; Putzier, M.; Haas, N.P.; Kandziora, F. Mechanical Concepts for Disc Regeneration. Eur. Spine J. 2006, 15 (Suppl. S3), S354–S360. [Google Scholar] [CrossRef] [Green Version]
- Kos, N.; Gradisnik, L.; Velnar, T.A. Brief Review of the Degenerative Intervertebral Disc Disease. Med. Arch. 2019, 73, 421–424. [Google Scholar] [CrossRef] [PubMed]
- De Geer, C.M. Cytokine Involvement in Biological Inflammation Related to Degenerative Disorders of the Intervertebral Disk: A Narrative Review. J. Chiropr. Med. 2018, 17, 54–62. [Google Scholar] [CrossRef]
- Molinos, M.; Almeida, C.R.; Caldeira, J.; Cunha, C.; Gonçalves, R.M.; Barbosa, M.A. Inflammation in intervertebral disc degeneration and regeneration. J. R. Soc. Interface 2015, 12, 20141191, Correction in J. R. Soc. Interface 2015, 12, 20150429. [Google Scholar] [CrossRef] [PubMed]
- Sive, J.I.; Baird, P.; Jeziorsk, M.; Watkins, A.; Hoyland, J.A.; Freemont, A.J. Expression of chondrocyte markers by cells of normal and degenerate intervertebral discs. Mol. Pathol. 2002, 55, 91–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pękala, P.; Taterra, D.; Krupa, K.; Paziewski, M.; Wojciechowski, W.; Konopka, T.; Walocha, J.A.; Tomaszewski, K.A. Correlation of morphological and radiological characteristics of degenerative disc disease in lumbar spine: A cadaveric study. Folia Morphol. 2022, 81, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Padilla, S. Biologic Therapies to Enhance Intervertebral Disc Repair. Regen. Med. 2018, 13, 55–72. [Google Scholar] [CrossRef]
- Quero, L.; Klawitter, M.; Schmaus, A.; Rothley, M.; Sleeman, J.; Tiaden, A.N.; Klasen, J.; Boos, N.; Hottiger, M.O.; Wuertz, K.; et al. Hyaluronic Acid Fragments Enhance the Inflammatory and Catabolic Response in Human Intervertebral Disc Cells through Modulation of Toll-like Receptor 2 Signalling Pathways. Arthritis Res. Ther. 2013, 15, R94. [Google Scholar] [CrossRef] [Green Version]
- Risbud, M.V.; Shapiro, I.M. Role of Cytokines in Intervertebral Disc Degeneration: Pain and Disc Content. Nat. Rev. Rheumatol. 2014, 10, 44–56. [Google Scholar] [CrossRef]
- Johnson, Z.I.; Schoepflin, Z.R.; Choi, H.; Shapiro, I.M.; Risbud, M.V. Disc in Flames: Roles of TNF-α and IL-1β in Intervertebral Disc Degeneration. Eur. Cell. Mater. 2015, 30, 104–116, discussion 116–117. [Google Scholar] [CrossRef]
- Kwon, W.-K.; Moon, H.J.; Kwon, T.-H.; Park, Y.-K.; Kim, J.H. The Role of Hypoxia in Angiogenesis and Extracellular Matrix Regulation of Intervertebral Disc Cells During Inflammatory Reactions. Neurosurgery 2017, 81, 867–875. [Google Scholar] [CrossRef]
- Hsu, Y.H.; Lin, R.M.; Chiu, Y.S.; Liu, W.L.; Huang, K.Y. Effects of IL-1β, IL-20, and BMP-2 on Intervertebral Disc Inflammation under Hypoxia. J. Clin. Med. 2020, 9, 140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gruber, H.E.; Hoelscher, G.L.; Ingram, J.A.; Norton, H.J.; Hanley, E.N. Increased IL-17 Expression in Degenerated Human Discs and Increased Production in Cultured Annulus Cells Exposed to IL-1ß and TNF-α. Biotech. Histochem. 2013, 88, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Paesold, G.; Nerlich, A.G.; Boos, N. Biological treatment strategies for disc degeneration: Potentials and shortcomings. Eur. Spine J. 2007, 16, 447–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noack, M.; Beringer, A.; Miossec, P. Additive or Synergistic Interactions Between IL-17A or IL-17F and TNF or IL-1β Depend on the Cell Type. Front. Immunol. 2019, 10, 1726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akiyama, S.; Sakuraba, A. Distinct roles of interleukin-17 and T helper 17 cells among autoimmune diseases. J. Transl. Autoimmun. 2021, 4, 100104. [Google Scholar] [CrossRef]
- Dai, J.; Rabie, A.B. VEGF: An essential mediator of both angiogenesis and endochondral ossification. J. Dent. Res. 2007, 86, 937–950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scholz, B.; Kinzelmann, C.; Benz, K.; Mollenhauer, J.; Wurst, H.; Schlosshauer, B. Suppression of adverse angiogenesis in an albumin-based hydrogel for articular cartilage and intervertebral disc regeneration. Eur. Cell. Mater. 2010, 20, 24–37. [Google Scholar] [CrossRef]
- Xiong, C.; Huang, B.; Cun, Y.; Aghdasi, B.G.; Zhou, Y. Migration inhibitory factor enhances inflammation via CD74 in cartilage end plates with Modic type 1 changes on MRI. Clin. Orthop. Relat. Res. 2014, 472, 1943–1954. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.L.; Ma, J.X.; Wang, T.; Tian, P.; Han, C. Possible role of autoimmune reaction in Modic Type I changes. Med. Hypotheses 2011, 76, 692–694. [Google Scholar] [CrossRef]
- Lotz, J.C.; Fields, A.J.; Liebenberg, E.C. The role of the vertebral end plate in low back pain. Glob. Spine J. 2013, 3, 153–164. [Google Scholar] [CrossRef] [Green Version]
- Novikova, A.V.; Pravdyuk, N.G.; Saklakova, V.S.; Lolomadze, E.A.; Feniksov, V.; Nikolaev, D.A.; Davygora, K.S.; Timofeev, V.T.; Shostak, N.A. Degenerative disc disease in young adults: Cytokine profile and angiogenic factors. Bull. RSMU 2021, 6, 75–83. [Google Scholar] [CrossRef]
- Fields, A.J.; Ballatori, A.; Liebenberg, E.C.; Lotz, J.C. Contribution of the endplates to disc degeneration. Curr. Mol. Biol. Rep. 2018, 4, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, L.M.R.; Oliveira, L.Z.; Silva, M.B.R.D.; Accardo, C.M.; Giglio, A.B.D.; Pinhal, M.A.D.S. Inflammatory biomarkers in sera of patients with intervertebral disc degeneration. Einstein 2019, 17, eAO4637. [Google Scholar] [CrossRef]
- Schroeder, G.D.; Markova, D.Z.; Koerner, J.D.; Rihn, J.A.; Hilibrand, A.S.; Vaccaro, A.R.; Anderson, D.G.; Kepler, C.K. Are Modic changes associated with intervertebral disc cytokine profiles? Spine J. 2017, 17, 129–134. [Google Scholar] [CrossRef]
- Baptista, J.S.; Traynelis, V.C.; Liberti, E.A.; Fontes, R.B.V. Expression of degenerative markers in intervertebral discs of young and elderly asymptomatic individuals. PLoS ONE 2020, 15, e0228155. [Google Scholar] [CrossRef] [Green Version]
- Risbud, M.V.; Guttapalli, A.; Tsai, T.T.; Lee, J.Y.; Danielson, K.G.; Vaccaro, A.R.; Albert, T.J.; Gazit, Z.; Gazit, D.; Shapiro, I.M. Evidence for skeletal progenitor cells in the degenerate human intervertebral disc. Spine 2007, 32, 2537–2544. [Google Scholar] [CrossRef]
- Bach, F.C.; Poramba-Liyanage, D.W.; Riemers, F.M.; Guicheux, J.; Camus, A.; Iatridis, J.C.; Chan, D.; Ito, K.; Le Maitre, C.L.; Tryfonidou, M.A. Notochordal Cell-Based Treatment Strategies and Their Potential in Intervertebral Disc Regeneration. Front. Cell Dev. Biol. 2022, 9, 780749. [Google Scholar] [CrossRef]
- Han, S.; Zhang, Y.; Zhang, X.; Zhang, H.; Meng, S.; Kong, M.; Liu, X.; Ma, X. Single-Cell RNA Sequencing of the Nucleus Pulposus Reveals Chondrocyte Differentiation and Regulation in Intervertebral Disc Degeneration. Front. Cell Dev. Biol. 2022, 10, 824771. [Google Scholar] [CrossRef] [PubMed]







| Characteristics of Patients | Indicators | MRI Data of the Lumbar Spine | Indicators |
|---|---|---|---|
| Men, women | 50%, 50% | Hernia localization, n (%) | |
| – | ||
| 8 (23.53) | ||
| 26 (76.47) | ||
| Pain intensity median (VAS, mm), Me [Q1;Q3] | 62 | The IVD height, mm, | |
| [46.75–87.00] | M ± SD | ||
| 0.86 ± 0.16 | ||
| 2.61 ± 0.15 | ||
| Variant of the course of pain syndrome, n (%) | DDD stage according to Pfirrmann, n (%) | ||
| 4 (11.76) | 3-rd | 2 (5.88) |
| 30 (88,24) | 4-th | 19 (55.88) |
| 5-th | 13 (38.24) | ||
| The nature of pain, n (%) | Modic-changes, n (%) | 19 (55.88) | |
| 25 (73.53) |
| 9 (26.47) |
| 9 (26.47) |
| 10 (29.41) |
| Duration of pain syndrome, years | from 0.1 to 25 years | Erosion of the EP, n (%) | 20 (58.82) |
| Degree of functional disorders (BP index (BAI)), n (%) | Modic + Erosion of the EP, n (%) | 16 (47.06) | |
| 6 (17.65%) | ||
| 7 (20.59%) | ||
| 21 (61.76%) |
| Marker | Expression Level in Patients, n = 34, Average Value % | Expression Level in Controls, n = 7, Average Value % |
|---|---|---|
| IL-1β | Diffuse in the matrix: 13.06 ± 3.65 (around the vessels 79.24 ± 8.12) Chondrocytes clusters in the NP: 56.15 ± 24.40 | Diffuse in the matrix: 1.17 ± 0.47 (around the vessels 4.18 ± 1.72) Chondrocytes in the NP: 16.43 ± 10.10 |
| IL-6 | Diffuse in the matrix: 16.33 ± 5.40 Chondrocytes clusters in the NP: 49.65 ± 21.41 | Diffuse in the matrix: 3.93 ± 1.17 Chondrocytes in the NP: 29.86 ± 8.45 |
| IL-17 | Diffuse in the matrix: 14.15 ± 5.12 (around the vessels 51.89 ± 7.35) Chondrocytes clusters in the NP: 63.71 ± 21.12 | Diffuse in the matrix: 0.57 ± 0.35 (around the vessels 3.51 ± 1.60) Chondrocytes in the NP: 8.71 ± 5.77 |
| VEGF-A | Diffuse in the matrix: 16.82 ± 5.70 Chondrocytes clusters in the NP: 74.68 ± 19.17 | Diffuse in the matrix: 0.51 ± 0.12 Chondrocytes in the NP: 17.43 ± 7.74 |
| CD31 | Diffuse in the matrix: 19.71 ± 9.25 | Diffuse in the matrix: 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pravdyuk, N.G.; Novikova, A.V.; Shostak, N.A.; Buianova, A.A.; Tairova, R.T.; Patsap, O.I.; Raksha, A.P.; Timofeyev, V.T.; Feniksov, V.M.; Nikolayev, D.A.; et al. Immunomorphogenesis in Degenerative Disc Disease: The Role of Proinflammatory Cytokines and Angiogenesis Factors. Biomedicines 2023, 11, 2184. https://doi.org/10.3390/biomedicines11082184
Pravdyuk NG, Novikova AV, Shostak NA, Buianova AA, Tairova RT, Patsap OI, Raksha AP, Timofeyev VT, Feniksov VM, Nikolayev DA, et al. Immunomorphogenesis in Degenerative Disc Disease: The Role of Proinflammatory Cytokines and Angiogenesis Factors. Biomedicines. 2023; 11(8):2184. https://doi.org/10.3390/biomedicines11082184
Chicago/Turabian StylePravdyuk, Natalya G., Anna V. Novikova, Nadezhda A. Shostak, Anastasiia A. Buianova, Raisa T. Tairova, Olga I. Patsap, Aleksandr P. Raksha, Vitaliy T. Timofeyev, Victor M. Feniksov, Dmitriy A. Nikolayev, and et al. 2023. "Immunomorphogenesis in Degenerative Disc Disease: The Role of Proinflammatory Cytokines and Angiogenesis Factors" Biomedicines 11, no. 8: 2184. https://doi.org/10.3390/biomedicines11082184
APA StylePravdyuk, N. G., Novikova, A. V., Shostak, N. A., Buianova, A. A., Tairova, R. T., Patsap, O. I., Raksha, A. P., Timofeyev, V. T., Feniksov, V. M., Nikolayev, D. A., & Senko, I. V. (2023). Immunomorphogenesis in Degenerative Disc Disease: The Role of Proinflammatory Cytokines and Angiogenesis Factors. Biomedicines, 11(8), 2184. https://doi.org/10.3390/biomedicines11082184