Comparison of Autografts and Biodegradable 3D-Printed Composite Scaffolds with Osteoconductive Properties for Tissue Regeneration in Bone Tuberculosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of 3D-Printed Composite Scaffolds with Adhered rMSCs
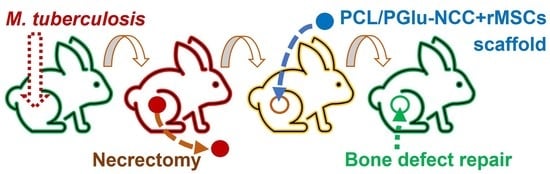
2.3. Experiments In Vivo
- -
- Group 1: infection control (IC)—infection, no surgical operation, no treatment, (n = 4);
- -
- Group 2: autoplastic (AP)—infection, necrectomy, autoplastic surgery, anti-TB treatment, (n = 12);
- -
- Group 3: scaffold implantation (SI)—infection, necrectomy, plastic surgery with the composite scaffold, anti-TB treatment, (n = 15).
2.3.1. Tuberculosis Modeling
2.3.2. Tuberculosis Verification
2.3.3. Treatment and Plastic Surgery
2.4. Biochemical Analysis
2.5. Microcomputed Tomography
2.6. Histomorphological Analysis
2.7. Statistical Analysis
3. Results
3.1. Scaffolds
3.2. Tuberculosis Modeling, Verification and Treatment
3.3. Monitoring of Biochemical Markers
3.4. Micro-Computed Tomography
3.5. Histomorphological Analysis
3.5.1. Group 1: Infection Control
3.5.2. Group 2: Autoplastic Bone Surgery
3.5.3. Group 3: Plastic Surgery with Composite Scaffold
3.6. Quantitative Assessment of Bone Regeneration
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pai, M.; Behr, M.A.; Dowdy, D.; Dheda, K.; Divangahi, M.; Boehme, C.C.; Ginsberg, A.; Swaminathan, S.; Spigelman, M.; Getahun, H.; et al. Tuberculosis. Nat. Rev. Dis. Prim. 2016, 2, 16076. [Google Scholar] [CrossRef] [PubMed]
- Gomez, J.E.; McKinney, J.D. M. tuberculosis persistence, latency, and drug tolerance. Tuberculosis 2004, 84, 29–44. [Google Scholar] [CrossRef] [PubMed]
- Tuberculosis. Available online: https://www.who.int/news-room/fact-sheets/detail/tuberculosis (accessed on 20 June 2023).
- Pigrau-Serrallach, C.; Rodríguez-Pardo, D. Bone and joint tuberculosis. Eur. Spine J. 2013, 22, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Yi, Z.; Song, Q.; Zhou, J.; Zhou, Y. The efficacy of single posterior debridement, bone grafting and instrumentation for the treatment of thoracic spinal tuberculosis. Sci. Rep. 2021, 11, 3591. [Google Scholar] [CrossRef]
- Muneer, A.; Macrae, B.; Krishnamoorthy, S.; Zumla, A. Urogenital tuberculosis—epidemiology, pathogenesis and clinical features. Nat. Rev. Urol. 2019, 16, 573–598. [Google Scholar] [CrossRef]
- Basu, S. Absence of Evidence as The Evidence of Absence: The Curious Case of Latent Infection Causing Ocular Tuberculosis. Front. Ophthalmol. 2022, 2, 874400. [Google Scholar] [CrossRef]
- Cao, T.; Liu, X.; Yang, C.; Mei, C.; Ou, J.; Du, R. Multidrug-resistant tuberculosis in middle ear: A case report. J. Clin. Tuberc. Other Mycobact. Dis. 2023, 31, 100355. [Google Scholar] [CrossRef]
- El Farhaoui, A.; Batou, Y.; Benalia, K.; Lachkar, A.; Abdeljaouad, N.; Yacoubi, H. Tuberculosis of navicular bone: An exceptional localization of osteoarticular tuberculosis. Radiol. Case Rep. 2023, 18, 1989–1992. [Google Scholar] [CrossRef]
- Amarnath Praphakar, R.; Sumathra, M.; Sam Ebenezer, R.; Vignesh, S.; Shakila, H.; Rajan, M. Fabrication of bioactive rifampicin loaded κ-Car-MA-INH/Nano hydroxyapatite composite for tuberculosis osteomyelitis infected tissue regeneration. Int. J. Pharm. 2019, 565, 543–556. [Google Scholar] [CrossRef]
- Vohra, R.; Kang, H.S.; Dogra, S.; Saggar, R.R.; Sharma, R. Tuberculous osteomyelitis. J. Bone Jt. Surg. 1997, 79, 562–566. [Google Scholar] [CrossRef]
- Dartois, V.A.; Rubin, E.J. Anti-tuberculosis treatment strategies and drug development: Challenges and priorities. Nat. Rev. Microbiol. 2022, 20, 685–701. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, K.; Kumar, M.; Jha, A.; Bharti, K.; Das, M.; Mishra, B. Nanocarriers for tuberculosis therapy: Design of safe and effective drug delivery strategies to overcome the therapeutic challenges. J. Drug Deliv. Sci. Technol. 2022, 67, 102850. [Google Scholar] [CrossRef]
- Churilov, L.; Korzhikov-Vlakh, V.; Sinitsyna, E.; Polyakov, D.; Darashkevich, O.; Poida, M.; Platonova, G.; Vinogradova, T.; Utekhin, V.; Zabolotnykh, N.; et al. Enhanced Delivery of 4-Thioureidoiminomethylpyridinium Perchlorate in Tuberculosis Models with IgG Functionalized Poly(Lactic Acid)-Based Particles. Pharmaceutics 2018, 11, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, W.; Wei, X.; Wei, K.; Cao, X.; Zhong, S. A mesoporous silicon/poly-(dl-lactic-co-glycolic) acid microsphere for long time anti-tuberculosis drug delivery. Int. J. Pharm. 2014, 476, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Skrahin, A.; Ahmed, R.K.; Ferrara, G.; Rane, L.; Poiret, T.; Isaikina, Y.; Skrahina, A.; Zumla, A.; Maeurer, M.J. Autologous mesenchymal stromal cell infusion as adjunct treatment in patients with multidrug and extensively drug-resistant tuberculosis: An open-label phase 1 safety trial. Lancet Respir. Med. 2014, 2, 108–122. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, Q.; Ye, Z.; Li, Y.; Che, Z.; Huang, M.; Zeng, J. Mesenchymal Stem Cells and Tuberculosis: Clinical Challenges and Opportunities. Front. Immunol. 2021, 12, 695278. [Google Scholar] [CrossRef]
- Khan, A.; Hunter, R.L.; Jagannath, C. Emerging role of mesenchymal stem cells during tuberculosis: The fifth element in cell mediated immunity. Tuberculosis 2016, 101, S45–S52. [Google Scholar] [CrossRef]
- Leonardi, E.; Ciapetti, G.; Baglìo, S.R.; Devescovi, V.; Baldini, N.; Granchi, D. Osteogenic properties of late adherent subpopulations of human bone marrow stromal cells. Histochem. Cell Biol. 2009, 132, 547–557. [Google Scholar] [CrossRef]
- Rahyussalim, A.J.; Kurniawati, T.; Siregar, N.C.; Syahrurachman, A.; Dilogo, I.H.; Iskandriati, D.; Fitri, A.D. New Bone Formation in Tuberculous-Infected Vertebral Body Defect after Administration of Bone Marrow Stromal Cells in Rabbit Model. Asian Spine J. 2016, 10, 1–5. [Google Scholar] [CrossRef]
- Henkel, J.; Woodruff, M.A.; Epari, D.R.; Steck, R.; Glatt, V.; Dickinson, I.C.; Choong, P.F.M.; Schuetz, M.A.; Hutmacher, D.W. Bone Regeneration Based on Tissue Engineering Conceptions—A 21st Century Perspective. Bone Res. 2013, 1, 216–248. [Google Scholar] [CrossRef] [Green Version]
- Kaya, A.; Kaya, B.; Aktas, A.; Fırat, E.T. Effect of rifampin in combination with allogeneic, alloplastic, and heterogenous bone grafts on bone regeneration in rat tibial bone defects. J. Oral Maxillofac. Surg. Med. Pathol. 2015, 27, 20–28. [Google Scholar] [CrossRef]
- Tang, G.; Liu, Z.; Liu, Y.; Yu, J.; Wang, X.; Tan, Z.; Ye, X. Recent Trends in the Development of Bone Regenerative Biomaterials. Front. Cell Dev. Biol. 2021, 9, 665813. [Google Scholar] [CrossRef]
- Narayanan, G.; Vernekar, V.N.; Kuyinu, E.L.; Laurencin, C.T. Poly (lactic acid)-based biomaterials for orthopaedic regenerative engineering. Adv. Drug Deliv. Rev. 2016, 107, 247–276. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Xu, Y.; Wang, Y.; Ito, Y.; Zhang, P.; Chen, X. Enhanced in Vitro Mineralization and in Vivo Osteogenesis of Composite Scaffolds through Controlled Surface Grafting of L-Lactic Acid Oligomer on Nanohydroxyapatite. Biomacromolecules 2016, 17, 818–829. [Google Scholar] [CrossRef]
- Seyednejad, H.; Gawlitta, D.; Dhert, W.J.A.; van Nostrum, C.F.; Vermonden, T.; Hennink, W.E. Preparation and characterization of a three-dimensional printed scaffold based on a functionalized polyester for bone tissue engineering applications. Acta Biomater. 2011, 7, 1999–2006. [Google Scholar] [CrossRef]
- Best, S.M.; Porter, A.E.; Thian, E.S.; Huang, J. Bioceramics: Past, present and for the future. J. Eur. Ceram. Soc. 2008, 28, 1319–1327. [Google Scholar] [CrossRef]
- Baino, F.; Novajra, G.; Vitale-Brovarone, C. Bioceramics and Scaffolds: A Winning Combination for Tissue Engineering. Front. Bioeng. Biotechnol. 2015, 3, 202. [Google Scholar] [CrossRef] [Green Version]
- Alam, F.; Verma, P.; Mohammad, W.; Teo, J.; Varadarajan, K.M.; Kumar, S. Architected poly(lactic acid)/poly(ε-caprolactone)/halloysite nanotube composite scaffolds enabled by 3D printing for biomedical applications. J. Mater. Sci. 2021, 56, 14070–14083. [Google Scholar] [CrossRef]
- Lu, L.; Zhang, Q.; Wootton, D.; Chiou, R.; Li, D.; Lu, B.; Lelkes, P.; Zhou, J. Mechanical study of polycaprolactone-hydroxyapatite porous scaffolds created by porogen-based solid freeform fabrication method. J. Appl. Biomater. Funct. Mater. 2014, 12, 145–154. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, X.; Chen, J.; Lin, K. The development of collagen based composite scaffolds for bone regeneration. Bioact. Mater. 2018, 3, 129–138. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, N.; Ma, Y.; Dai, H.; Han, B. Preparation and study of 3D printed dipyridamole/β-tricalcium phosphate/polyvinyl alcohol composite scaffolds in bone tissue engineering. J. Drug Deliv. Sci. Technol. 2022, 68, 103053. [Google Scholar] [CrossRef]
- Stepanova, M.; Eremin, A.; Averianov, I.; Gofman, I.; Lavrentieva, A.; Korzhikov-Vlakh, V.; Korzhikova-Vlakh, E. Comparison of Supermacroporous Polyester Matrices Fabricated by Thermally Induced Phase Separation and 3D Printing Techniques. Key Eng. Mater. 2019, 822, 277–283. [Google Scholar] [CrossRef]
- Averianov, I.V.; Korzhikov, V.A.; Tennikova, T.B. Synthesis of poly(lactic acid) and the formation of poly(lactic acid)-based supraporous biofunctional materials for tissue engineering. Polym. Sci. Ser. B 2015, 57, 336–348. [Google Scholar] [CrossRef]
- Liu, X.; Wang, T.; Chow, L.C.; Yang, M.; Mitchell, J.W. Effects of inorganic fillers on the thermal and mechanical properties of poly(lactic acid). Int. J. Polym. Sci. 2014, 2014, 827028. [Google Scholar] [CrossRef] [Green Version]
- Aliotta, L.; Cinelli, P.; Coltelli, M.B.; Lazzeri, A. Rigid filler toughening in PLA-Calcium Carbonate composites: Effect of particle surface treatment and matrix plasticization. Eur. Polym. J. 2019, 113, 78–88. [Google Scholar] [CrossRef]
- Belaid, H.; Nagarajan, S.; Teyssier, C.; Barou, C.; Barés, J.; Balme, S.; Garay, H.; Huon, V.; Cornu, D.; Cavaillès, V.; et al. Development of new biocompatible 3D printed graphene oxide-based scaffolds. Mater. Sci. Eng. C 2020, 110, 110595. [Google Scholar] [CrossRef]
- Piekarska, K.; Sowinski, P.; Piorkowska, E.; Haque, M.M.U.; Pracella, M. Structure and properties of hybrid PLA nanocomposites with inorganic nanofillers and cellulose fibers. Compos. Part A Appl. Sci. Manuf. 2016, 82, 34–41. [Google Scholar] [CrossRef]
- Murizan, N.I.S.; Mustafa, N.S.; Ngadiman, N.H.A.; Mohd Yusof, N.; Idris, A. Review on Nanocrystalline Cellulose in Bone Tissue Engineering Applications. Polymers 2020, 12, 2818. [Google Scholar] [CrossRef]
- Vlakh, E.G.; Panarin, E.F.; Tennikova, T.B.; Suck, K.; Kasper, C. Development of multifunctional polymer-mineral composite materials for bone tissue engineering. J. Biomed. Mater. Res.—Part A 2005, 75, 333–341. [Google Scholar] [CrossRef]
- Averianov, I.V.; Stepanova, M.A.; Gofman, I.V.; Lavrentieva, A.; Korzhikov-Vlakh, V.A.; Korzhikova-Vlakh, E.G. Osteoconductive biocompatible 3D-printed composites of poly-d,l-lactide filled with nanocrystalline cellulose modified by poly(glutamic acid). Mendeleev Commun. 2022, 32, 810–812. [Google Scholar] [CrossRef]
- Korzhikov, V.; Roeker, S.; Vlakh, E.; Kasper, C.; Tennikova, T. Synthesis of multifunctional polyvinylsaccharide containing controllable amounts of biospecific ligands. Bioconjug. Chem. 2008, 19, 617–625. [Google Scholar] [CrossRef]
- Averianov, I.; Stepanova, M.; Solomakha, O.; Gofman, I.; Serdobintsev, M.; Blum, N.; Kaftuirev, A.; Baulin, I.; Nashchekina, J.; Lavrentieva, A.; et al. 3D-Printed composite scaffolds based on poly(ε-caprolactone) filled with poly(glutamic acid)-modified cellulose nanocrystals for improved bone tissue regeneration. J. Biomed. Mater. Res. Part B Appl. Biomater. 2022, 110, 2422–2437. [Google Scholar] [CrossRef]
- Kunwong, N.; Tangjit, N.; Rattanapinyopituk, K.; Dechkunakorn, S.; Anuwongnukroh, N.; Arayapisit, T.; Sritanaudomchai, H. Optimization of poly (lactic-co-glycolic acid)-bioactive glass composite scaffold for bone tissue engineering using stem cells from human exfoliated deciduous teeth. Arch. Oral Biol. 2021, 123, 105041. [Google Scholar] [CrossRef]
- Bilem, I.; Chevallier, P.; Plawinski, L.; Sone, E.D.; Durrieu, M.C.; Laroche, G. RGD and BMP-2 mimetic peptide crosstalk enhances osteogenic commitment of human bone marrow stem cells. Acta Biomater. 2016, 36, 132–142. [Google Scholar] [CrossRef]
- Firdhausi Wardhani, I.; Mega Rizki Samudra, R.; Katherine; Hikmawati, D. In vitro study of Nano Hydroxyapatite/Streptomycin -Gelatin-Based Injectable Bone Substitute Associated- 3D printed Bone Scaffold for Spinal Tuberculosis Case. J. Phys. Conf. Ser. 2020, 1445, 012003. [Google Scholar] [CrossRef]
- Huang, D.; Li, D.; Wang, T.; Shen, H.; Zhao, P.; Liu, B.; You, Y.; Ma, Y.; Yang, F.; Wu, D.; et al. Isoniazid conjugated poly(lactide-co-glycolide): Long-term controlled drug release and tissue regeneration for bone tuberculosis therapy. Biomaterials 2015, 52, 417–425. [Google Scholar] [CrossRef]
- Zhu, M.; Li, K.; Zhu, Y.; Zhang, J.; Ye, X. 3D-printed hierarchical scaffold for localized isoniazid/rifampin drug delivery and osteoarticular tuberculosis therapy. Acta Biomater. 2015, 16, 145–155. [Google Scholar] [CrossRef]
- Xie, T.; Song, Y.; Peng, H.; Dai, Z.; Kang, Y.; Xiu, P.; Wang, L.; Li, H.; Yang, X. A bioactive implant combining isoniazid with nanohydroxyapatite/polyamide 66 for the treatment of osteoarticular tuberculosis. Mater. Des. 2021, 210, 110064. [Google Scholar] [CrossRef]
- Stepanova, M.; Averianov, I.; Serdobintsev, M.; Gofman, I.; Blum, N.; Semenova, N.; Nashchekina, Y.; Vinogradova, T.; Korzhikov-Vlakh, V.; Karttunen, M.; et al. PGlu-Modified Nanocrystalline Cellulose Improves Mechanical Properties, Biocompatibility, and Mineralization of Polyester-Based Composites. Materials 2019, 12, 3435. [Google Scholar] [CrossRef] [Green Version]
- Averianov, I.V.; Stepanova, M.A.; Gofman, I.V.; Nikolaeva, A.L.; Korzhikov-Vlakh, V.A.; Karttunen, M.; Korzhikova-Vlakh, E.G. Chemical modification of nanocrystalline cellulose for enhanced interfacial compatibility with poly(lactic acid). Mendeleev Commun. 2019, 29, 220–222. [Google Scholar] [CrossRef]
- Vasilyeva, S.N.; Kaftyrev, A.S.; Vinogradova, T.I.; Serdobintsev, M.S.; Zabolotnykh, N.M. A Method for Modeling Tuberculous Ostitis of Varying Severity. RU 2421823 C1, 20 June 2011. (In Russian)
- Giusti, G. Adenosine Deaminase. In Methods of Enzymatic Analysis; Bergmeyer, H.U., Ed.; Academic Press: New York, NY, USA, 1974; pp. 1092–1099. [Google Scholar]
- Visser, L.; Blout, E.R. The use of p-nitrophenyl N-tert-butyloxycarbonyl-L-alaninate as substrate for elastase. Biochim. Biophys. Acta—Enzymol. 1972, 268, 257–260. [Google Scholar] [CrossRef] [PubMed]
- Charan, J.; Kantharia, N.D. How to calculate sample size in animal studies? J. Pharmacol. Pharmacother. 2013, 4, 303–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stepanova, M.; Dobrodumov, A.; Averianov, I.; Gofman, I.; Nashchekina, J.; Guryanov, I.; Klyukin, I.; Zhdanov, A.; Korzhikova-Vlakh, E.; Zhizhin, K. Design, Fabrication and Characterization of Biodegradable Composites Containing Closo-Borates as Potential Materials for Boron Neutron Capture Therapy. Polymers 2022, 14, 3864. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, S.A. The mechanical properties of trabecular bone: Dependence on anatomic location and function. J. Biomech. 1987, 20, 1055–1061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, K.; Li, J.; Huang, T.; Zhong, W.; Luo, X.; Quan, Z. Clinical efficacy of three types of autogenous bone grafts in treatment of single-segment thoracic tuberculosis: A retrospective cohort study. Int. J. Med. Sci. 2020, 17, 2844–2849. [Google Scholar] [CrossRef]
- Sohn, H.-S.; Oh, J.-K. Review of bone graft and bone substitutes with an emphasis on fracture surgeries. Biomater. Res. 2019, 23, 9. [Google Scholar] [CrossRef] [Green Version]
- Hochepied, T.; Berger, F.G.; Baumann, H.; Libert, C. α1-Acid glycoprotein: An acute phase protein with inflammatory and immunomodulating properties. Cytokine Growth Factor Rev. 2003, 14, 25–34. [Google Scholar] [CrossRef]
- Reinhardt, R.A.; Sanderfer, V.J.; Meinberg, T.A.; Nummikoski, P.; Lee, H.; Marx, D.B. Local biochemical markers of bone turnover: Relationship to subsequent density of healing alveolar bone defects. J. Clin. Periodontol. 2004, 31, 223–228. [Google Scholar] [CrossRef]
- Chapurlat, R.D.; Confavreux, C.B. Novel biological markers of bone: From bone metabolism to bone physiology. Rheumatology 2016, 55, 1714–1725. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Yoon, H.; Park, J.; Che, X.; Jin, X.; Choi, J. G protein-coupled receptor 119 is involved in RANKL-induced osteoclast differentiation and fusion. J. Cell. Physiol. 2019, 234, 11490–11499. [Google Scholar] [CrossRef]
- Pellegatti, P.; Falzoni, S.; Donvito, G.; Lemaire, I.; Di Virgilio, F. P2X7 receptor drives osteoclast fusion by increasing the extracellular adenosine concentration. FASEB J. 2011, 25, 1264–1274. [Google Scholar] [CrossRef]
- Mediero, A.; Kara, F.M.; Wilder, T.; Cronstein, B.N. Adenosine A2A Receptor Ligation Inhibits Osteoclast Formation. Am. J. Pathol. 2012, 180, 775–786. [Google Scholar] [CrossRef] [Green Version]
- Barletta, K.E.; Ley, K.; Mehrad, B. Regulation of Neutrophil Function by Adenosine. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 856–864. [Google Scholar] [CrossRef] [Green Version]
- Nathan, C.; Xie, Q.; Halbwachs-Mecarelli, L.; Jin, W. Albumin inhibits neutrophil spreading and hydrogen peroxide release by blocking the shedding of CD43 (sialophorin, leukosialin). J. Cell Biol. 1993, 122, 243–256. [Google Scholar] [CrossRef]
- Kälvegren, H.; Fridfeldt, J.; Bengtsson, T. The role of plasma adenosine deaminase in chemoattractant-stimulated oxygen radical production in neutrophils. Eur. J. Cell Biol. 2010, 89, 462–467. [Google Scholar] [CrossRef]
- Shcherbak, V.A.; Kargina, I.G.; Shcherbak, N.M. Markers of bone metabolism in children with rickets. Ross. Vestn. Perinatol. i Pediatr. (Russian Bull. Perinatol. Pediatr.) 2020, 65, 71–77. [Google Scholar] [CrossRef]
- St. Hilaire, C.; Ziegler, S.G.; Markello, T.C.; Brusco, A.; Groden, C.; Gill, F.; Carlson-Donohoe, H.; Lederman, R.J.; Chen, M.Y.; Yang, D.; et al. NT5E Mutations and Arterial Calcifications. N. Engl. J. Med. 2011, 364, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Mazzon, E.; Esposito, E.; Impellizzeri, D.; Di Paola, R.; Melani, A.; Bramanti, P.; Pedata, F.; Cuzzocrea, S. CGS 21680, an Agonist of the Adenosine (A2A) Receptor, Reduces Progression of Murine Type II Collagen-induced Arthritis. J. Rheumatol. 2011, 38, 2119–2129. [Google Scholar] [CrossRef]
- Nurullina, G.M.; Akhmadullina, G.I. Bone remodeling in norm and in primary osteoporosis: The significance of bone remodeling markers. Russ. Arch. Intern. Med. 2018, 8, 100–110. [Google Scholar] [CrossRef] [Green Version]
- van der Hoeven, D.; Wan, T.C.; Auchampach, J.A. Activation of the A 3 Adenosine Receptor Suppresses Superoxide Production and Chemotaxis of Mouse Bone Marrow Neutrophils. Mol. Pharmacol. 2008, 74, 685–696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Margerrison, E.; Argentieri, M.; Kommala, D.; Schabowsky, C.N. Polycaprolactone (PCL) Safety Profile Report Details Date of Submission ECRI Corporate Governance Project Manager; European Commission against Racism and Intolerance (ECRI): Plymouth Meeting, PA, USA, 2021. [Google Scholar]
- Manoukian, O.S.; Arul, M.R.; Sardashti, N.; Stedman, T.; James, R.; Rudraiah, S.; Kumbar, S.G. Biodegradable polymeric injectable implants for long-term delivery of contraceptive drugs. J. Appl. Polym. Sci. 2018, 135, 46068. [Google Scholar] [CrossRef] [PubMed]
- Roman, M. Toxicity of Cellulose Nanocrystals: A Review. Ind. Biotechnol. 2015, 11, 25–33. [Google Scholar] [CrossRef]
- Park, S.-B.; Sung, M.-H.; Uyama, H.; Han, D.K. Poly(glutamic acid): Production, composites, and medical applications of the next-generation biopolymer. Prog. Polym. Sci. 2021, 113, 101341. [Google Scholar] [CrossRef]
- Bhattarai, D.P.; Kim, M.H.; Park, H.; Park, W.H.; Kim, B.S.; Kim, C.S. Coaxially fabricated polylactic acid electrospun nanofibrous scaffold for sequential release of tauroursodeoxycholic acid and bone morphogenic protein2 to stimulate angiogenesis and bone regeneration. Chem. Eng. J. 2020, 389, 123470. [Google Scholar] [CrossRef]
- Wang, L.; Xu, L.; Peng, C.; Teng, G.; Wang, Y.; Xie, X.; Wu, D. The effect of bone marrow mesenchymal stem cell and nano-hydroxyapatite/collagen I/poly-L-lactic acid scaffold implantation on the treatment of avascular necrosis of the femoral head in rabbits. Exp. Ther. Med. 2019, 18, 2021–2028. [Google Scholar] [CrossRef]
- Rabiee, S.M.; Mortazavi, S.M.J.; Moztarzadeh, F.; Sharifi, D.; Fakhrejahani, F.; Khafaf, A.; Houshiar Ahmadi, S.A.; Nosoudi, N.; Ravarian, R. Association of a synthetic bone graft and bone marrow cells as a composite biomaterial. Biotechnol. Bioprocess Eng. 2009, 14, 1–5. [Google Scholar] [CrossRef]
- Sun, H.; Mei, L.; Song, C.; Cui, X.; Wang, P. The in vivo degradation, absorption and excretion of PCL-based implant. Biomaterials 2006, 27, 1735–1740. [Google Scholar] [CrossRef]
- Bartnikowski, M.; Dargaville, T.R.; Ivanovski, S.; Hutmacher, D.W. Degradation mechanisms of polycaprolactone in the context of chemistry, geometry and environment. Prog. Polym. Sci. 2019, 96, 1–20. [Google Scholar] [CrossRef]
- Almeida, B.C.; Figueiredo, P.; Carvalho, A.T.P. Polycaprolactone Enzymatic Hydrolysis: A Mechanistic Study. ACS Omega 2019, 4, 6769–6774. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Diaz, S.; Garcia-Giralt, N.; Lebourg, M.; Gómez-Tejedor, J.-A.; Vila, G.; Caceres, E.; Benito, P.; Monleón Pradas, M.; Nogues, X.; Gómez Ribelles, J.L.; et al. In Vivo Evaluation of 3-Dimensional Polycaprolactone Scaffolds for Cartilage Repair in Rabbits. Am. J. Sports Med. 2010, 38, 509–519. [Google Scholar] [CrossRef]
- Abdelfatah, J.; Paz, R.; Alemán-Domínguez, M.E.; Monzón, M.; Donate, R.; Winter, G. Experimental Analysis of the Enzymatic Degradation of Polycaprolactone: Microcrystalline Cellulose Composites and Numerical Method for the Prediction of the Degraded Geometry. Materials 2021, 14, 2460. [Google Scholar] [CrossRef] [PubMed]
- Stepanova, M.; Korzhikova-Vlakh, E. Modification of Cellulose Micro- and Nanomaterials to Improve Properties of Aliphatic Polyesters/Cellulose Composites: A Review. Polymers 2022, 14, 1477. [Google Scholar] [CrossRef] [PubMed]











| Group | Average Rabbit Weight (g) | ||||
|---|---|---|---|---|---|
| Initial Weight | 18 Days | 2 Months | 4 Months | 6 Months | |
| 1 (IC) | 3518 [3256; 3676] | 3348 [3164; 3572] | 2949 (p = 0.02 *) [2816; 3082] | 2713 (p = 0.04 **) [2713; 2713] | – |
| 2 (AP) | 3809 (p = 0.02 *; p = 0.003 **) [3486; 4025] | 4251 (p = 0.00003 *; p = 0.00001 **) [3943; 4435] | 3995 (p = 0.03 *; p = 0.007 **) [3635; 4395] | ||
| 3 (SI) | 3912 (p = 0.0008 *; p = 0.0001 **) [3574; 4144] | 3959, (p = 0.002 *; p = 0.0001 **) [3646; 4356] | 4270 (p = 0.01 *; p = 0.001 **) [3660; 4280] | ||
| Biochemical Parameter | Initial Values | Groups | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 (n = 4) | 2 (n = 12) | 3 (n = 15) | ||||||||||
| Time Periods after Infection for Biochemical Screening | ||||||||||||
| 18 Days | 2 Months | 4 Months | 18 Days | 2 Months | 4 Months | 6 Months | 18 Days | 2 Months | 4 Months | 6 Months | ||
| EL (IU) | 293.4 [251.9; 323.3] | 347.7 (p = 0.0008 *) [293.4; 423.8] | 239.0 [239.0; 239.0] | 130.0 [130.0; 130.0] | 347.7 (p = 0.0008 *) [293.4; 423.8] | 297.0 [233.9; 459.2] | 298.6 [279.8; 364.0] | 293.3 [249.9; 396.5] | 347.7 (p = 0.0008 *) [293.4; 423.8] | 484.6, (p = 0.000001 *; p = 0.01 **; p = 0.01 #; p = 0.02 &) [384,6; 532,5] | 342.2 [288.0; 434.7] | 362.2 (p = 0.056 *) [282.5; 402.0] |
| TP (g/L) | 71.0 [66.0; 77.0] | 65.6 (p = 0.05 *) [62.5; 70.4] | 64.5 [63,0; 66,0] | 67.0 [67.0; 67.0] | 65.6 (p = 0.05 *) [62.5; 70.4] | 66.0 [64.0; 71.0] | 77.0 (p = 0.015 *; p = 0.002 **) [72.5; 86.5] | 76.0 (p = 0.05 **) [71.0; 82.5] | 65.6 (p = 0.05 *) [62.5; 70.4] | 65.0 [63.0; 72.0] | 72.0 (p = 0.05 **) [67.0; 82.0] | 70.0 [66.0; 71.0] |
| AL (g/L) | 47.0 [45.0; 50.0] | 46.0 [45.0; 49.0] | 46.0 [46.0; 46.0] | 42.0 [42.0; 42.0] | 46.0 [45.0; 49.0] | 46.0 [44.0; 49.0] | 50.5 (p = 0.01 *; p = 0.0002 **) [49.0; 51.0] | 47.0 [46.0;49.5] | 46.0 [45.0; 49.0] | 47.0 [45.0; 50.0] | 48.0 (p = 0.01 **) [47.0; 52.0] | 47.0 [46.0; 47.0] |
| CP (g/L) | 0.34 [0.28; 0.45] | 0.42 (p = 0.01 *) [0.35; 0.52] | 0.46 [0.45; 0.48] | 0.95 [0.95; 0.95] | 0.42 (p = 0.01 *) [0.35; 0.52] | 0.32, (p = 0.0009 *; p = 0.03 **) [0.26; 0.39] | 0.23 (p = 0.00001 **) [0.15; 0.24] | 0.28 (p = 0.01 **) [0.25; 0.34] | 0.42 (p = 0.01 *) [0.35; 0.52] | 0.33 (p = 0.0007 **; p = 0.01 #) [0.24; 0.38] | 0.26 (p = 0.008 *; p = 0.000008 **; p = 0.03 &) [0.24; 0.28] | 0.35 [0.29; 0.39] |
| Total ADA (U/L) | 9.6 [6.8;11.6] | 9.8 [6.7; 12.2] | 17.0 [10.2; 23.9] | 27.7 [27.7; 27.7] | 9.8 [6.7; 12.2] | 14.9 (p = 0.04 *) [6.9; 18.4] | 17.0 (p = 0.001 *; p = 0.006 **) [11.3; 22.9] | 13.8 [8.1; 17.7] | 9.8 [6.7; 12.2] | 13.4 (p = 0.0027 *; p = 0.02 **) [8.4; 16.2] | 11.6 [8.8; 17.0] | 11.6 [5.4; 14.0] |
| ekto-ADA-1 (U/L) | 8.5 [6.6; 9.4] | 8.3 [5.1; 11.2] | 13.8 [7.7; 19.9] | 23.5 [23.5; 23.5] | 8.3 [5.1; 11.2] | 12.0 (p = 0.004 *) [5.5; 17.0] | 15.4 (p = 0.008 **) [8.8; 18.4] | 10.5 [6.7; 15.8] | 8.3 [5.1; 11.2] | 12.1 (p = 0.004 *; p = 0.02 **) [7.9; 14.3] | 10.6 (p = 0.02 *) [9.1; 17.0] | 6.0 [3.1; 11.4] |
| ekto-ADA-2 (U/L) | 0.45 [0; 2.0] | 0.8 [0; 1.4] | 3.3 [2.5; 4.0] | 4.1 [4.1; 4.1] | 0.8 [0; 1.4] | 0.55 [0; 2.7] | 0 [0; 3.6] | 1.3 [0; 3.2] | 0.8 [0; 1.4] | 0.75 [0; 1.9] | 0.35 [0; 1.8] | 3.3 (p = 0.004 *; p = 0.004 **) [2.6; 3.5] |
| RANKL (ng/L) | 99.7 n = 12 [87.5; 102.8] | 76.7 (p = 0.002 *) n = 14 [57.6; 91.4] | 101.0 (p = 0.04 *; p = 0.04 **) n = 2 [99.0; 103.0] | 83.2 n = 2 [83.2; 83.2] | 76.7 (p = 0.002 *) n = 14 [57.6; 91.4] | 83.7 n = 12 [70.3; 94.3] | 86.3 n = 8 [82.5; 92.4] | 89.4 n = 4 [72.1; 92.4] | 76.7 (p = 0.002 *) n = 14 [57.6; 91.4] | 83.5 (p = 0.02 *) n = 15 [64.4; 92.5] | 84.5 n = 10 (p = 0.04 **) [66.5; 100,0] | 103.0 n = 5 (p = 0.01 **) [95.5; 106.0] |
| ALPL (ng/L) | 0.32 n = 11 [0.2; 1.1] | 0.13 n = 5 [0.1; 0.23] | n = 0 | 0.22 n = 2 [0.22; 0.22] | 0.13 n = 5 [0.1; 0.23] | 0.32 n = 5 [0.28; 0.62] | 1.12 (p = 0.026 *; p = 0.04 **) n = 8 [0.54; 1.47] | 1.35 (p = 0.04 *) n = 4 [0.6; 2.13] | 0.13 n = 5 [0.1; 0.23] | 0.24 n = 8 [0.18; 0.33] | 0.42 n = 9 (p = 0.046 &) [0.35; 0.65] | 0.64 n = 4 [0.36; 1.87] |
| Group * | Indicator (2/4/6 Months, Respectively) 1: The Indicator Is Positive; 0: The Indicator Is Negative | |||||
|---|---|---|---|---|---|---|
| Bone Trabeculae Sprouting | Structurality of Bone Beams | Bone Callus | Marginal Bone Growths | Resorption Zone | Sclerosis Area along the Defect | |
| 1 (IC) | 0/1 | 0/0 | 0/1 | 1/1 | 1/1 | 0/0 |
| 2 (AP) | 1/1/1 | 0/1/1 | 1/1/1 | 1/1/1 | 1/1/1 | 0/1/0 |
| 3 (SI) | 1/1/1 | 0/1/1 | 1/1/1 | 1/1/0 | 1/1/0 | 1/0/0 |
| Time, Months | Cells (%) | |||||
|---|---|---|---|---|---|---|
| Macrophages | Fibroblasts | Lymphocytes | Plasmacytes | Eosinophiles | Multinucleated Cells | |
| 2 | 50.0 | 4.0 | 12.2 | 21.1 | 12.6 | 0.1 |
| 4 | 33.7 | 13.6 | 29.3 | 11.1 | 12.3 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vinogradova, T.I.; Serdobintsev, M.S.; Korzhikova-Vlakh, E.G.; Korzhikov-Vlakh, V.A.; Kaftyrev, A.S.; Blum, N.M.; Semenova, N.Y.; Esmedlyaeva, D.S.; Dyakova, M.E.; Nashchekina, Y.A.; et al. Comparison of Autografts and Biodegradable 3D-Printed Composite Scaffolds with Osteoconductive Properties for Tissue Regeneration in Bone Tuberculosis. Biomedicines 2023, 11, 2229. https://doi.org/10.3390/biomedicines11082229
Vinogradova TI, Serdobintsev MS, Korzhikova-Vlakh EG, Korzhikov-Vlakh VA, Kaftyrev AS, Blum NM, Semenova NY, Esmedlyaeva DS, Dyakova ME, Nashchekina YA, et al. Comparison of Autografts and Biodegradable 3D-Printed Composite Scaffolds with Osteoconductive Properties for Tissue Regeneration in Bone Tuberculosis. Biomedicines. 2023; 11(8):2229. https://doi.org/10.3390/biomedicines11082229
Chicago/Turabian StyleVinogradova, Tatiana I., Mikhail S. Serdobintsev, Evgenia G. Korzhikova-Vlakh, Viktor A. Korzhikov-Vlakh, Alexander S. Kaftyrev, Natalya M. Blum, Natalya Yu. Semenova, Dilyara S. Esmedlyaeva, Marina E. Dyakova, Yulia A. Nashchekina, and et al. 2023. "Comparison of Autografts and Biodegradable 3D-Printed Composite Scaffolds with Osteoconductive Properties for Tissue Regeneration in Bone Tuberculosis" Biomedicines 11, no. 8: 2229. https://doi.org/10.3390/biomedicines11082229
APA StyleVinogradova, T. I., Serdobintsev, M. S., Korzhikova-Vlakh, E. G., Korzhikov-Vlakh, V. A., Kaftyrev, A. S., Blum, N. M., Semenova, N. Y., Esmedlyaeva, D. S., Dyakova, M. E., Nashchekina, Y. A., Dogonadze, M. Z., Zabolotnykh, N. V., & Yablonsky, P. K. (2023). Comparison of Autografts and Biodegradable 3D-Printed Composite Scaffolds with Osteoconductive Properties for Tissue Regeneration in Bone Tuberculosis. Biomedicines, 11(8), 2229. https://doi.org/10.3390/biomedicines11082229