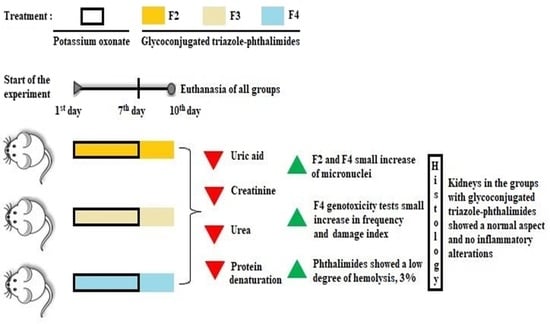
Anti-Hyperuricemic, Anti-Arthritic, Hemolytic Activity and Therapeutic Safety of Glycoconjugated Triazole-Phthalimides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Glycoconjugated Triazole-Phthalimides
2.1.1. General
2.1.2. Typical Procedure to Prepare F2, F3 and F4
2.1.3. 4-(4,6-Di-O-acetyl-2,3-dideoxy-α-D-erythro-hex-2-enopyranoside)-O-methyl-1-(2-phthalimidoethyl)-1,2,3-triazole (F2)
2.1.4. 4-(4,6-Di-O-acetyl-2,3-dideoxy-α-D-erythro-hex-2-enopyranoside)-O-methyl-1-(3-phthalimidopropyl)-1,2,3-triazole (F3)
2.1.5. 4-(4,6-Di-O-acetyl-2,3-dideoxy-α-D-erythro-hex-2-enopyranoside)-O-methyl-1-(4-phthalimidobutyl)-1,2,3-triazole (F4)
2.2. Model of Hyperuricemia in Mice
2.3. Biochemical Analysis
2.4. Histological Analysis
2.5. In Vitro Anti-Arthritic Activity
2.6. Assessment of Genotoxicity and Mutagenicity
2.7. Hemolytic Activity
3. Results
3.1. Chemistry
3.2. Hyperuricemia in Mice
3.3. Histological Analysis
3.4. In Vitro Anti-Arthritic Activity
3.5. Assessment of Genotoxicity and Mutagenicity
3.6. Hemolytic Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ridi, R.E.; Tallima, H. Physiological functions and pathogenic potential of uric acid: A review. J. Adv. Rev. 2017, 8, 487–493. [Google Scholar] [CrossRef]
- Jordheim, L.P.; Peters, G.J. New insights in research on purine and pyrimidine metabolism. Nucl. Nucleot. Nucl. 2022, 41, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Nouri, A.; Ghatreh-Samani, K.; Amini-Khoei, H.; Mohammadi, A.; Heidarian, E.; Najafi, M. Ferulic acid prevents cyclosporine-induced nephrotoxicity in rats through exerting anti-oxidant and anti-inflammatory effects via activation of Nrf2/HO-1 signaling and suppression of NF-κB/TNF-α axis. Naunyn Schmiedebergs Arch. Pharmacol. 2022, 395, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Cui, R.; Zhou, Y.; Ma, Y.; Jin, Y.; Gou, X.; Yang, J.; Wu, X. Uric acid accumulation in the kidney triggers mast cell degranulation and aggravates renal oxidative stress. Toxicology 2023, 483, 153387. [Google Scholar] [CrossRef] [PubMed]
- Helget, L.N.; Mikuls, T.R. Environmental Triggers of Hyperuricemia and Gout. Rheum. Dis. Clin. N. Am. 2022, 48, 891–906. [Google Scholar] [CrossRef]
- Diaz-Torne, C.; Ortiz, M.A.; Garcia-Guillen, A.; Jeria-Navarro, S.; Sainz, L.; Fernandez-Sanchez, S.; Corominas, H.; Vidal, S. The inflammatory role of silent urate crystal deposition in intercritical gout. Rheumatology 2021, 60, 5463–5472. [Google Scholar] [CrossRef]
- Crawley, W.T.; Jungels, C.G.; Stenmark, K.R.; Fini, M.A. U-shaped association of uric acid to overall-cause mortality and its impact on clinical management of hyperuricemia. Redox Biol. 2022, 51, 102271. [Google Scholar] [CrossRef]
- Zitt, E.; Fischer, A.; Lhotta, K.; Concin, H.; Nagel, G. Sex- and age-specific variations, temporal trends and metabolic determinants of serum uric acid concentrations in a large population-based Austrian cohort. Sci. Rep. 2020, 10, 7578. [Google Scholar] [CrossRef]
- Tang, Y.M.; Zhang, L.; Zhu, S.Z.; Pan, J.J.; Zhou, S.H.; He, T.J.; Li, Q. Gout in China, 1990–2017: The global burden of disease study 2017. Public Health 2021, 191, 33–38. [Google Scholar] [CrossRef]
- Light, J.; Wellman, L.L.; Conran, R.M. Educational case: Gout. Acad. Pathol. 2023, 10, 100065. [Google Scholar] [CrossRef]
- Ji, P.; Zhu, J.; Feng, J.; Li, H.; Yu, Q.; Qin, H.; Wei, L.; Zhang, J. Serum uric acid levels and diabetic kidney disease in patients with type 2 diabetes mellitus: A dose-response meta-analysis. Prim. Care Diabetes 2022, 16, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Ohta, Y.; Kamide, K.; Hanada, H.; Morimoto, S.; Nakahashi, T.; Takiuchi, S.; Ishimitsu, T.; Tsuchihashi, T.; Soma, M.; Katsuya, T.T.; et al. Genetic factors associated with elevation of uric acid after treatment with thiazide-like diuretic in patients with essential hypertension. Hypertens. Res. 2020, 43, 220–226. [Google Scholar] [CrossRef]
- Hassanein, E.H.M.; Mohamed, W.R.; Khalaf, M.M.; Shalkami, A.S.; Sayed, A.M.; Hemeida, R.A.M. Diallyl disulfide ameliorates methotrexate-induced nephropathy in rats: Molecular studies and network pharmacology analysis. J. Food Biochem. 2021, 45, e13765. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.H.; Chung, S.J.; Yoo, H.S.; Lee, Y.H.; Baik, K.; Ye, B.S.; Sohn, Y.H.; Lee, P.H. Sex-specific association of urate and levodopa-induced dyskinesia in Parkinson’s disease. Eur. J. Neurol. 2020, 27, 1948–1956. [Google Scholar] [CrossRef]
- Ben Salem, C.; Slim, R.; Fathallah, N.; Hmouda, H. Drug-induced hyperuricaemia and gout. Rheumatology 2017, 56, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Mishima, E.; Anzai, N.; Miyazaki, M.; Abe, T. Uric acid elevation by favipiravir, an antiviral drug. Tohoku J. Exp. Med. 2020, 251, 87–90. [Google Scholar] [CrossRef]
- Ribeiro, H.; Rodrigues, I.; Napoleão, L.; Lira, L.; Marques, D.; Veríssimo, M.; Andrade, J.P.; Dourado, M. Non-steroidal anti-inflammatory drugs (NSAIDs), pain and aging: Adjusting prescription to patient features. Biomed. Pharmacother. 2022, 150, 112958. [Google Scholar] [CrossRef]
- Owusu, Y.B.; Elkhalifa, W.H.; Awaisu, A.; Kheir, N. Assessment of Qatar community pharmacists’ competence and practices related to renal and gastrointestinal adverse effects of nonprescription NSAIDs. Saudi Pharm. J. 2022, 30, 1396–1404. [Google Scholar] [CrossRef]
- Abubakr, N.; Salem, Z.; Ali, Z.; El Assali, M. Comparative evaluation of the early effects of the low-level laser therapy versus intra-articular steroids on temporomandibular joint acute osteoarthriritis in rats: A histochemical, molecular and iamginig evaluation. Dent. Med. Probl. 2018, 55, 359–366. [Google Scholar] [CrossRef]
- Hammadi, S.H.; Hassan, M.A.; Allam, E.A.; Elsharkawy, A.M.; Shams, S.S. Effect of sacubitril/valsartan on cognitive impairment in colchicine-induced Alzheimer’s model in rats. Fundam. Clin. Pharmacol. 2023, 37, 275–286. [Google Scholar] [CrossRef]
- Nataraj, N.; Manivasagam, T.; Thenmozhi, A.J.; Essa, M.M. Neurotrophic Effect of Asiatic acid, a Triterpene of Centella asiatica Against Chronic 1-Methyl 4-Phenyl 1, 2, 3,6-Tetrahydropyridine Hydrochloride/Probenecid Mouse Model of Parkinson’s disease: The Role of MAPK, PI3K-Akt-GSK3β nd mTOR Signalling Pathways. Neurochem. Res. 2017, 42, 1354–1365. [Google Scholar] [CrossRef] [PubMed]
- Perez-Gomez, M.V.; Bartsch, L.A.; Castillo-Rodriguez, E.; Fernandez-Prado, R.; Kanbay, M.; Ortiz, A. Potential dangers of serum urate-lowering therapy. Am. J. Med. 2019, 132, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Imtiaz, M.; Shah, S.A.A.; Rehman, Z. A review of arthritis diagnosis techniques in artificial intelligence era: Current trends and research challenges. Neurosci. Inform. 2022, 2, 100079. [Google Scholar] [CrossRef]
- Tan, H.; Li, Z.; Zhang, S.; Zhang, J.; Jia, E. Novel perception of neutrophil extracellular traps in gouty inflammation. Int. Immunopharmacol. 2023, 115, 109642. [Google Scholar] [CrossRef]
- Ponticelli, C.; Podestà, M.A.; Moroni, G. Hyperuricemia as a trigger of immune response in hypertension and chronic kidney disease. Kidney Int. 2020, 98, 1149–1159. [Google Scholar] [CrossRef]
- Lang, J.; Li, L.; Chen, S.; Quan, Y.; Yi, J.; Zeng, J.; Li, Y.; Zhao, J.; Yin, Z. Mechanism Investigation of Wuwei Shexiang Pills on Gouty Arthritis via Network Pharmacology, Molecule Docking, and Pharmacological Verification. Evid. Based Complement. Alternat. Med. 2022, 2022, 2377692. [Google Scholar] [CrossRef]
- Chen, P.N.; Hao, M.J.; Li, H.J.; Xu, J.; Mahmud, T.; Lan, W.J. Biotransformations of anthranilic acid and phthalimide to potent antihyperlipidemic alkaloids by the marine-derived fungus Scedosporium apiospermum F41-1. Bioorg. Chem. 2021, 116, 105375. [Google Scholar] [CrossRef]
- Almeida, M.L.; Oliveira, M.C.V.A.; Pitta, I.R.; Pitta, M.G.R. Advances in Synthesis and Medicinal Applications of Compounds Derived from Phthalimide. Curr. Org. Synth. 2020, 17, 252–270. [Google Scholar] [CrossRef]
- Pan, L.; Li, X.; Gong, C.; Jin, H.; Qin, B. Synthesis of N-substituted phthalimides and their antifungal activity against Alternaria solani and Botrytis cinerea. Microb. Pathog. 2016, 95, 186–192. [Google Scholar] [CrossRef]
- Gomes, P.A.T.M.; Cardoso, M.V.O.; Santos, I.R.; Sousa, F.A.; Conceicao, J.M.; Silva, V.G.M.; Duarte, D.; Pereira, R.; Oliveira, R.; Nogueira, F.; et al. Dual parasiticidal activities of new Phthalimides: Synthesis and biological profile against Trypanosoma cruzi and Plasmodium falciparum. ChemMedChem 2020, 15, 2164–2175. [Google Scholar] [CrossRef]
- Holanda, V.N.; Lima, E.M.A.; Silva, W.V.; Maia, R.T.; Medeiros, R.L.; Ghosh, A.; Lima, V.L.M.; Figueiredo, R.C.B.Q. Identification of 1,2,3-triazole-phthalimide derivatives as potential drugs against COVID-19: A virtual screening, docking and molecular dynamic study. J. Biomol. Struct. Dyn. 2022, 40, 5462–5480. [Google Scholar] [CrossRef]
- Guan, L.P.; Jin, Q.H.; Tian, G.R.; Chai, K.Y.; Quan, Z.S. Synthesis of some quinoline-2(1H)-one and 1,2,4-triazolo [4,3-a] quinoline derivatives as potent anticonvulsants. J. Pharm. Pharm. Sci. 2007, 10, 254–262. [Google Scholar] [PubMed]
- Assis, S.P.O.; Silva, M.T.; Oliveira, R.N.; Lima, V.L.M. Synthesis and antiinflammatory activity of new alkyl-substituted phthalimide 1h-1,2,3-triazole derivatives. Sci. World J. 2012, 2012, 925925. [Google Scholar] [CrossRef] [PubMed]
- Silva Júnior, J.G.; Araújo, H.D.A.; Rocha, I.G.; Silva Neto, J.C.; Oliveira, R.N.; Assis, S.P.O.; Lima, V.L.M. Antihyperlipidemic activity of glycoconjugated phthalimides in mice submitted to a model of dyslipidemia and insulin resistance. Chem. Biodivers. 2022, 19, e202200119. [Google Scholar] [CrossRef] [PubMed]
- Lavanya, R.; Maheshwari, S.U.; Harish, G.; Raj, J.B.; Kamali, S.; Hemamalani, D.; Varma, J.B.; Reddy, C.U. Investigation of in-vitro anti-inflammatory, anti-platelet and antiarthritic activities in the leaves of Anisomeles malabarica Linn. Res. J. Pharm. Biol. Chem. Sci. 2010, 1, 745–752. [Google Scholar]
- Collins, A.R.; Oscoz, A.A.; Brunborg, G.; Gaivão, I.; Giovannelli, L.; Kruszewski, M.; Smith, C.C.; Stetina, R. The comet assay: Topical issues. Mutagenesis 2008, 23, 143–151. [Google Scholar] [CrossRef]
- Costa, W.K.; do Nascimento, M.F.; Barbosa, É.L.S.; Souza, T.G.d.S.; Chagas, C.A.; Napoleão, T.H.; Correia, M.T.d.S.; Brayner, F.A.; de Oliveira, A.M.; da Silva, M.V. Cytotoxicity, oral toxicity, genotoxicity, and mutagenicity evaluation of essential oil from Psidium glaziovianum Kiaersk leaves. J. Ethnopharmacol. 2023, 303, 115955. [Google Scholar] [CrossRef]
- OECD. Test Nº. 474: Mammalian Erythrocyte Micronucleus Test; OECD: Paris, France, 2016. [Google Scholar]
- Alencar, D.B.; Melo, A.A.; Silva, G.C.; Lima, R.L.; Pires-Cavalcante, K.M.; Carneiro, R.F.; Rabelo, A.S.; Sousa, O.V.; Vieira, R.H.; Viana, F.A.; et al. Antioxidant, hemolytic, antimicrobial, and cytotoxic activities of the tropical Atlantic marine zoanthid Palythoa caribaeorum. An. Acad. Bras. Cienc. 2015, 87, 1113–1123. [Google Scholar] [CrossRef]
- Melo, V.N.; Dantas, W.M.; Camara, C.A.; De Oliveira, R.N. Synthesis of 2,3-unsaturated alkynyl O-glucosides from tri-O-acetyl-d-glucal by using montmorillonite K-10/iron(III) chloride hexahydrate with subsequent copper(I)-catalyzed 1,3-dipolar cycloaddition. Synthesis 2015, 47, 3529–3541. [Google Scholar] [CrossRef]
- Wang, X.; Wang, C.P.; Há, Q.H.; Lv, Y.Z.; Zhang, X.; OuYang, Z.; Kong, L.D. The dual actions of Sanmiao wan as a hypouricemic agent: Down-regulation of hepatic XOD and renal mURAT1 in hyperuricemic mice. J. Ethnopharmacol. 2010, 128, 107–115. [Google Scholar] [CrossRef]
- Habu, Y.; Yanoa, A.; Takeuchia, H.; Saitoa, M.; Okudaa, A.; Fukatsub, K. Decreased activity of basolateral organic ion transports in hyperuricemic rat kidney: Roles of organic ion transporters, rOAT1, rOAT3 and rOCT2. Biochem. Pharmacol. 2003, 66, 1107–1114. [Google Scholar] [CrossRef] [PubMed]
- Habu, Y.; Yanoa, A.; Takeuchia, H.; Saitoa, M.; Okudaa, A.; Fukatsub, K. Restored expression and activity of organic ion transporters rOAT1, rOAT3 and rOCT2 after hyperuricemia in the rat kidney. Biochem. Pharmacol. 2005, 69, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Lanaspa, M.A.; Sanchez-Lozada, L.G.; Choi, Y.J.; Cicerchi, C.; Kanbay, M.; Roncal-Jimenez, C.A.; Ishimoto, T.; Li, N.; Marek, G.; Duranay, M.; et al. Uric acid induces hepatic steatosis by generation of mitochondrial oxidative stress: Potential role in fructose-dependent and -independent fatty liver. J. Biol. Chem. 2012, 287, 40732–40744. [Google Scholar] [CrossRef]
- Lanaspa, M.A.; Sanchez-Lozada, L.G.; Cicerchi, C.; Li, N.; Roncal-Jimenez, C.A.; Ishimoto, T.; Le, M.; Garcia, G.E.; Thomas, J.B.; Rivard, C.J.; et al. Uric acid stimulates fructokinase and accelerates fructose metabolism in the development of fatty liver. PLoS ONE 2012, 7, e47948. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.J.; Nakagawa, T.; Jalal, D.; Sánchez-Lozada, L.G.; Kang, D.H.; Ritz, E. Uric acid and chronic kidney disease: Which is chasing which? Nephrol. Dial. Transplant. 2013, 28, 2221–2228. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.J.; Nakagawa, T.; Sanchez-Lozada, L.G.; Shafiu, M.; Sundaram, S.; Le, M.; Ishimoto, T.; Sautin, Y.Y.; Lanaspa, M.A. Sugar, uric acid, and the etiology of diabetes and obesity. Diabetes 2013, 62, 3307–3315. [Google Scholar] [CrossRef]
- Zhi, L.; Yuzhang, Z.; Tianliang, H.; Hisatome, I.; Yamamoto, T.; Jidong, C. High uric acid induces insulin resistance in cardiomyocytes In Vitro and In Vivo. PLoS ONE 2016, 11, e147737. [Google Scholar] [CrossRef]
- Nakagawa, T.; Hu, H.; Zharikov, S.; Tuttle, K.R.; Short, R.A.; Glushakova, O.; Ouyang, X.; Feig, D.I.; Block, E.R.; Herrera-Acosta, J.; et al. A causal role for uric acid in fructose-induced metabolic syndrome. Am. J. Physiol. Renal. Physiol. 2006, 290, 625–631. [Google Scholar] [CrossRef]
- Zhu, B.; Zhang, J.; Song, N.; Shi, Y.; Fang, Y.; Ding, X.; Li, Y. Distinct Prognostic Role of Serum Uric Acid Levels for Predicting All-Cause Mortality Among Chinese Adults Aged 45~75 Years with and Without Diabetes. Front. Endocrinol. 2021, 12, 782230. [Google Scholar] [CrossRef]
- Lai, Y.J.; Chen, Y.Y.; Ku, P.W.; Chen, L.J.; Yen, Y.F. Association between uric acid level and incidence of albuminuria in patients with type 2 diabetes mellitus: A 4.5-year cohort study. Medicine 2021, 100, e27496. [Google Scholar] [CrossRef]
- Yu, S.; Chen, Y.; Hou, X.; Xu, D.; Che, K.; Li, C.; Yan, S.; Wang, Y.; Wang, B. Serum Uric Acid Levels and Diabetic Peripheral Neuropathy in Type 2 Diabetes: A Systematic Review and Meta-analysis. Mol. Neurobiol. 2016, 53, 1045–1051. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Rong, S.; Wang, Q.; Sun, T.; Bao, W.; Chen, L.; Liu, L. Association between plasma uric acid and insulin resistance in type 2 diabetes: A Mendelian randomization analysis. Diabetes Res. Clin. Pract. 2021, 171, 108542. [Google Scholar] [CrossRef] [PubMed]
- Hall, I.H.; Scoville, J.P.; Reynolds, D.J.; Simlot, R.; Duncan, P. Substituted cyclic imides as potential anti-gout agents. Life Sci. 1990, 46, 1923–1927. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, Y.; Ou, J.; Wan, Q.; Shi, L.; Li, Y.; He, F.; Wang, H.; He, L.; Gao, J. Effect and mechanism of shizhifang on uric acid metabolism in hyperuricemic rats. Evid. Based Compl. Alternat. Med. 2018, 25, 6821387. [Google Scholar] [CrossRef]
- Perez-Ruiz, F.; Herrero-Beites, A.M. Evaluation and treatmentof gout as a chronic disease. Adv. Ther. 2012, 29, 935–946. [Google Scholar] [CrossRef]
- Bardin, T.; Richette, P. Definition of hyperuricemia and goutyconditions. Curr. Opin. Rheumatol. 2014, 26, 186–191. [Google Scholar] [CrossRef]
- Li, T.; Chen, S.L.; Dai, Q.; Han, X.H.; Li, Z.G.; Wu, D.H.; Zhang, X.; Gu, J.R.; Yang, N.P.; Sun, L.Y.; et al. Etoricoxib versus indometacin in the treatment of Chinese patientswith acute gouty arthritis: A randomized double-blind trial. Chin. Med. J. 2013, 126, 1867–1871. [Google Scholar] [CrossRef]
- Thakkar, S.S.; Thakor, P.; Doshi, H.; Ray, A. 1,2,4-Triazole and 1,3,4-oxadiazole analogues: Synthesis, MO studies, in silico molecular docking studies, antimalarial as DHFR inhibitor and antimicrobial activities. Bioorg. Med. Chem. 2017, 25, 4064–4075. [Google Scholar] [CrossRef]
- Castro, T.F.D.; Souza, J.G.S.; Carvalho, A.F.S.; Assis, I.L.; Palmieri, M.J.; Vieira, L.F.A.; Marcussi, S.; Machado, M.R.F.; Murgas, L.D.S. Anxiety-associated behavior and genotoxicity found in adult Danio rerio exposed to tebuconazole-based commercial product. Environ. Toxicol. Pharmacol. 2018, 62, 140–146. [Google Scholar] [CrossRef]
- Teo, S.; Morgan, M.; Stirling, D.; Thomas, S. Assessment of the in vitro and in vivo genotoxicity of thalomid® (Thalidomide). Teratogen. Carcinogen. Mutagen. 2000, 20, 301–311. [Google Scholar] [CrossRef]
- Nayab, P.S.; Irfan, M.; Abid, M.; Pulaganti, M.; Nagaraju, C.; Chitta, S.K.; Rahisuddin. Experimental and molecular docking investigation on DNA interaction of N-substituted phthalimides: Antibacterial, antioxidant and hemolytic activities. Luminescence 2017, 32, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zhao, L.; Liu, L.; Li, Y.; Sun, J.; Liu, Y. Relationship between serum uric acid level and nonalcoholic fatty liver disease in type 2 diabetes patients. Medicine 2021, 100, e26946. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Song, Q.; Yang, X. Lack of associations between elevated serum uric acid and components of metabolic syndrome such as hypertension, dyslipidemia, and T2DM in overweight and obese chinese adults. J. Diabetes Res. 2019, 2019, 3175418. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Zhao, Q.; Zhang, M.; Ban, B.; Tao, H. Association between serum uric acid and triglycerides in Chinese children and adolescents with short stature. Lipids Health Dis. 2021, 20, 1. [Google Scholar] [CrossRef]
- Cibičková, L.; Langová, K.; Vaverková, H.; Kubíčková, V.; Karásek, D. Correlation of uric acid levels and parameters of metabolic syndrome. Physiol. Res. 2017, 66, 481–487. [Google Scholar] [CrossRef]
- Schumacher, H.R., Jr. Management strategies for osteoarthritis, ankylosing spondylitis, and gouty arthritis. J. Clin. Rheumatol. 2004, 10, 18–25. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Y.; Sun, L.; Yan, J.; Mao, H.Q. Nanomedicine Strategies for Anti-Inflammatory Treatment of Noninfectious Arthritis. Adv. Healthc. Mater. 2021, 10, e2001732. [Google Scholar] [CrossRef]
- Cohn, L.A. Acute hemolytic disorders. In Small Animal Critical Care Medicine, 3rd ed.; W.B. Saunders: Philadelphia, PA, USA, 2023; pp. 632–639. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, J.G.d., Júnior; Aires, A.d.L.; Cunha, R.X.d.; Monte, T.V.S.d.; Assis, S.P.d.O.; Oliveira, R.N.d.; Souza, T.G.d.S.; Chagas, C.A.; Silva Neto, J.d.C.; Araújo, H.D.A.d.; et al. Anti-Hyperuricemic, Anti-Arthritic, Hemolytic Activity and Therapeutic Safety of Glycoconjugated Triazole-Phthalimides. Biomedicines 2023, 11, 2537. https://doi.org/10.3390/biomedicines11092537
Silva JGd Júnior, Aires AdL, Cunha RXd, Monte TVSd, Assis SPdO, Oliveira RNd, Souza TGdS, Chagas CA, Silva Neto JdC, Araújo HDAd, et al. Anti-Hyperuricemic, Anti-Arthritic, Hemolytic Activity and Therapeutic Safety of Glycoconjugated Triazole-Phthalimides. Biomedicines. 2023; 11(9):2537. https://doi.org/10.3390/biomedicines11092537
Chicago/Turabian StyleSilva, José Guedes da, Júnior, André de Lima Aires, Rebeca Xavier da Cunha, Talyta Valéria Siqueira do Monte, Shalom Pôrto de Oliveira Assis, Ronaldo Nascimento de Oliveira, Talita Giselly dos Santos Souza, Cristiano Aparecido Chagas, Jacinto da Costa Silva Neto, Hallysson Douglas Andrade de Araújo, and et al. 2023. "Anti-Hyperuricemic, Anti-Arthritic, Hemolytic Activity and Therapeutic Safety of Glycoconjugated Triazole-Phthalimides" Biomedicines 11, no. 9: 2537. https://doi.org/10.3390/biomedicines11092537
APA StyleSilva, J. G. d., Júnior, Aires, A. d. L., Cunha, R. X. d., Monte, T. V. S. d., Assis, S. P. d. O., Oliveira, R. N. d., Souza, T. G. d. S., Chagas, C. A., Silva Neto, J. d. C., Araújo, H. D. A. d., & Lima, V. L. d. M. (2023). Anti-Hyperuricemic, Anti-Arthritic, Hemolytic Activity and Therapeutic Safety of Glycoconjugated Triazole-Phthalimides. Biomedicines, 11(9), 2537. https://doi.org/10.3390/biomedicines11092537