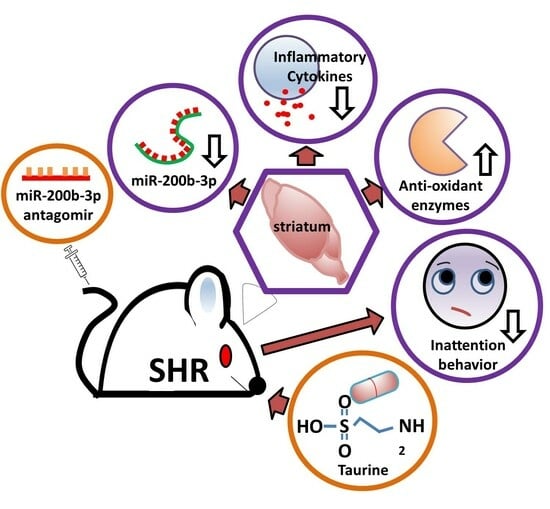
Unraveling the Role of miR-200b-3p in Attention-Deficit/Hyperactivity Disorder (ADHD) and Its Therapeutic Potential in Spontaneously Hypertensive Rats (SHR)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Procedure
2.2. MicroRNA and Striatal Stereotaxic Injection
2.3. Quantitative Real-Time PCR (RT-qPCR)
2.4. Enzyme-Linked Immunosorbent Assay (ELISA)
2.5. Immunohistochemistry (IHC)
2.6. Immunoblotting
2.7. Spontaneous Alternation
2.8. Statistical Analysis
3. Results
3.1. Effect of High-Dose Taurine on Expressions of miR-200b-3p and Silt2 in the Striatum of WKY and SHR
3.2. Effects of miR-200b-3p Antagomir on miR-200b-3p and Slit2 Protein Expressions in Striatum of SHR
3.3. MiR-200b-3p Antagomir Attenuated the Expressions of Inflammatory Cytokines and Increased the Activity of Antioxidant Enzymes
3.4. MiR-200b-3p Antagomir Improves Working Memory in SHR
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Danielson, M.L.; Bitsko, R.H.; Ghandour, R.M.; Holbrook, J.R.; Kogan, M.D.; Blumberg, S.J. Prevalence of parent-reported ADHD diagnosis and associated treatment among U.S. children and adolescents, 2016. J. Clin. Child Adolesc. Psychol. 2018, 53, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Spekker, E.; Szabó, Á.; Polyák, H.; Vécsei, L. Modelling the neurodevelopmental pathogenesis in neuropsychiatric disorders. Bioactive kynurenines and their analogues as neuroprotective agents-in celebration of 80th birthday of Professor Peter Riederer. J. Neural Transm. 2022, 129, 627–642. [Google Scholar] [CrossRef] [PubMed]
- Biederman, J.; Mick, E.; Faraone, S.V. Age-dependent decline of symptoms of attention deficit hyperactivity disorder: Impact of remission definition and symptom type. Am. J. Psychiatry 2000, 157, 816–818. [Google Scholar] [CrossRef]
- Asherson, P.; IMAGE Consortium. Attention-Deficit Hyperactivity Disorder in the post-genomic era. Eur. Child Adolesc. Psychiatry 2004, 13 (Suppl. S1), 150–170. [Google Scholar] [CrossRef]
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993, 75, 843–854. [Google Scholar] [CrossRef]
- Wightman, B.; Ha, I.; Ruvkun, G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell 1993, 75, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Li, S.C.; Chan, W.C.; Hu, L.Y.; Lai, C.H.; Hsu, C.N.; Lin, W.C. Identification of homologous microRNAs in 56 animal genomes. Genomics 2010, 96, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Friedlander, M.R.; Lizano, E.; Houben, A.J.; Bezdan, D.; Banez-Coronel, M.; Kudla, G.; Mateu-Huertas, E.; Kagerbauer, B.; González, J.; Chen, K.C.; et al. Evidence for the biogenesis of more than 1000 novel human microRNAs. Genome Biol. 2014, 15, R57. [Google Scholar] [CrossRef] [PubMed]
- Forman, J.J.; Legesse-Miller, A.; Coller, H.A. A search for conserved sequences in coding regions reveals that the let-7 microRNA targets Dicer within its coding sequence. Proc. Natl. Acad. Sci. USA 2008, 105, 14879–14884. [Google Scholar] [CrossRef] [PubMed]
- Dharap, A.; Pokrzywa, C.; Murali, S.; Pandi, G.; Vemuganti, R. MicroRNA miR-324-3p induces promoter-mediated expression of RelA gene. PLoS ONE 2013, 8, e79467. [Google Scholar] [CrossRef] [PubMed]
- Juvale, I.I.A.; Che Has, A.T. The Potential Role of miRNAs as Predictive Biomarkers in Neurodevelopmental Disorders. J. Mol. Neurosci. 2021, 71, 1338–1355. [Google Scholar] [CrossRef] [PubMed]
- Abdolahi, S.; Zare-Chahoki, A.; Noorbakhsh, F.; Gorji, A. A Review of Molecular Interplay between Neurotrophins and miRNAs in Neuropsychological Disorders. Mol. Neurobiol. 2022, 59, 6260–6280. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Szabó, Á.; Vécsei, L.; Giménez-Llort, L. Emerging Translational Research in Neurological and Psychiatric Diseases: From In Vitro to In Vivo Models. Int. J. Mol. Sci. 2023, 24, 15739. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Pan, J.; Cai, Q.Q.; Zhang, F.; Peng, M.; Fan, X.L.; Ji, H.; Dong, Y.W.; Wu, X.Z.; Wu, L.H. MicroRNA profile as potential molecular signature for attention deficit hyperactivity disorder in children. Biomarkers 2022, 27, 230–239. [Google Scholar] [CrossRef]
- Wang, L.J.; Kuo, H.C.; Lee, S.Y.; Huang, L.H.; Lin, Y.; Lin, P.H.; Li, S.C. MicroRNAs serve as prediction and treatment-response biomarkers of attention-deficit/hyperactivity disorder and promote the differentiation of neuronal cells by repressing the apoptosis pathway. Transl. Psychiatry 2022, 12, 67. [Google Scholar] [CrossRef] [PubMed]
- Davison, A.N.; Kaczmarek, L.K. Taurine—A possible neurotransmitter? Nature 1971, 234, 107–108. [Google Scholar] [CrossRef]
- Wu, J.Y.; Prentice, H. Role of taurine in the central nervous system. J. Biomed. Sci. 2010, 17 (Suppl. S1), S1. [Google Scholar] [CrossRef]
- Kumari, N.; Prentice, H.; Wu, J.Y. Taurine and its neuroprotective role. In Taurine 8, 18th International Taurine Meeting, Marrakech, Morocco, 7–13 April 2012; Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2013; Volume 775, pp. 19–27. [Google Scholar] [CrossRef]
- Jakaria, M.; Azam, S.; Haque, M.E.; Jo, S.H.; Uddin, M.S.; Kim, I.S.; Choi, D.K. Taurine and its analogs in neurological disorders: Focus on therapeutic potential and molecular mechanisms. Redox Biol. 2019, 24, 101223. [Google Scholar] [CrossRef]
- Chung, M.C.; Malatesta, P.; Bosquesi, P.L.; Yamasaki, P.R.; Santos, J.L.; Vizioli, E.O. Advances in drug design based on the amino Acid approach: Taurine analogues for the treatment of CNS diseases. Pharmaceuticals 2012, 5, 1128–1146. [Google Scholar] [CrossRef] [PubMed]
- De Luca, A.; Pierno, S.; Camerino, D.C. Taurine: The appeal of a safe amino acid for skeletal muscle disorders. J. Transl. Med. 2015, 13, 243. [Google Scholar] [CrossRef]
- Ito, T.; Schaffer, S.; Azuma, J. The effect of taurine on chronic heart failure: Actions of taurine against catecholamine and angiotensin II. Amino Acids. 2014, 46, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, S.; Kim, H.W. Effects and Mechanisms of Taurine as a Therapeutic Agent. Biomol. Ther. 2018, 26, 225–241. [Google Scholar] [CrossRef]
- Malek Mahdavi, A.; Javadivala, Z. A systematic review of preclinical studies on the efficacy of taurine for the treatment of rheumatoid arthritis. Amino Acids 2021, 53, 783–800. [Google Scholar] [CrossRef] [PubMed]
- Roşca, A.E.; Vlădăreanu, A.M.; Mirica, R.; Anghel-Timaru, C.M.; Mititelu, A.; Popescu, B.O.; Căruntu, C.; Voiculescu, S.E.; Gologan, Ş.; Onisâi, M.; et al. Taurine and Its Derivatives: Analysis of the Inhibitory Effect on Platelet Function and Their Antithrombotic Potential. J. Clin. Med. 2022, 11, 666. [Google Scholar] [CrossRef]
- Kp, A.D.; Martin, A. Recent insights into the molecular regulators and mechanisms of taurine to modulate lipid metabolism: A review. Crit. Rev. Food Sci. Nutr. 2022, 18, 6005–6017. [Google Scholar] [CrossRef] [PubMed]
- Surai, P.F.; Earle-Payne, K.; Kidd, M.T. Taurine as a Natural Antioxidant: From Direct Antioxidant Effects to Protective Action in Various Toxicological Models. Antioxidants 2021, 10, 1876. [Google Scholar] [CrossRef]
- Jia, H.; Miyoshi, M.; Li, X.; Furukawa, K.; Otani, L.; Shirahige, K.; Miura, F.; Ito, T.; Kato, H. The Epigenetic Legacy of Maternal Protein Restriction: Renal Ptger1 DNA Methylation Changes in Hypertensive Rat Offspring. Nutrients 2023, 15, 3957. [Google Scholar] [CrossRef]
- Sagvolden, T.; Russell, V.A.; Aase, H.; Johansen, E.B.; Farshbaf, M. Rodent models of attention-deficit/hyperactivitydisorder. Biol. Psychiatry 2005, 57, 1239–1247. [Google Scholar] [CrossRef]
- Chen, V.C.; Hsu, T.C.; Chen, L.J.; Chou, H.C.; Weng, J.C.; Tzang, B.S. Effects of taurine on resting-state fMRI activity in spontaneously hypertensive rats. PLoS ONE 2017, 12, e0181122. [Google Scholar] [CrossRef]
- Chen, V.C.; Chiu, C.C.; Chen, L.J.; Hsu, T.C.; Tzang, B.S. Effects of taurine on striatal dopamine transporter expression and dopamine uptake in SHR rats. Behav. Brain Res. 2018, 348, 219–226. [Google Scholar] [CrossRef]
- Sim, M.S.; Soga, T.; Pandy, V.; Wu, Y.S.; Parhar, I.S.; Mohamed, Z. MicroRNA expression signature of methamphetamine use and addiction in the rat nucleus accumbens. Metab. Brain Dis. 2017, 32, 1767–1783. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.W.; Rodriguez-Ortiz, C.J.; Lim, S.L.; Zumkehr, J.; Kilian, J.G.; Vidal, J.; Kitazawa, M. Copper-Induced Upregulation of MicroRNAs Directs the Suppression of Endothelial LRP1 in Alzheimer’s Disease Model. Toxicol. Sci. 2019, 170, 144–156. [Google Scholar] [CrossRef]
- Regan, S.L.; Williams, M.T.; Vorhees, C.V. Review of rodent models of attention deficit hyperactivity disorder. Neurosci. Biobehav. Rev. 2022, 132, 621–637. [Google Scholar] [CrossRef]
- Dabrowska, S.; Andrzejewska, A.; Kozlowska, H.; Strzemecki, D.; Janowski, M.; Lukomska, B. Neuroinflammation evoked by brain injury in a rat model of lacunar infarct. Exp. Neurol. 2021, 336, 113531. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Chou, Y.H.; Liu, Y.L.; Hsu, T.C.; Yow, J.L.; Tzang, B.S.; Chiang, W.H. Tumor acidity-responsive polymeric nanoparticles to promote intracellular delivery of zoledronic acid by PEG detachment and positive charge exposure for enhanced antitumor potency. J. Mater. Chem. B 2022, 10, 4363–4374. [Google Scholar] [CrossRef] [PubMed]
- Katz, R.J.; Schmaltz, K. Dopaminergic involvement in attention. A novel animal model. Prog. Neuropsychopharmacol. 1980, 4, 585–590. [Google Scholar] [CrossRef]
- Pasquinelli, A.E. MicroRNAs: Deviants no longer. Trends Genet. 2002, 18, 171–173. [Google Scholar] [CrossRef]
- Miska, E.A. How microRNAs control cell division, differentiation and death. Curr. Opin. Genet. Dev. 2005, 15, 563–568. [Google Scholar] [CrossRef]
- Iguchi, H.; Kosaka, N.; Ochiya, T. Versatile applications of microRNA in anti-cancer drug discovery: From therapeutics to biomarkers. Curr. Drug Discov. Technol. 2010, 7, 95–105. [Google Scholar] [CrossRef]
- Long, J.M.; Lahiri, D.K. Advances in microRNA experimental approaches to study physiological regulation of gene products implicated in CNS disorders. Exp. Neurol. 2012, 235, 402–418. [Google Scholar] [CrossRef]
- Gehrau, R.C.; Mas, V.R.; Maluf, D.G. Hepatic disease biomarkers and liver transplantation: What is the potential utility of microRNAs? Expert. Rev. Gastroenterol. Hepatol. 2013, 7, 157–170. [Google Scholar] [CrossRef]
- Harris, V.K.; Sadiq, S.A. Biomarkers of therapeutic response in multiple sclerosis: Current status. Mol. Diagn. Ther. 2014, 18, 605–617. [Google Scholar] [CrossRef] [PubMed]
- De Gonzalo-Calvo, D.; Vea, A.; Bär, C.; Fiedler, J.; Couch, L.S.; Brotons, C.; Llorente-Cortes, V.; Thum, T. Circulating non-coding RNAs in biomarker-guided cardiovascular therapy: A novel tool for personalized medicine? Eur. Heart J. 2019, 40, 1643–1650. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Tu, Y.; Yuan, H.; Shi, Z.; Guo, Y.; Gong, W.; Tu, S. Regulatory functions of miR-200b-3p in tumor development (Review). Oncol. Rep. 2022, 47, 96. [Google Scholar] [CrossRef]
- Chen, W.; Jiang, W.; Dong, J.; Wang, J.; Wang, B. miR-200b-3p Induces the Formation of Insulin-Producing Cells from Umbilical Cord Mesenchymal Stem Cells by Targeting ZEB2. Crit. Rev. Eukaryot. Gene Expr. 2022, 32, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; He, C.; Bao, C.; Li, Z.; Jin, W.; Li, C.; Chen, Y. MiRNA Profiling and Its Potential Roles in Rapid Growth of Velvet Antler in Gansu Red Deer (Cervus elaphus kansuensis). Genes 2023, 14, 424. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X. MiR-200b-3p is upregulated in the placental tissues from patients with preeclampsia and promotes the development of preeclampsia via targeting profilin 2. Cell Cycle 2022, 21, 1945–1957. [Google Scholar] [CrossRef]
- Huang, W.; Chen, J.; Xu, E.; Zhu, T.; Cai, X. KCNQ1OT1 mediates keratinocyte migration to promote skin wound healing through the miR-200b-3p/SERP1 axis. Burns 2022, 49, 415–424. [Google Scholar] [CrossRef]
- Zheng, Y.L.; Su, X.; Chen, Y.M.; Guo, J.B.; Song, G.; Yang, Z.; Chen, P.J.; Wang, X.Q. microRNA-Based Network and Pathway Analysis for Neuropathic Pain in Rodent Models. Front. Mol. Biosci. 2022, 8, 780730. [Google Scholar] [CrossRef]
- Florian, I.A.; Buruiana, A.; Timis, T.L.; Susman, S.; Florian, I.S.; Balasa, A.; Berindan-Neagoe, I. An Insight into the microRNAs Associated with Arteriovenous and Cavernous Malformations of the Brain. Cells 2021, 10, 1373. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Yang, L.; Meng, L.; Cui, H. Inhibition of miR-200b-3p alleviates hypoxia-ischemic brain damage via targeting Slit2 in neonatal rats. Biochem. Biophys. Res. Commun. 2020, 523, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Zhou, L.; Liu, M.; Zhang, D.; Yan, Y.; Chang, Y.F.; Zhang, X.; Xie, Q.; Luo, Q. gga-miR-200b-3p Promotes Macrophage Activation and Differentiation via Targeting Monocyte to Macrophage Differentiation-Associated in HD11 Cells. Front. Immunol. 2020, 11, 563143. [Google Scholar] [CrossRef] [PubMed]
- Kozak, J.; Jonak, K.; Maciejewski, R. The function of miR-200 family in oxidative stress response evoked in cancer chemotherapy and radiotherapy. Biomed. Pharmacother. 2020, 125, 110037. [Google Scholar] [CrossRef]
- Lin, Y.; Liu, X.; Cheng, Y.; Yang, J.; Huo, Y.; Zhang, C. Involvement of MicroRNAs in hydrogen peroxide-mediated gene regulation and cellular injury response in vascular smooth muscle cells. J. Biol. Chem. 2009, 284, 7903–7913. [Google Scholar] [CrossRef]
- Kim, C.; Cha, Y.N. Taurine chloramine produced from taurine under inflammation provides anti-inflammatory and cytoprotective effects. Amino Acids 2014, 46, 89–100. [Google Scholar] [CrossRef]
- Ng, H.M.; Ho, J.C.H.; Nong, W.; Hui, J.H.L.; Lai, K.P.; Wong, C.K.C. Genome-wide analysis of MicroRNA-messenger RNA interactome in ex-vivo gill filaments, Anguilla japonica. BMC Genom. 2020, 21, 208. [Google Scholar] [CrossRef]
- Kofler, M.J.; Singh, L.J.; Soto, E.F.; Chan, E.S.M.; Miller, C.E.; Harmon, S.L.; Spiegel, J.A. Working memory and short-term memory deficits in ADHD: A bifactor modeling approach. Neuropsychology 2020, 34, 686–698. [Google Scholar] [CrossRef]
- Meneses, A.; Perez-Garcia, G.; Ponce-Lopez, T.; Tellez, R.; Gallegos-Cari, A.; Castillo, C. Spontaneously hypertensive rat (SHR) as an animal model for ADHD: A short overview. Rev. Neurosci. 2011, 22, 365–371. [Google Scholar] [CrossRef]
- Lee, W.S.; Yoon, B.E. Necessity of an Integrative Animal Model for a Comprehensive Study of Attention-Deficit/Hyperactivity Disorder. Biomedicines 2023, 11, 1260. [Google Scholar] [CrossRef]
- Yoshida, M.; Watanabe, Y.; Yamanishi, K.; Yamashita, A.; Yamamoto, H.; Okuzaki, D.; Shimada, K.; Nojima, H.; Yasunaga, T.; Okamura, H.; et al. Analysis of genes causing hypertension and stroke in spontaneously hypertensive rats: Gene expression profiles in the brain. Int. J. Mol. Med. 2014, 33, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Arnsten, A.F. Toward a new understanding of attention-deficit hyperactivity disorder pathophysiology: An important role for prefrontal cortex dysfunction. CNS Drugs 2009, 23, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Womersley, J.S.; Dimatelis, J.J.; Russell, V.A. Proteomic analysis of maternal separation-induced striatal changes in a rat model of ADHD: The spontaneously hypertensive rat. J. Neurosci. Methods 2015, 252, 64–74. [Google Scholar] [CrossRef]
- Rana, S.K.; Sanders, T.A.B. Taurine concentrations in the diet, plasma, urine and breast milk of vegans compared with omnivores. Br. J. Nutr. 1986, 56, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Purchas, R.W.; Rutherfurd, S.M.; Pearce, P.D.; Vather, R.; Wilkinson, B.H. Concentrations in beef and lamb of taurine, carnosine, coenzyme Q(10), and creatine. Meat Sci. 2004, 66, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Kontro, P. Interactions of taurine and dopamine in the striatum. In The Biology of Taurine; Advances in Experimental Medicine and Biology; Springer: Boston, MA, USA, 1987; Volume 217, pp. 347–355. [Google Scholar] [CrossRef]
- Ahmadian, M.; Roshan, V.D.; Aslani, E.; Stannard, S.R. Taurine supplementation has anti-atherogenic and anti-inflammatory effects before and after incremental exercise in heart failure. Ther. Adv. Cardiovasc. Dis. 2017, 11, 185–194. [Google Scholar] [CrossRef]
- Azuma, J.; Sawamura, A.; Awata, N.; Ohta, H.; Hamaguchi, T.; Harada, H.; Takihara, K.; Hasegawa, H.; Yamagami, T.; Ishiyama, T.; et al. Therapeutic effect of taurine in congestive heart failure: A double-blind crossover trial. Clin. Cardiol. 1985, 8, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T.; Ando, K.; Noda, H.; Ito, Y.; Sato, Y. Effects of increased adrenomedullary activity and taurine in young patients with borderline hypertension. Circulation 1987, 75, 525–532. [Google Scholar] [CrossRef]
- Durelli, L.; Mutani, R.; Fassio, F. The treatment of myotonia: Evaluation of chronic oral taurine therapy. Neurology 1983, 33, 599–603. [Google Scholar] [CrossRef]
- Bergamini, L.; Mutani, R.; Delsedime, M.; Durelli, L. First clinical experience on the antiepileptic action of taurine. Eur. Neurol. 1974, 11, 261–269. [Google Scholar] [CrossRef]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Saré, R.M.; Lemons, A.; Smith, C.B. Behavior Testing in Rodents: Highlighting Potential Confounds Affecting Variability and Reproducibility. Brain Sci. 2021, 11, 522. [Google Scholar] [CrossRef] [PubMed]
- Améndola, L.; Weary, D.; Zobel, G. Effects of personality on assessments of anxiety and cognition. Neurosci. Biobehav. Rev. 2022, 141, 104827. [Google Scholar] [CrossRef]
- Oenarto, J.; Karababa, A.; Castoldi, M.; Bidmon, H.J.; Görg, B.; Häussinger, D. Ammonia-induced miRNA expression changes in cultured rat astrocytes. Sci. Rep. 2016, 6, 18493. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Qiu, Z.; Inam-U-Llah; Zhang, M.; Li, K.; Wu, P.; Suleman, R.; Aadil, R.M.; Piao, F. The microRNAs Expression Profile in Sciatic Nerves of Diabetic Neuropathy Rats After Taurine Treatment by Sequencing. In Taurine 11, Proceedings of the 21st International Taurine Meeting, Shenyang, China, 20–26 May 2018; Advances in Experimental Medicine and Biology; Springer: Singapore, 2019; Volume 1155, pp. 935–947. [Google Scholar] [CrossRef]
- Nabi, A.A.; Atta, S.A.; El-Ahwany, E.; Elzayat, E.; Saleh, H. Taurine Upregulates miRNA-122-5p Expression and Suppresses the Metabolizing Enzymes of Glycolytic Pathway in Hepatocellular Carcinoma. Mol. Biol. Rep. 2021, 48, 5549–5559. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Lu, C.; Zhang, D.; Liu, H.; Cui, S. Taurine promotes estrogen synthesis by regulating microRNA-7a2 in mice ovarian granulosa cells. Biochem. Biophys. Res. Commun. 2022, 626, 129–134. [Google Scholar] [CrossRef]
- Song, Q.; Guo, J.X.; Ma, Y.X.; Ou, T.; Zhang, J.; Li, H.Z.; Mi, S.Q.; Zhang, Y.Z.; Oda, H.; Chen, W. Taurine alleviated hepatic steatosis in oleic acid-treated-HepG2 cells and rats fed a high-fat diet. Heliyon 2023, 9, e16401. [Google Scholar] [CrossRef]
- Grodzka, O.; Procyk, G.; Gąsecka, A. The Role of MicroRNAs in Myocarditis-What Can We Learn from Clinical Trials? Int. J. Mol. Sci. 2022, 23, 16022. [Google Scholar] [CrossRef]
- Zhai, W.; Zhao, M.; Zhang, G.; Wang, Z.; Wei, C.; Sun, L. MicroRNA-Based Diagnosis and Therapeutics for Vascular Cognitive Impairment and Dementia. Front. Neurol. 2022, 13, 895316. [Google Scholar] [CrossRef]
- Cools, R.; Froböse, M.; Aarts, E.; Hofmans, L. Dopamine and the motivation of cognitive control. Handb. Clin. Neurol. 2019, 163, 123–143. [Google Scholar] [CrossRef]







| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| Slit2 | 5′-CGCCAAAGGGATTCAAGTGT-3′ | 5′-CACTGGCATATTGGTTCATTCA-3′ |
| β-actin | 5′-CCCATCTATGAGGGTTACGC-3′ | 5′-TTTAATGTCACGCACGATTTC-3′ |
| TNF-α | 5′-TCAGCCGATTTGCCATTTCAT-3′ | 5′-ACACGCCAGTCGCTTCACAGA-3′ |
| IL-1β | 5′-GTCCTTTCACTTGCCCTCAT-3′ | 5′-CAAACTGGTCACAGCTTTCGA-3′ |
| IL-6 | 5′-AATGCCTCGTGCTGTCTGACC-3′ | 5′-GGTGGGTGTGCCGTCTTTCATC-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, T.-M.; Lin, H.-L.; Tzang, C.-C.; Liang, J.-A.; Hsu, T.-C.; Tzang, B.-S. Unraveling the Role of miR-200b-3p in Attention-Deficit/Hyperactivity Disorder (ADHD) and Its Therapeutic Potential in Spontaneously Hypertensive Rats (SHR). Biomedicines 2024, 12, 144. https://doi.org/10.3390/biomedicines12010144
Chang T-M, Lin H-L, Tzang C-C, Liang J-A, Hsu T-C, Tzang B-S. Unraveling the Role of miR-200b-3p in Attention-Deficit/Hyperactivity Disorder (ADHD) and Its Therapeutic Potential in Spontaneously Hypertensive Rats (SHR). Biomedicines. 2024; 12(1):144. https://doi.org/10.3390/biomedicines12010144
Chicago/Turabian StyleChang, Tung-Ming, Hsiu-Ling Lin, Chih-Chen Tzang, Ju-An Liang, Tsai-Ching Hsu, and Bor-Show Tzang. 2024. "Unraveling the Role of miR-200b-3p in Attention-Deficit/Hyperactivity Disorder (ADHD) and Its Therapeutic Potential in Spontaneously Hypertensive Rats (SHR)" Biomedicines 12, no. 1: 144. https://doi.org/10.3390/biomedicines12010144
APA StyleChang, T. -M., Lin, H. -L., Tzang, C. -C., Liang, J. -A., Hsu, T. -C., & Tzang, B. -S. (2024). Unraveling the Role of miR-200b-3p in Attention-Deficit/Hyperactivity Disorder (ADHD) and Its Therapeutic Potential in Spontaneously Hypertensive Rats (SHR). Biomedicines, 12(1), 144. https://doi.org/10.3390/biomedicines12010144