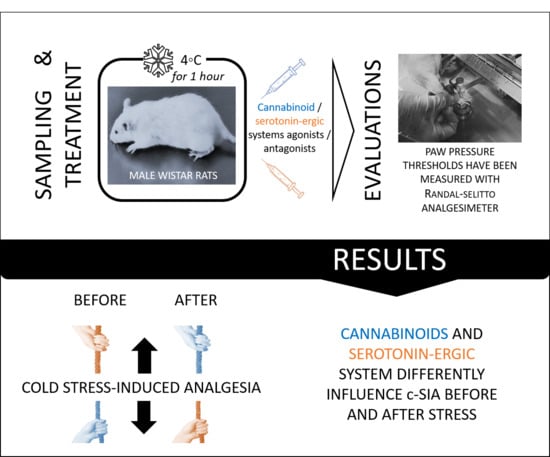
Cannabinoid and Serotonergic Systems: Unraveling the Pathogenetic Mechanisms of Stress-Induced Analgesia
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Study Design
2.2. Animals
2.3. Methodology
2.4. Experimental Groups
2.5. Acute Model of Cold Stress
2.6. Drugs
2.7. Nociceptive Test
2.8. Statistical Analysis
3. Results
3.1. Antinociceptive Effect of AEA and DPAT before and after 1 h of Cold Exposure
3.2. Effects of Agonist/Antagonist Co-Administration before and after Cold Exposure on Cold-SIA
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, S.M.; Vale, W.W. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin. Neurosci. 2022, 8, 367–381. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.S. The physiology of stress and the human body’s response to stress. In Epigenetics of Stress and Stress Disorders; Academic Press: Cambridge, MA, USA, 2022; Volume 31, pp. 1–18. [Google Scholar] [CrossRef]
- Oh, J.; Lee, H.Y.; Khuong, Q.L.; Markuns, J.F.; Bullen, C.; Barrios, O.E.A.; Hwang, S.S.; Suh, Y.S.; McCool, J.; Kachur, S.P.; et al. Mobility restrictions were associated with reductions in COVID-19 incidence early in the pandemic: Evidence from a real-time evaluation in 34 countries. Sci. Rep. 2021, 11, 13717. [Google Scholar] [CrossRef] [PubMed]
- Koolhaas, J.M.; Bartolomucci, A.; Buwalda, B.; de Boer, S.F.; Flügge, G.; Korte, S.M.; Meerlo, P.; Murison, R.; Olivier, B.; Palanza, P.; et al. Stress revisited: A critical evaluation of the stress concept. Neurosci. Biobehav. Rev. 2011, 35, 1291–1301. [Google Scholar] [CrossRef] [PubMed]
- Beecher, H.K. Pain in men wounded in battle. Ann. Surg. 1946, 123, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Ford, G.K.; Finn, D.P. Clinical correlates of stress-induced analgesia: Evidence from pharmacological studies. Pain 2008, 140, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Willer, J.C.; Dehen, H.; Cambier, J. Stress-induced analgesia in humans: Endogenous opioids and naloxone-reversible depression of pain reflexes. Science 1981, 212, 689–691. [Google Scholar] [CrossRef]
- Butler, R.K.; Finn, D.P. Stress-induced analgesia. Prog. Neurobiol. 2009, 88, 184–202. [Google Scholar] [CrossRef]
- Calcagnetti, D.J.; Helmstetter, F.J.; Fanselow, M.S. Quaternary naltrexone reveals the central mediation of conditional opioid analgesia. Pharmacol. Biochem. Behav. 1987, 27, 529–531. [Google Scholar] [CrossRef]
- Watkins, L.R.; Mayer, D.J. Multiple endogenous opiate and non-opiate analgesia systems: Evidence of their existence and clinical implications. Ann. N. Y. Acad. Sci. 1986, 467, 273–299. [Google Scholar] [CrossRef]
- Helmstetter, F.J.; Landeira-Fernandez, J. Conditional hypoalgesia is attenuated by naltrexone applied to the periaqueductal gray. Brain Res. 1990, 537, 88–92. [Google Scholar] [CrossRef]
- Helmstetter, F.J. The amygdala is essential for the expression of conditional hypoalgesia. Behav. Neurosci. 1992, 106, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Hohmann, A.G.; Suplita, R.L.; Bolton, N.M.; Neely, M.H.; Fegley, D.; Mangieri, R.; Krey, J.F.; Walker, J.M.; Holmes, P.V.; Crystal, J.D.; et al. An endocannabinoid mechanism for stress-induced analgesia. Nature 2005, 435, 1108–1112. [Google Scholar] [CrossRef] [PubMed]
- Woodhams, S.G.; Chapman, V.; Finn, D.P.; Hohmann, A.G.; Neugebauer, V. The cannabinoid system and pain. Neuropharmacology 2017, 124, 105–120. [Google Scholar] [CrossRef] [PubMed]
- Micale, V.; Drago, F. Endocannabinoid system, stress and HPA axis. Eur. J. Pharmacol. 2018, 834, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Rothner, A.; Gov, T.; Hinden, L.; Nemirovski, A.; Tam, J.; Rosenzweig, B. Systemic Changes in Endocannabinoids and Endocannabinoid-like Molecules in Response to Partial Nephrectomy-Induced Ischemia in Humans. Int. J. Mol. Sci. 2023, 24, 4216. [Google Scholar] [CrossRef] [PubMed]
- Immke, D.C.; Gavva, N.R. The TRPV1 receptor and nociception. Semin. Cell Dev. Biol. 2006, 17, 582–591. [Google Scholar] [CrossRef]
- Herkenham, M.; Lynn, A.B.; Little, M.D.; Johnson, M.R.; Melvin, L.S.; de Costa, B.R.; Rice, K.C. Cannabinoid receptor localization in brain. Proc. Natl. Acad. Sci. USA 1990, 87, 1932–1936. [Google Scholar] [CrossRef]
- Herkenham, M.; Lynn, A.; Johnson, M.R.; Melvin, L.; de Costa, B.; Rice, K. Characterization and localization of cannabinoid receptors in rat brain: A quantitative in vitro autoradiographic study. J. Neurosci. 1991, 11, 563–583. [Google Scholar] [CrossRef]
- Ziegler, C.G.; Mohn, C.; Lamounier-Zepter, V.; Rettori, V.; Bornstein, S.R.; Krug, A.W.; Ehrhart-Bornstein, M. Expression and Function of Endocannabinoid Receptors in the Human Adrenal Cortex. Horm. Metab. Res. 2009, 42, 88–92. [Google Scholar] [CrossRef]
- Van Sickle, M.D.; Duncan, M.; Kingsley, P.J.; Mouihate, A.; Urbani, P.; Mackie, K.; Stella, N.; Makriyannis, A.; Piomelli, D.; Davison, J.S.; et al. Identification and Functional Characterization of Brainstem Cannabinoid CB2 Receptors. Science 2005, 310, 329–332. [Google Scholar] [CrossRef]
- Gong, J.-P.; Onaivi, E.S.; Ishiguro, H.; Liu, Q.-R.; Tagliaferro, P.A.; Brusco, A.; Uhl, G.R. Cannabinoid CB2 receptors: Immunohistochemical localization in rat brain. Brain Res. 2006, 1071, 10–23. [Google Scholar] [CrossRef]
- Wang, M.; Hill, M.N.; Zhang, L.; Gorzalka, B.B.; Hillard, C.J.; Alger, B.E. Acute restraint stress enhances hippocampal endo-cannabinoid function via glucocorticoid receptor activation. J. Psychopharmacol. 2011, 26, 56–70. [Google Scholar] [CrossRef] [PubMed]
- Cota, D.; Steiner, M.-A.; Marsicano, G.; Cervino, C.; Herman, J.; Grübler, Y.; Stalla, J.; Pasquali, R.; Lutz, B.; Stalla, G.K.; et al. Requirement of Cannabinoid Receptor Type 1 for the Basal Modulation of Hypothalamic-Pituitary-Adrenal Axis Function. Endocrinology 2007, 148, 1574–1581. [Google Scholar] [CrossRef] [PubMed]
- Finn, D.P.; Haroutounian, S.; Hohmann, A.G.; Krane, E.; Soliman, N.; Rice, A.S.C. Cannabinoids, the endocannabinoid system and pain: A review of preclinical studies. Pain 2021, 162, S5–S25. [Google Scholar] [CrossRef] [PubMed]
- Viveros, M.P.; Marco, E.M.; File, S.E. Endocannabinoid system and stress and anxiety responses. Pharmacol. Biochem. Behav. 2005, 81, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Aviram, J.; Samuelly-Leichtag, G. Efficacy of Cannabis-Based Medicines for Pain Management: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Pain Physician 2017, 20, E755–E796. [Google Scholar] [CrossRef] [PubMed]
- Petzke, F.; Tölle, T.; Fitzcharles, M.A.; Häuser, W. Cannabis-Based Medicines and Medical Cannabis for Chronic Neuropathic Pain. CNS Drugs 2022, 36, 31–44. [Google Scholar] [CrossRef]
- Tournier, M.; Sorbara, F.; Gindre, C.; Swendsen, J.D.; Verdoux, H. Cannabis use and anxiety in daily life: A naturalistic investigation in a non-clinical population. Psychiatry Res. 2003, 118, 1–8. [Google Scholar] [CrossRef]
- Arevalo, C.; De Miguel, R.; Hernandez-Tristan, R. Cannabinoid effects on anxiety-related behaviors and hypothalamic neurotransmitters. Pharmacol. Biochem. Behav. 2001, 70, 123–131. [Google Scholar] [CrossRef]
- Haller, J.; Varga, B.; Ledent, C.; Barna, I.; Freund, T. Context-dependent effects of CB1 cannabinoid gene disruption on anxiety-like and social behavior in mice. Eur. J. Neurosci. 2004, 16, 1906–1912. [Google Scholar] [CrossRef]
- Marsicano, G.; Wotjak, C.T.; Azad, S.C.; Bisogno, T.; Rammes, G.; Cascio, M.G.; Hermann, H.; Tang, J.; Hofmann, C.; Zieg-lgänsberger, W.; et al. The endogenous cannabinoid system controls extinction of aversive memories. Nature 2002, 418, 530–534. [Google Scholar] [CrossRef]
- Kathuria, S.; Gaetani, S.; Fegley, D.; Valino, F.; Duranti, A.; Tontini, A.; Mor, M.; Tarzia, G.; La Rana, G.; Calignano, A.; et al. Modulation of anxiety through blockade of anandamide hydrolysis. Nat. Med. 2003, 9, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Hall, W.; Solowij, N. Adverse effects of cannabis. Lancet 1998, 352, 1611–1616. [Google Scholar] [CrossRef] [PubMed]
- Nocheva, H.H.; Encheva-Stoykova, E.N.; Grigorov, E.E. Interaction between endocannabinoids and the adrenergic system before and after stress-exposure. Pharmacia 2021, 69, 249–254. [Google Scholar] [CrossRef]
- Nocheva, H.; Encheva-Stoykova, E.N.; Bogdanov, G.; Tashev, R.; Nikolov, R. The endogenous cannabinoid system and nitric oxide interact in modulation of cold stress-induced analgesia. CR Acad. Bulg. Sci. 2022, 75, 1672–1679. [Google Scholar] [CrossRef]
- Nocheva, H.H.; Tashev, R.E.; Bocheva, A.I.; Atanasova, D.Y.; Dandov, A.D.; Lazarov, N.E. Interactions between the endogenous cannabinoid system and the peptides of the Tyr-MIF-1 family modulate heat stress-induced analgesia. Biomed. Rev. 2020, 31, 91–103. [Google Scholar] [CrossRef]
- Donner, N.C.; Siebler, P.H.; Johnson, D.T.; Villarreal, M.D.; Mani, S.; Matti, A.J.; Lowry, C.A. Serotonergic systems in the balance: CRHR1 and CRHR2 differentially control stress-induced serotonin synthesis. Psychoneuroendocrinology 2015, 63, 178–190. [Google Scholar] [CrossRef]
- Leonard, B. The HPA and immune axes in stress: The involvement of the serotonergic system. Eur. Psychiatry 2005, 20, S302–S306. [Google Scholar] [CrossRef]
- Marks, D.; Shah, M.; Patkar, A.; Masand, P.; Park, G.-Y.; Pae, C.-U. Serotonin-Norepinephrine Reuptake Inhibitors for Pain Control: Premise and Promise. Curr. Neuropharmacol. 2009, 7, 331–336. [Google Scholar] [CrossRef]
- Chae, J.W.; Kang, D.H.; Li, Y.; Kim, S.H.; Lee, H.G.; Choi, J.I.; Yoon, M.H.; Kim, W.M. Antinociceptive effects of nefopam modulating serotonergic, adrenergic, and glutamatergic neurotransmission in the spinal cord. Neurosci. Lett. 2020, 731, 135057. [Google Scholar] [CrossRef]
- Nunes-de-Souza, R.L.; Canto-de-Souza, A.; da-Costa, M.; Fornari, R.V.; Graeff, F.G.; Pela, I.R. Anxiety-induced antinociception in mice: Effects of systemic and intra-amygdala administration of 8-OH-DPAT and midazolam. Psychopharmacology 2000, 150, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Haj-Dahmane, S.; Shen, R.-Y. Modulation of the serotonin system by endocannabinoid signaling. Neuropharmacology 2011, 61, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Häring, M.; Marsicano, G.; Lutz, B.; Monory, K. Identification of the cannabinoid receptor type 1 in serotonergic cells of raphe nuclei in mice. Neuroscience 2007, 146, 1212–1219. [Google Scholar] [CrossRef] [PubMed]
- Jahanshahi, A.; Le Maitre, E.; Temel, Y.; Lanfumey, L.; Hamon, M.; Lesch, K.-P.; Tordera, R.M.; Del Río, J.; Aso, E.; Maldonado, R.; et al. Altered expression of neuronal tryptophan hydroxylase-2 mRNA in the dorsal and median raphe nuclei of three genetically modified mouse models relevant to depression and anxiety. J. Chem. Neuroanat. 2011, 41, 227–233. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, R.J.; Hill, M.N.; Gorzalka, B.B. Monoaminergic neurotransmission contributes to cannabinoid-induced activation of the hypothalamic-pituitary-adrenal axis. Eur. J. Pharmacol. 2009, 624, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Coderre, T.J.; Rollman, G.B. Stress analgesia: Effects of PCPA, yohimbine, and naloxone. Pharmacol. Biochem. Behav. 1984, 21, 681–686. [Google Scholar] [CrossRef]
- Schlereth, T.; Birklein, F. The sympathetic nervous system and pain. Neuromolecular Med. 2008, 10, 141–147. [Google Scholar] [CrossRef]
- Heinricher, M.M.; Tavares, I.; Leith, J.L.; Lumb, B.M. Descending control of nociception: Specificity, recruitment and plasticity. Brain Res. Rev. 2009, 60, 214–225. [Google Scholar] [CrossRef]
- Saeki, Y. Effect of local application of cold or heat for relief of pricking pain. Nurs. Health Sci. 2002, 4, 97–105. [Google Scholar] [CrossRef]
- Ernst, E.; Fialka, V. Ice freezes pain? A review of the clinical effectiveness of analgesic cold therapy. J. Pain Symptom Manag. 1994, 9, 56–59. [Google Scholar] [CrossRef]
- Choi, J.C.; Park, H.J.; Park, J.A.; Kang, D.R.; Choi, Y.S.; Choi, S.; Lee, H.G.; Choi, J.H.; Choi, I.H.; Yoon, M.; et al. The increased analgesic efficacy of cold therapy after an unsuccessful analgesic experience is associated with inferior parietal lobule activation. Sci. Rep. 2022, 12, 14687. [Google Scholar] [CrossRef] [PubMed]
- Nocheva, H.; Krastev, N.S.; Krastev, D.S.; Mileva, M. The Endogenous Cannabinoid and the Nitricoxidergic Systems in the Modulation of Stress Responses. Int. J. Mol. Sci. 2023, 24, 2886. [Google Scholar] [CrossRef] [PubMed]
- Miczek, K.A.; Thompson, M.L.; Shuster, L. Opioid-like analgesia in defeated mice. Science 1982, 215, 1520–1522. [Google Scholar] [CrossRef] [PubMed]
- Zeisberger, E. Interdependence of peripheral and central noradrenaline action in thermal adaption. J. Physiol. 1978, 284, 41P. [Google Scholar] [PubMed]
- Wiesenfeld, Z.; Hallin, R.G. Influence of nerve lesions, strain differences and continuous cold stress on chronic pain behavior in rats. Physiol. Behav. 1981, 27, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Nocheva, H.; Kochev, D.; Krastev, D.; Bocheva, A. Cold stress-induced analgesia. Interactions between the Tyr-MIF-1 family of peptides and the endocannabinoid system. CR Acad. Bulg. Sci. 2013, 66, 1639–1644. [Google Scholar]
- Marzouki, H.; Aboussaleh, Y.; Najimi, M.; Chigr, F.; Ahami, A. Effect of Cold Stress on Neurobehavioral and Physiological Parameters in Rats. Front. Physiol. 2021, 12, 660124. [Google Scholar] [CrossRef] [PubMed]
- Kayser, V. Randall-Selitto Paw Pressure Test. In Encyclopedia of Pain; Gebhart, G.F., Schmidt, R.F., Eds.; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar] [CrossRef]
- ARRP Guideline 20: Guidelines for the Housing of Rats in Scientific Institutions. Available online: http://www.animalethics.org.au (accessed on 1 August 2021).
- Solinas, M.; Justinova, Z.; Goldberg, S.R.; Tanda, G. Anandamide administration alone and after inhibition of fatty acid amide hydrolase (FAAH) increases dopamine levels in the nucleus accumbens shell in rats. J. Neurochem. 2006, 98, 408–419. [Google Scholar] [CrossRef]
- da Veiga, M.A.L.C.; Fonseca Bloise, F.; Costa-e-Sousa, R.H.; Souza, L.L.; Almeida, N.A.d.S.; Oliveira, K.J.; Pazos-Moura, C.C. Acute effects of endocannabinoid anandamide and CB1 receptor antagonist, AM251 in the regulation of thyrotropin secretion. J. Endocrinol. 2008, 199, 235–242. [Google Scholar] [CrossRef]
- Randall, L.O.; Selitto, J.J. A method for measurement of analgesic activity on inflamed tissue. Arch. Int. Pharmacodyn. Ther. 1957, 111, 409–419. [Google Scholar] [PubMed]
- Pääkkönen, T.; Leppäluoto, J. Cold exposure, and hormonal secretion: A review. Int. J. Circumpolar Health 2002, 61, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Lichtman, A.H.; Cook, S.A.; Martin, B.R. Investigation of brain sites mediating cannabinoid induced antinociception in rats: Evidence supporting periaqueductal gray involvement. J. Pharmacol. Exp. Ther. 1996, 276, 585–593. [Google Scholar] [PubMed]
- Martin, W.J.; Patrick, S.L.; Coffin, P.O.; Tsou, K.; Walker, J. An examination of the central sites of action of cannabinoid-induced antinociception in the rat. Life Sci. 1995, 56, 2103–2109. [Google Scholar] [CrossRef] [PubMed]
- Martin, W.J.; Hohmann, A.G.; Walker, J.M. Suppression of Noxious Stimulus-Evoked Activity in the Ventral Posterolateral Nucleus of the Thalamus by a Cannabinoid Agonist: Correlation between Electrophysiological and Antinociceptive Effects. J. Neurosci. 1996, 16, 6601–6611. [Google Scholar] [CrossRef] [PubMed]
- Martin, W.J.; Tsou, K.; Walker, J.M. Cannabinoid receptor-mediated inhibition of the rat tail-flick reflex after microinjection into the rostral ventromedial medulla. Neurosci. Lett. 1998, 242, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Meng, I.D.; Manning, B.H.; Martin, W.J.; Fields, H.L. An analgesia circuit activated by cannabinoids. Nature 1998, 395, 381–383. [Google Scholar] [CrossRef] [PubMed]
- Martin, W.J.; Coffin, P.O.; Attias, E.; Balinsky, M.; Tsou, K.; Walker, J. Anatomical basis for cannabinoid-induced antinociception as revealed by intracerebral microinjections. Brain Res. 1999, 822, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Guindon, J.; Hohmann, A.G. The Endocannabinoid System and Pain. CNS Neurol. Disord. Drug Targets 2009, 8, 403–421. [Google Scholar] [CrossRef]
- Smith, P.B.; Martin, B.R. Spinal mechanisms of delta9-tetrahydrocannabinol-induced analgesia. Brain Res. 1992, 578, 8–12. [Google Scholar] [CrossRef]
- Yaksh, T.L. The Antinociceptive Effects of Intrathecally Administered Levonantradol and Desacetyllevonantradol in the Rat. J. Clin. Pharmacol. 1981, 21, 334S–340S. [Google Scholar] [CrossRef]
- Hohmann, A.G.; Tsou, K.; Walker, J.M. Cannabinoid modulation of wide dynamic range neurons in the lumbar dorsal horn of the rat by spinally administered WIN55,212-2. Neurosci. Lett. 1998, 257, 119–122. [Google Scholar] [CrossRef]
- Johanek, L.M.; Simone, D.A. Cannabinoid Agonist, CP 55,940, Prevents Capsaicin-Induced Sensitization of Spinal Cord Dorsal Horn Neurons. J. Neurophysiol. 2005, 93, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Sokal, D.M.; Elmes, S.J.R.; Kendall, D.A.; Chapman, V. Intraplantar injection of anandamide inhibits mechanically evoked responses of spinal neurones via activation of CB2 receptors in anaesthetised rats. Neuropharmacology 2003, 45, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Hohmann, A.G.; Tsou, K.; Walker, J.M. Intrathecal cannabinoid administration suppresses noxious stimulus-evoked Fos protein-like immunoreactivity in rat spinal cord: Comparison with morphine. Acta Pharmacol. Sin. 1999, 20, 1132–1136. [Google Scholar]
- Richardson, J.D.; Aanonsen, L.; Hargreaves, K.M. Hypoactivity of the Spinal Cannabinoid System Results in NMDA-Dependent Hyperalgesia. J. Neurosci. 1998, 18, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Hohmann, A.G. Spinal and peripheral mechanisms of cannabinoid antinociception: Behavioral, neurophysio-logical, and neu-roanatomical perspectives. Chem. Phys. Lipids 2002, 121, 173–190. [Google Scholar] [CrossRef] [PubMed]
- Guindon, J.; Hohmann, A.G. Cannabinoid CB2 receptors: A therapeutic target for the treatment of inflamma-tory and neuro-pathic pain. Br. J. Pharmacol. 2008, 153, 319–334. [Google Scholar] [CrossRef] [PubMed]
- Rice, A.S.C.; Farquhar-Smith, W.P.; Nagy, I. Endocannabinoids and pain: Spinal and peripheral analgesia in inflammation and neuropathy. Prostaglandins Leukot. Essent. Fat. Acids 2002, 66, 243–256. [Google Scholar] [CrossRef]
- Patel, S.; Roelke, C.T.; Rademacher, D.J.; Cullinan, W.E.; Hillard, C.J. Endocannabinoid Signaling Negatively Modulates Stress-Induced Activation of the Hypothalamic-Pituitary-Adrenal Axis. Endocrinology 2004, 145, 5431–5438. [Google Scholar] [CrossRef]
- Mariotti, A. The effects of chronic stress on health: New insights into the molecular mechanisms of brain-body communication. Future Sci. OA 2015, 1, FSO23. [Google Scholar] [CrossRef]
- Harris, R.B.S. Chronic and acute effects of stress on energy balance: Are there appropriate animal models? Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 308, R250–R265. [Google Scholar] [CrossRef] [PubMed]
- James, K.A.; Stromin, J.I.; Steenkamp, N.; Combrinck, M.I. Understanding the relationships between physiological and psychosocial stress, cortisol and cognition. Front. Endocrinol. 2023, 14, 1085950. [Google Scholar] [CrossRef] [PubMed]
- Stuart, K.E.; Padgett, C. A systematic review of the association between psychological stress and dementia risk in humans. J. Alzheimer’s Dis. 2020, 78, 335–352. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Wang, J.-H.; Geng, Y.; Shen, L.; Wang, H.-L.; Wang, Y.-Y.; Wang, M.-W. Chronic stress contributes to cognitive dysfunction and hippocampal metabolic abnormalities in app/ps1 mice. Cell Physiol. Biochem. 2017, 41, 1766–1776. [Google Scholar] [CrossRef] [PubMed]
- Kvetnansky, R.; McCarty, R. Immobilization Stress. In Encyclopedia of Stress, 2nd ed.; Fink, G., Ed.; Academic Press: Cambridge, MA, USA, 2007; pp. 445–449. ISBN 9780123739476. [Google Scholar] [CrossRef]
- Becker, M.; Pinhasov, A.; Ornoy, A. Animal models of depression: What can they teach us about the human disease? Diagnostics 2021, 11, 123. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Meng, G.; Zhu, L.; Zhu, J.; Dong, N.; Zhou, X.; Zhang, S.; Zhang, Y. Susceptibility to chronic immobilization stress-induced depressive-like behaviour in middle-aged female mice and accompanying changes in dopamine D1 and GABAA receptors in related brain regions. Behav. Brain Funct. 2021, 17, 2. [Google Scholar] [CrossRef] [PubMed]
- Stasiulewicz, A.; Znajdek, K.; Grudzień, M.; Pawiński, T.; Sulkowska, A.J.I. A Guide to Targeting the Endocannabinoid System in Drug Design. Int. J. Mol. Sci. 2020, 21, 2778. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, L.; Bailey, I.; Toms, N.J.; Clarke, G.D.; Kitchen, I.; Hourani, S.M. Paracetamol inhibits nitric oxide synthesis in murine spinal cord slices. Eur. J. Pharmacol. 2007, 562, 68–71. [Google Scholar] [CrossRef]
- Ottani, A.; Leone, S.; Sandrini, M.; Ferrari, A.; Bertolini, A. The analgesic activity of paracetamol is prevented by the blockade of cannabinoid CB1 receptors. Eur. J. Pharmacol. 2006, 531, 280–281. [Google Scholar] [CrossRef]
- Pickering, G.; Loriot, M.A.; Libert, F.; Eschalier, A.; Beaune, P.; Dubray, C. Analgesic effect of acetaminophen in humans: First, evidence of a central serotonergic mechanism. Clin. Pharmacol. Ther. 2006, 79, 371–378. [Google Scholar] [CrossRef]
- Baldo, B.A.; Rose, M.A. The anaesthetist, opioid analgesic drugs, and serotonin toxicity: A mechanistic and clinical review. Br. J. Anaesth. 2020, 124, 44–62. [Google Scholar] [CrossRef]
- Gillman, P.K. Extracting value from case reports: Lessons from serotonin toxicity. Biol. Psychiatr. 2006, 61, 419–422. [Google Scholar] [CrossRef]
- Bouaboula, M.; Perrachon, S.; Milligan, L.; Canat, X.; Rinaldi-Carmona, M.; Portier, M.; Barth, F.; Calandra, B.; Pecceu, F.; Lupker, J. A Selective Inverse Agonist for Central Cannabinoid Receptor Inhibits Mitogen-activated Protein Kinase Activation Stimulated by Insulin or Insulin-like Growth Factor 1. Evidence for a new model of receptor/ligand interactions. J. Biol. Chem. 1997, 272, 22330–22339. [Google Scholar] [CrossRef]
- Console-Bram, L.; Marcu, J.; Abood, M.E. Cannabinoid receptors: Nomenclature and pharmacological principles. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2012, 38, 4–15. [Google Scholar] [CrossRef]
- Ibsen, M.S.; Connor, M.; Glass, M. Cannabinoid CB1 and CB2 receptor signaling and bias. Cannabis Cannabinoid Res. 2017, 2, 48–60. [Google Scholar] [CrossRef]
- Kalant, H. Adverse effects of cannabis on health: An update of the literature since 1996. Prog. Neuropsychopharmacol. Biol. Psychiatry 2004, 28, 849–863. [Google Scholar] [CrossRef] [PubMed]
- Whiting, P.F.; Wolff, R.F.; Deshpande, S.; Di Nisio, M.; Duffy, S.; Hernandez, A.V.; Keurentjes, J.C.; Lang, S.; Misso, K.; Ryder, S.; et al. Cannabinoids for medical use: A systematic review and meta-analysis. J. Am. Med. Assoc. 2015, 313, 2456–2473. [Google Scholar] [CrossRef] [PubMed]
- Todaro, B. Cannabinoids in the treatment of chemotherapy-induced nausea and vomiting. J. Natl. Compr. Cancer Netw. 2012, 10, 487–492. [Google Scholar] [CrossRef]
- Lutge, E.E.; Gray, A.; Siegfried, N. The medical use of cannabis for reducing morbidity and mortality in patients with HIV/AIDS. Cochrane Database Syst. Rev. 2013, 4, CD005175. [Google Scholar] [CrossRef] [PubMed]
- Marx, W.; McGuinness, A.J.; Rocks, T.; Ruusunen, A.; Cleminson, J.; Walker, A.J.; Gomes-da-Costa, S.; Lane, M.; Sanches, M.; Diaz, A.P.; et al. The kynurenine pathway in major depressive disorder, bipolar disorder, and schizophrenia: A meta-analysis of 101 studies. Mol. Psychiatry 2021, 26, 4158–4178. [Google Scholar] [CrossRef] [PubMed]
- Savitz, J. The kynurenine pathway: A finger in every pie. Mol. Psychiatry 2020, 25, 131–147. [Google Scholar] [CrossRef]




| Before 1 h Cold Stress | After 1 h Cold Stress | |
|---|---|---|
| AEA+DPAT |
|
|
| AEA+NAN |
|
|
| AM+DPAT |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nocheva, H.; Stoynev, N.; Vodenicharov, V.; Krastev, D.; Krastev, N.; Mileva, M. Cannabinoid and Serotonergic Systems: Unraveling the Pathogenetic Mechanisms of Stress-Induced Analgesia. Biomedicines 2024, 12, 235. https://doi.org/10.3390/biomedicines12010235
Nocheva H, Stoynev N, Vodenicharov V, Krastev D, Krastev N, Mileva M. Cannabinoid and Serotonergic Systems: Unraveling the Pathogenetic Mechanisms of Stress-Induced Analgesia. Biomedicines. 2024; 12(1):235. https://doi.org/10.3390/biomedicines12010235
Chicago/Turabian StyleNocheva, Hristina, Nikolay Stoynev, Vlayko Vodenicharov, Dimo Krastev, Nikolay Krastev, and Milka Mileva. 2024. "Cannabinoid and Serotonergic Systems: Unraveling the Pathogenetic Mechanisms of Stress-Induced Analgesia" Biomedicines 12, no. 1: 235. https://doi.org/10.3390/biomedicines12010235
APA StyleNocheva, H., Stoynev, N., Vodenicharov, V., Krastev, D., Krastev, N., & Mileva, M. (2024). Cannabinoid and Serotonergic Systems: Unraveling the Pathogenetic Mechanisms of Stress-Induced Analgesia. Biomedicines, 12(1), 235. https://doi.org/10.3390/biomedicines12010235