Maternal Exposure to Endocrine-Disrupting Chemicals: Analysis of Their Impact on Infant Gut Microbiota Composition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Maternal Anthropometric Parameters
2.3. Maternal Phthalate, BPA, and Creatinine Level Determination
2.4. Type of Feeding and Dietary Habits of Infants
2.5. Sample Processing for Gut Microbiota Analysis
2.6. Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt2)
2.7. Statistical Analyses
3. Results
3.1. Population
3.2. Infants’ Gut Microbiota and Maternal Urinary EDC Correlations
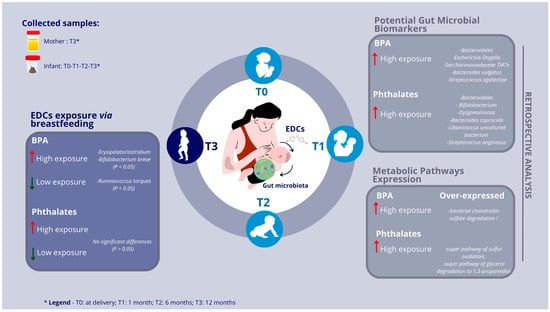
3.3. Exposure to High BPA and EDC Concentrations via Breastfeeding
3.4. Retrospective Profiling of Infants’ Gut Microbiota
3.5. Potential Gut Microbial Biomarkers of High-Risk Exposure to EDCs in Pediatric Age
3.6. Over/Under-Expressed Metabolic Pathways in Pediatric Gut Microbiota under High-Risk Exposure to EDCs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Papalou, O.; Kandaraki, E.A.; Papadakis, G.; Diamanti-Kandarakis, E. Endocrine Disrupting Chemicals: An Occult Mediator of Metabolic Disease. Front. Endocrinol. 2019, 10, 112. [Google Scholar] [CrossRef] [PubMed]
- Di Napoli, I.; Tagliaferri, S.; Sommella, E.; Salviati, E.; Porri, D.; Raspini, B.; Cena, H.; Campiglia, P.; La Rocca, C.; Cerbo, R.M.; et al. Lifestyle Habits and Exposure to BPA and Phthalates in Women of Childbearing Age from Northern Italy: A Pilot Study. Int. J. Environ. Res. Public. Health 2021, 18, 9710. [Google Scholar] [CrossRef] [PubMed]
- Obata, Y.; Castaño, Á.; Boeing, S.; Bon-Frauches, A.C.; Fung, C.; Fallesen, T.; de Agüero, M.G.; Yilmaz, B.; Lopes, R.; Huseynova, A.; et al. Neuronal programming by microbiota regulates intestinal physiology. Nature 2020, 578, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Lucaccioni, L.; Trevisani, V.; Passini, E.; Righi, B.; Plessi, C.; Predieri, B.; Iughetti, L. Perinatal Exposure to Phthalates: From Endocrine to Neurodevelopment Effects. Int. J. Mol. Sci. 2021, 22, 4063. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, C.M.; Beserra, B.T.S.; Silva, N.G.; Lima, C.L.; Rocha, P.R.S.; Coelho, M.S.; Neves, F.A.R.; Amato, A.A. Exposure to endocrine-disrupting chemicals and anthropometric measures of obesity: A systematic review and meta-analysis. BMJ Open 2020, 21, e033509. [Google Scholar] [CrossRef] [PubMed]
- Predieri, B.; Iughetti, L.; Bernasconi, S.; Street, M.E. Endocrine Disrupting Chemicals’ Effects in Children: What We Know and What We Need to Learn? Int. J. Mol. Sci. 2022, 23, 11899. [Google Scholar] [CrossRef] [PubMed]
- Geens, T.; Aerts, D.; Berthot, C.; Bourguignon, J.P.; Goeyens, L.; Lecomte, P.; Maghuin-Rogister, G.; Pironnet, A.M.; Pussemier, L.; Scippo, M.L.; et al. A review of dietary and non-dietary exposure to bisphenol-A. Food Chem. Toxicol. 2012, 50, 3725–3740. [Google Scholar] [CrossRef] [PubMed]
- Braun, J.M. Early-life exposure to EDCs: Role in childhood obesity and neurodevelopment. Nat. Rev. Endocrinol. 2017, 13, 161–173. [Google Scholar] [CrossRef]
- Puttabyatappa, M.; Banker, M.; Zeng, L.; Goodrich, J.M.; Domino, S.E.; Dolinoy, D.C.; Meeker, J.D.; Pennathur, S.; Song, P.X.K.; Padmanabhan, V. Maternal Exposure to Environmental Disruptors and Sexually Dimorphic Changes in Maternal and Neonatal Oxidative Stress. J. Clin. Endocrinol. Metab. 2020, 105, 492–505. [Google Scholar] [CrossRef]
- Wu, A.H.; Franke, A.A.; Wilkens, L.R.; Tseng, C.; Conroy, S.M.; Li, Y.; Sangaramoorthy, M.; Polfus, L.M.; DeRouen, M.C.; Caberto, C.; et al. Risk of breast cancer and prediagnostic urinary excretion of bisphenol A, triclosan and parabens: The Multiethnic Cohort Study. Int. J. Cancer. 2021, 149, 1426–1434. [Google Scholar] [CrossRef]
- Kolatorova, L.; Vitku, J.; Hampl, R.; Adamcova, K.; Skodova, T.; Simkova, M.; Parizek, A.; Starka, L.; Duskova, M. Exposure to bisphenols and parabens during pregnancy and relations to steroid changes. Env. Res. 2018, 163, 115–122. [Google Scholar] [CrossRef]
- Street, M.E.; Bernasconi, S. Endocrine-Disrupting Chemicals in Human Fetal Growth. Int. J. Mol. Sci. 2020, 21, 1430. [Google Scholar] [CrossRef]
- Raspini, B.; Porri, D.; De Giuseppe, R.; Chieppa, M.; Liso, M.; Cerbo, R.M.; Civardi, E.; Garofoli, F.; Monti, M.C.; Vacca, M.; et al. Prenatal and postnatal determinants in shaping offspring’s microbiome in the first 1000 days: Study protocol and preliminary results at one month of life. Ital. J. Pediatr. 2020, 46, 45. [Google Scholar] [CrossRef] [PubMed]
- Cresci, G.A.; Bawden, E. Gut Microbiome: What We Do and Don’t Know. Nutr. Clin. Pract. 2015, 30, 734–746. [Google Scholar] [PubMed]
- Liu, B.N.; Liu, X.T.; Liang, Z.H.; Wang, J.H. Gut microbiota in obesity. World J. Gastroenterol. 2021, 27, 3837–3850. [Google Scholar] [CrossRef] [PubMed]
- Heintz-Buschart, A.; Wilmes, P. Human Gut Microbiome: Function Matters. Trends Microbiol. 2018, 26, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Shin, H.S.; Lee, W.H. Impact of Endocrine-Disrupting Chemicals in Breast Milk on Postpartum Depression in Korean Mothers. Int. J. Environ. Res. Public. Health 2021, 18, 4444. [Google Scholar] [CrossRef] [PubMed]
- Biagi, E.; Quercia, S.; Aceti, A.; Beghetti, I.; Rampelli, S.; Turroni, S.; Faldella, G.; Candela, M.; Brigidi, P.; Corvaglia, L. The Bacterial Ecosystem of Mother’s Milk and Infant’s Mouth and Gut. Front. Microbiol. 2017, 8, 1214. [Google Scholar] [CrossRef] [PubMed]
- Genuis, S.J.; Beesoon, S.; Lobo, R.A.; Birkholz, D. Human elimination of phthalate compounds: Blood, urine, and sweat (BUS) study. Sci. World J. 2012, 2012, 615068. [Google Scholar] [CrossRef]
- Frederiksen, H.; Skakkebaek, N.E.; Andersson, A.M. Metabolism of phthalates in humans. Mol. Nutr. Food Res. 2007, 51, 899–911. [Google Scholar] [CrossRef]
- Mendonca, K.; Hauser, R.; Calafat, A.M.; Arbuckle, T.E.; Duty, S.M. Bisphenol A concentrations in maternal breast milk and infant urine. Int. Arch. Occup. Env. Health 2014, 87, 13–20. [Google Scholar] [CrossRef]
- Witczak, A.; Pohoryło, A.; Abdel-Gawad, H. Endocrine-Disrupting Organochlorine Pesticides in Human Breast Milk: Changes during Lactation. Nutrients 2021, 13, 229. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Jacobs, D.R., Jr. Methodological issues in human studies of endocrine disrupting chemicals. Rev. Endocr. Metab. Disord. 2015, 4, 289–297. [Google Scholar] [CrossRef] [PubMed]
- WHO. World Health Organization: Biological Monitoring of Chemical Exposure in the Workplace; WHO: Geneva, Switzerland, 1996. [Google Scholar]
- Papageorghiou, A.T.; Kennedy, S.H.; Salomon, L.J.; Altman, D.G.; Ohuma, E.O.; Stones, W.; Gravett, M.G.; Barros, F.C.; Victora, C.; Purwar, M.; et al. International Fetal and Newborn Growth Consortium for the 21(st) Century (INTERGROWTH-21(st)). The INTERGROWTH-21st fetal growth standards: Toward the global integration of pregnancy and pediatric care. Am. J. Obstet. Gynecol. 2018, 218, S630–S640. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed]
- Langille, M.G.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Vega Thurber, R.L.; Knight, R.; et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013, 31, 814–821. [Google Scholar] [CrossRef] [PubMed]
- La Merrill, M.A.; Vandenberg, L.N.; Smith, M.T.; Goodson, W.; Browne, P.; Patisaul, H.B.; Guyton, K.Z.; Kortenkamp, A.; Cogliano, V.J.; Woodruff, T.J.; et al. Consensus on the key characteristics of endocrine-disrupting chemicals as a basis for hazard identification. Nat. Rev. Endocrinol. 2020, 16, 45–57. [Google Scholar] [CrossRef]
- Neuman, H.; Debelius, J.W.; Knight, R.; Koren, O. Microbial endocrinology: The interplay between the microbiota and the endocrine system. FEMS Microbiol. Rev. 2015, 39, 509–521. [Google Scholar] [CrossRef]
- Ahn, J.; Hayes, R.B. Environmental Influences on the Human Microbiome and Implications for Noncommunicable Disease. Annu. Rev. Public. Health 2021, 42, 277–292. [Google Scholar] [CrossRef]
- Birnbaum, L.S.; Fenton, S.E. Cancer and developmental exposure to endocrine disruptors. Env. Health Perspect. 2003, 111, 389–394. [Google Scholar] [CrossRef]
- Rubin, B.S.; Soto, A.M. Bisphenol A: Perinatal exposure and body weight. Mol. Cell Endocrinol. 2009, 304, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Raspini, B.; Vacca, M.; Porri, D.; De Giuseppe, R.; Calabrese, F.M.; Chieppa, M.; Liso, M.; Cerbo, R.M.; Civardi, E.; Garofoli, F.; et al. Early life microbiota colonization at six months of age: A transitional time point. Front. Cell Infect. Microbiol. 2021, 11, 204. [Google Scholar] [CrossRef] [PubMed]
- Vacca, M.; Raspini, B.; Calabrese, F.M.; Porri, D.; De Giuseppe, R.; Chieppa, M.; Liso, M.; Cerbo, R.M.; Civardi, E.; Garofoli, F.; et al. The establishment of the gut microbiota in 1-year-aged infants: From birth to family food. Eur. J. Nutr. 2023, 62, 2705. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Cai, D.; Li, X.; Liu, B.; Chen, J.; Jiang, X.; Li, H.; Li, Z.; Teerds, K.; Sun, J.; et al. Effects of Bisphenol A on reproductive toxicity and gut microbiota dysbiosis in male rats. Ecotoxicol. Env. Saf. 2022, 239, 113623. [Google Scholar] [CrossRef] [PubMed]
- Javurek, A.B.; Spollen, W.G.; Johnson, S.A.; Bivens, N.J.; Bromert, K.H.; Givan, S.A.; Rosenfeld, C.S. Effects of exposure to bisphenol A and ethinyl estradiol on the gut microbiota of parents and their offspring in a rodent model. Gut Microbes. 2016, 7, 471–485. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Guo, Y.; Hu, C.; Lam, P.K.S.; Lam, J.C.W.; Zhou, B. Dysbiosis of gut microbiota by chronic coexposure to titanium dioxide nanoparticles and bisphenol A: Implications for host health in zebrafish. Environ. Pollut. 2018, 234, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.P.; Chung, Y.T.; Li, R.; Wan, H.T.; Wong, C.K. Bisphenol A alters gut microbiome: Comparative metagenomics analysis. Environ. Pollut. 2016, 218, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.A.; Metz, L.; Yong, V.W. Review: Endocrine disrupting chemicals and immune responses: A focus on bisphenol-A and its potential mechanisms. Mol. Immunol. 2013, 53, 421–430. [Google Scholar] [CrossRef]
- González-Casanova, J.E.; Bermúdez, V.; Caro Fuentes, N.J.; Angarita, L.C.; Caicedo, N.H.; Rivas Muñoz, J.; Rojas-Gómez, D.M. New Evidence on BPA’s Role in Adipose Tissue Development of Proinflammatory Processes and Its Relationship with Obesity. Int. J. Mol. Sci. 2023, 24, 8231. [Google Scholar] [CrossRef]
- Leite, A.Z.; Rodrigues, N.C.; Gonzaga, M.I.; Paiolo, J.C.C.; de Souza, C.A.; Stefanutto, N.A.V.; Omori, W.P.; Pinheiro, D.G.; Brisotti, J.L.; Matheucci Junior, E.; et al. Detection of Increased Plasma Interleukin-6 Levels and Prevalence of Prevotella copri and Bacteroides vulgatus in the Feces of Type 2 Diabetes Patients. Front. Immunol. 2017, 8, 1107. [Google Scholar] [CrossRef]
- Wang, Y.; Kang, J.; Wang, R.; Ramezani, K.; Bonakdar, M.; Moghimi, N.; Salimi, M.; Yao, Y.; Wang, K. Bisphenol A interacts with DLGAP5 and regulates IL-6/JAK2/STAT3 signaling pathway to promote tumorigenesis and progression of osteosarcoma. Chemosphere. 2023, 312, 136545. [Google Scholar] [CrossRef] [PubMed]
- Prueitt, R.L.; Hixon, M.L.; Fan, T.; Olgun, N.S.; Piatos, P.; Zhou, J.; Goodman, J.E. Systematic review of the potential carcinogenicity of bisphenol A in humans. Regul. Toxicol. Pharmacol. 2023, 142, 105414. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.; Liu, G.; Lin, Y.; Guo, C.L.; Han, J.; Chu, E.S.H.; Shi, C.; Li, Y.; Zhang, H.; Hu, C.; et al. Altered gut microbiome composition by appendectomy contributes to colorectal cancer. Oncogene 2023, 42, 530–540. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Liang, S.; Jia, H.; Stadlmayr, A.; Tang, L.; Lan, Z.; Xia, H.; Xu, X.; Jie, Z.; Su, L.; et al. Gut microbiome development along the colorectal adenoma-carcinoma sequence. Nat. Commun. 2015, 6, 6528. [Google Scholar] [CrossRef]
- Kaczmarczyk, M.; Löber, U.; Adamek, K.; Węgrzyn, D.; Skonieczna-Żydecka, K.; Malinowski, D.; Łoniewski, I.; Markó, L.; Ulas, T.; Forslund, S.K.; et al. The gut microbiota is associated with the small intestinal paracellular permeability and the development of the immune system in healthy children during the first two years of life. J. Transl. Med. 2021, 19, 177. [Google Scholar] [CrossRef] [PubMed]
- Glover, J.S.; Ticer, T.D.; Engevik, M.A. Characterizing the mucin-degrading capacity of the human gut microbiota. Sci. Rep. 2022, 12, 8456. [Google Scholar] [CrossRef] [PubMed]
- Palm, N.W.; de Zoete, M.R.; Cullen, T.W.; Barry, N.A.; Stefanowski, J.; Hao, L.; Degnan, P.H.; Hu, J.; Peter, I.; Zhang, W.; et al. Immunoglobulin A coating identifies colitogenic bacteria in inflammatory bowel disease. Cell 2014, 158, 1000–1010. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Peng, Y.; Huang, Y.; Xie, M.; Dai, Z.; Cai, H.; Dong, W.; Xu, W.; Xie, Z.; Chen, D.; et al. Fluoride induced leaky gut and bloom of Erysipelatoclostridium ramosum mediate the exacerbation of obesity in high-fat-diet fed mice. J. Adv. Res. 2023, 50, 35–54. [Google Scholar] [CrossRef]
- Kaakoush, N.O. Insights into the Role of Erysipelotrichaceae in the Human Host. Front. Cell Infect. Microbiol. 2015, 5, 84. [Google Scholar] [CrossRef]
- Saturio, S.; Nogacka, A.M.; Alvarado-Jasso, G.M.; Salazar, N.; de Los Reyes-Gavilán, C.G.; Gueimonde, M.; Arboleya, S. Role of Bifidobacteria on Infant Health. Microorganisms 2021, 9, 2415. [Google Scholar] [CrossRef]
- Henrick, B.M.; Rodriguez, L.; Lakshmikanth, T.; Pou, C.; Henckel, E.; Arzoomand, A.; Olin, A.; Wang, J.; Mikes, J.; Tan, Z.; et al. Bifidobacteria-mediated immune system imprinting early in life. Cell 2021, 184, 3884–3898. [Google Scholar] [PubMed]
- Li, G.; Feng, H.; Mao, X.L.; Deng, Y.J.; Wang, X.B.; Zhang, Q.; Guo, Y.; Xiao, S.M. The effects of probiotics supplementation on glycaemic control among adults with type 2 diabetes mellitus: A systematic review and meta-analysis of randomised clinical trials. J. Transl. Med. 2023, 21, 442. [Google Scholar] [CrossRef] [PubMed]
- Saleem, A.; Ikram, A.; Dikareva, E.; Lahtinen, E.; Matharu, D.; Pajari, A.M.; de Vos, W.M.; Hasan, F.; Salonen, A.; Jian, C. Unique Pakistani gut microbiota highlights population-specific microbiota signatures of type 2 diabetes mellitus. Gut Microbes. 2022, 14, 2142009. [Google Scholar] [CrossRef] [PubMed]
- Doumatey, A.P.; Adeyemo, A.; Zhou, J.; Lei, L.; Adebamowo, S.N.; Adebamowo, C.; Rotimi, C.N. Gut Microbiome Profiles Are Associated with Type 2 Diabetes in Urban Africans. Front. Cell Infect. Microbiol. 2020, 10, 63. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, A.; van Sinderen, D. Bifidobacteria and Their Role as Members of the Human Gut Microbiota. Front. Microbiol. 2016, 7, 925. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Xu, K.; Jiang, Y.; Zhu, C.; Suo, C.; Cui, M.; Wang, Y.; Yuan, Z.; Xue, J.; Wang, J.; et al. The gut microbiome in subclinical atherosclerosis: A population-based multiphenotype analysis. Rheumatology 2021, 61, 258–269. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Peters, B.A.; Usyk, M.; Xing, J.; Hanna, D.B.; Wang, T.; Post, W.S.; Landay, A.L.; Hodis, H.N.; Weber, K.; et al. Gut Microbiota, Plasma Metabolomic Profiles, and Carotid Artery Atherosclerosis in HIV Infection. Arterioscler. Thromb. Vasc. Biol. 2022, 42, 1081–1093. [Google Scholar] [CrossRef] [PubMed]
- Verburgt, C.M.; Dunn, K.A.; Ghiboub, M.; Lewis, J.D.; Wine, E.; Sigall Boneh, R.; Gerasimidis, K.; Shamir, R.; Penny, S.; Pinto, D.M.; et al. Successful Dietary Therapy in Paediatric Crohn’s Disease is Associated with Shifts in Bacterial Dysbiosis and Inflammatory Metabotype Towards Healthy Controls. J. Crohns Colitis 2023, 17, 61–72. [Google Scholar] [CrossRef]
- Zhong, W.; Wu, K.; Long, Z.; Zhou, X.; Zhong, C.; Wang, S.; Lai, H.; Guo, Y.; Lv, D.; Lu, J.; et al. Gut dysbiosis promotes prostate cancer progression and docetaxel resistance via activating NF-κB-IL6-STAT3 axis. Microbiome 2022, 10, 94. [Google Scholar] [CrossRef]
- Mao, W.; Mao, L.; Zhou, F.; Shen, J.; Zhao, N.; Jin, H.; Hu, J.; Hu, Z. Influence of Gut Microbiota on Metabolism of Bisphenol A, a Major Component of Polycarbonate Plastics. Toxics 2023, 11, 340. [Google Scholar] [CrossRef]
- Pranzini, E.; Pardella, E.; Paoli, P.; Fendt, S.M.; Taddei, M.L. Metabolic Reprogramming in Anticancer Drug Resistance: A Focus on Amino Acids. Trends Cancer 2021, 7, 682–699. [Google Scholar] [CrossRef] [PubMed]
- Walker, V. Ammonia metabolism and hyperammonemic disorders. Adv. Clin. Chem. 2014, 67, 73–150. [Google Scholar] [PubMed]
- Gropman, A.L.; Summar, M.; Leonard, J.V. Neurological implications of urea cycle disorders. J. Inherit. Metab. Dis. 2007, 30, 865–879. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Zheng, J.; Zhang, S.; Wang, B.; Wu, C.; Guo, X. Advances in the Involvement of Gut Microbiota in Pathophysiology of NAFLD. Front. Med. 2020, 7, 361. [Google Scholar] [CrossRef]
- Croze, M.L.; Géloën, A.; Soulage, C.O. Abnormalities in myo-inositol metabolism associated with type 2 diabetes in mice fed a high-fat diet: Benefits of a dietary myo-inositol supplementation. Br. J. Nutr. 2015, 113, 1862–1875. [Google Scholar] [CrossRef] [PubMed]
- Croze, M.L.; Soulage, C.O. Potential role and therapeutic interests of myo-inositol in metabolic diseases. Biochimie 2013, 95, 1811–1827. [Google Scholar] [CrossRef] [PubMed]
- Turconi, G.; Celsa, M.; Rezzani, C.; Biino, G.; Sartirana, M.A.; Roggi, C. Reliability of a dietary questionnaire on food habits, eating behaviour and nutritional knowledge of adolescents. Eur. J. Clin. Nutr. 2003, 57, 753–763. [Google Scholar] [CrossRef]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef]
- La Rocca, C.; Maranghi, F.; Tait, S.; Tassinari, R.; Baldi, F.; Bottaro, G.; Buzzigoli, E.; Carli, F.; Cianfarani, S.; Conte, R.; et al. The LIFE PERSUADED project approach on phthalates and bisphenol A biomonitoring in Italian mother-child pairs linking exposure and juvenile diseases. Environ. Sci. Pollut. Res. 2018, 25, 25618–25625. [Google Scholar] [CrossRef]





| BPA | MEP * | MIbP * | MEHHP * | MBzP * | |
|---|---|---|---|---|---|
| median | 0.96 | 7.73 | 0.89 | 1.24 | 0.64 |
| min–max | 0.34–2.98 | 0.01–83.67 | 0–10.4 | 0.1–10.28 | 0.16–6.77 |
| IQR | 0.73–1.63 | 3.05–12.84 | 0–2.99 | 0.79–2.23 | 0.42–1.03 |
| O |vs.| B | HR |vs.| L | |||||
|---|---|---|---|---|---|---|
| Taxonomic Level | F-Value | R-Squared | p-Value | F-Value | R-Squared | p-Value |
| Phylum | 1.687 | 0.021 | 0.150 | 1.574 | 0.019 | 0.161 |
| Class | 1.191 | 0.015 | 0.299 | 1.662 | 0.020 | 0.113 |
| Order | 1.036 | 0.013 | 0.405 | 1.418 | 0.017 | 0.144 |
| Family | 1.133 | 0.014 | 0.319 | 1.539 | 0.019 | 0.105 |
| Genus | 1.716 | 0.021 | 0.049 * | 1.483 | 0.018 | 0.090 |
| Species | 1.263 | 0.015 | 0.239 | 1.360 | 0.017 | 0.178 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vacca, M.; Calabrese, F.M.; Loperfido, F.; Maccarini, B.; Cerbo, R.M.; Sommella, E.; Salviati, E.; Voto, L.; De Angelis, M.; Ceccarelli, G.; et al. Maternal Exposure to Endocrine-Disrupting Chemicals: Analysis of Their Impact on Infant Gut Microbiota Composition. Biomedicines 2024, 12, 234. https://doi.org/10.3390/biomedicines12010234
Vacca M, Calabrese FM, Loperfido F, Maccarini B, Cerbo RM, Sommella E, Salviati E, Voto L, De Angelis M, Ceccarelli G, et al. Maternal Exposure to Endocrine-Disrupting Chemicals: Analysis of Their Impact on Infant Gut Microbiota Composition. Biomedicines. 2024; 12(1):234. https://doi.org/10.3390/biomedicines12010234
Chicago/Turabian StyleVacca, Mirco, Francesco Maria Calabrese, Federica Loperfido, Beatrice Maccarini, Rosa Maria Cerbo, Eduardo Sommella, Emanuela Salviati, Luana Voto, Maria De Angelis, Gabriele Ceccarelli, and et al. 2024. "Maternal Exposure to Endocrine-Disrupting Chemicals: Analysis of Their Impact on Infant Gut Microbiota Composition" Biomedicines 12, no. 1: 234. https://doi.org/10.3390/biomedicines12010234
APA StyleVacca, M., Calabrese, F. M., Loperfido, F., Maccarini, B., Cerbo, R. M., Sommella, E., Salviati, E., Voto, L., De Angelis, M., Ceccarelli, G., Di Napoli, I., Raspini, B., Porri, D., Civardi, E., Garofoli, F., Campiglia, P., Cena, H., & De Giuseppe, R. (2024). Maternal Exposure to Endocrine-Disrupting Chemicals: Analysis of Their Impact on Infant Gut Microbiota Composition. Biomedicines, 12(1), 234. https://doi.org/10.3390/biomedicines12010234