The Role of GLP-1, GIP, MCP-1 and IGFBP-7 Biomarkers in the Development of Metabolic Disorders: A Review and Predictive Analysis in the Context of Diabetes and Obesity
Abstract
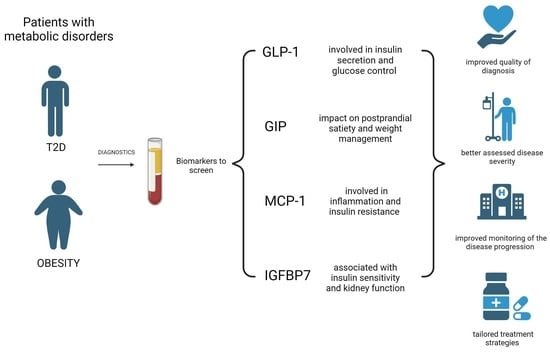
:1. Introduction
2. Materials and Methods
3. Results
3.1. GLP-1
3.1.1. GLP-1 and Its Impaired Function in T2D Patients
3.1.2. Dysregulated GLP-1 Secretion in Obese Patients
3.1.3. GLP-1 as a Therapeutic Hope
3.2. GIP
3.2.1. GIP’s Role in T2D Dysregulations
3.2.2. Lipid Metabolism and GIP Correlation
3.2.3. GIP as the Main Object of Interest in Clinical Research
3.3. MCP-1
MCP-1 and Its Correlation with Insulin Resistance
3.4. IGFBP7
The Urine-Based Marker IGFBP7 with Its Association with Insulin Resistance and Metabolic Syndrome
3.5. New Treatment Strategies: Are They Connected to Identified Biomarkers?
3.5.1. Bifunctional Agonists Targeting GLP-1 and Glucagon Receptors: A Dual Approach to Managing Glucose Intolerance and Obesity
3.5.2. Innovative Approaches in Diabetes and Obesity Therapeutics
3.5.3. Advancements in Diabetes and Obesity Therapeutics: Polyagonists and Structurally Selective Agonists Pioneering New Treatment Modalities
3.5.4. Potential Diagnostic, Prognostic, and Monitoring Significance of These Biomarkers in the Context of Diabetes and Obesity
4. Discussion
4.1. General Information
4.2. Innovations in Diagnostics and Therapeutics
4.2.1. Specificity
4.2.2. Personalization
4.2.3. Insight into Mechanisms
4.2.4. Monitoring and Progress Assessment
4.3. Clinical Implications
4.3.1. Early Detection
4.3.2. Tailored Treatment
4.3.3. Preventive Measures
4.3.4. Innovative Therapies
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Laakso, M. Biomarkers for type 2 diabetes. Mol. Metab. 2019, 27, S139–S146. [Google Scholar] [CrossRef]
- Chatterjee, S.; Khunti, K.; Davies, M.J. Type 2 diabetes. Lancet 2017, 389, 2239–2251, Erratum in Lancet 2017, 389, 2192. [Google Scholar] [CrossRef] [PubMed]
- The Emerging Risk Factors Collaboration; Sarwar, N.; Gao, P.; Seshasai, S.R.; Gobin, R.; Kaptoge, S.; Di Angelantonio, E.; Ingelsson, E.; Lawlor, D.A.; Selvin, E.; et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: A collaborative meta-analysis of 102 prospective studies. Lancet 2010, 375, 2215–2222. [Google Scholar] [CrossRef] [PubMed]
- Hoogeveen, E.K. The Epidemiology of Diabetic Kidney Disease. Kidney Dial. 2022, 2, 433–442. [Google Scholar] [CrossRef]
- Zilliox, L.A.; Chadrasekaran, K.; Kwan, J.Y.; Russell, J.W. Diabetes and Cognitive Impairment. Curr. Diabetes Rep. 2016, 16, 87. [Google Scholar] [CrossRef]
- Chevillet, J.R.; Lee, I.; Briggs, H.A.; He, Y.; Wang, K. Issues and prospects of microRNA-based biomarkers in blood and other body fluids. Molecules 2014, 19, 6080–6105. [Google Scholar] [CrossRef]
- Bray, G.A.; Kim, K.K.; Wilding, J.P.H.; World Obesity Federation. Obesity: A chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obes. Rev. 2017, 18, 715–723. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Classification and diagnosis of diabetes: Standards of medical care in diabetes—2021. Diabetes Care 2021, 44, S15–S33. [Google Scholar] [CrossRef]
- The GBD 2015 Obesity Collaborators. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar] [CrossRef]
- Guh, D.P.; Zhang, W.; Bansback, N.; Amarsi, Z.; Birmingham, C.L.; Anis, A.H. The incidence of co-morbidities related to obesity and overweight: A systematic review and meta-analysis. BMC Public Health 2009, 9, 88. [Google Scholar] [CrossRef]
- Hruby, A.; Hu, F.B. The Epidemiology of Obesity: A Big Picture. PharmacoEconomics 2015, 33, 673–689. [Google Scholar] [CrossRef]
- Trayhurn, P.; Wood, I.S. Adipokines: Inflammation and the pleiotropic role of white adipose tissue. Br. J. Nutr. 2004, 92, 347–355. [Google Scholar] [CrossRef]
- Drucker, D.J.; Nauck, M.A. The incretin system: Glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006, 368, 1696–1705. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.A.; Quast, D.R.; Wefers, J.; Meier, J.J. GLP-1 receptor agonists in the treatment of type 2 diabetes—State-of-the-art. Mol. Metab. 2021, 46, 101102. [Google Scholar] [CrossRef] [PubMed]
- Drucker, D.J. Enhancing incretin action for the treatment of type 2 diabetes. Diabetes Care 2003, 26, 2929–2940. [Google Scholar] [CrossRef] [PubMed]
- Drucker, D.J. The biology of incretin hormones. Cell Metab. 2006, 3, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Derosa, G.; Maffioli, P. GLP-1 Agonists Exenatide and Liraglutide: A Review about Their Safety and Efficacy. Curr. Clin. Pharmacol. 2012, 7, 214–228. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.A.; Meier, J.J.; Cavender, M.A.; El Aziz, M.A.; Drucker, D.J. Cardiovascular actions and clinical outcomes with glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Circulation 2017, 136, 849–870. [Google Scholar] [CrossRef]
- Steinert, R.E.; Feinle-Bisset, C.; Asarian, L.; Horowitz, M.; Beglinger, C.; Geary, N. Ghrelin, CCK, GLP-1, and PYY(3–36): Secretory controls and physiological roles in eating and glycemia in health, obesity, and after RYGB. Physiol. Rev. 2017, 97, 411–463. [Google Scholar] [CrossRef]
- Ard, J.; Fitch, A.; Fruh, S.; Herman, L. Weight Loss and Maintenance Related to the Mechanism of Action of Glucagon-like Peptide 1 Receptor Agonists. Adv. Ther. 2021, 38, 2821–2839. [Google Scholar] [CrossRef]
- Marso, S.P.; Daniels, G.H.; Brown-Frandsen, K.; Kristensen, P.; Mann, J.F.E.; Nauck, M.A.; Nissen, S.E.; Pocock, S.; Poulter, N.R.; Ravn, L.S.; et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. Drug Ther. Bull. 2016, 54, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J.; Bergenstal, R.; Bode, B.; Kushner, R.F.; Lewin, A.; Skjøth, T.V.; Andreasen, A.H.; Jensen, C.B.; DeFronzo, R.A.; for the NN8022-1922 Study Group. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: The SCALE diabetes randomized clinical trial. JAMA J. Am. Med. Assoc. 2015, 314, 687–699. [Google Scholar] [CrossRef] [PubMed]
- Vilsbøll, T.; Christensen, M.; Junker, A.E.; Knop, F.K.; Gluud, L.L. Effects of glucagon-like peptide-1 receptor agonists on weight loss: Systematic review and meta-analyses of randomised controlled trials. BMJ 2012, 344, d7771. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Luo, J.; Jiang, M.; Wang, K. The Efficacy and Safety of the Combination Therapy with GLP-1 Receptor Agonists and SGLT-2 Inhibitors in Type 2 Diabetes Mellitus: A Systematic Review and Meta-analysis. Front. Pharmacol. 2022, 13, 838277. [Google Scholar] [CrossRef]
- Nauck, M.; Weinstock, R.S.; Umpierrez, G.E.; Guerci, B.; Skrivanek, Z.; Milicevic, Z. Efficacy and safety of dulaglutide versus sitagliptin after 52 weeks in type 2 diabetes in a randomized controlled trial (AWARD-5). Diabetes Care 2014, 37, 2149–2158. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Wu, S.; Wang, J.; Guo, S.; Chai, S.; Yang, Z.; Li, L.; Zhang, Y.; Ji, L.; Zhan, S. Effect of glucagon-like peptide-1 receptor agonists on lipid profiles among type 2 diabetes: A systematic review and network meta-analysis. Clin. Ther. 2015, 37, 225–241.e8. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.D.; Finan, B.; Clemmensen, C.; DiMarchi, R.D.; Tschöp, M.H. The new biology and pharmacology of glucagon. Physiol. Rev. 2017, 97, 721–766. [Google Scholar] [CrossRef]
- Finan, B.; Müller, T.D.; Clemmensen, C.; Perez-Tilve, D.; DiMarchi, R.D.; Tschöp, M.H. Reappraisal of GIP Pharmacology for Metabolic Diseases. Trends Mol. Med. 2016, 22, 359–376. [Google Scholar] [CrossRef]
- Miyawaki, K.; Yamada, Y.; Ban, N.; Ihara, Y.; Tsukiyama, K.; Zhou, H.; Fujimoto, S.; Oku, A.; Tsuda, K.; Toyokuni, S.; et al. Inhibition of gastric inhibitory polypeptide signaling prevents obesity. Nat. Med. 2002, 8, 738–742. [Google Scholar] [CrossRef]
- Højberg, P.V.; Vilsbøll, T.; Rabøl, R.; Knop, F.K.; Bache, M.; Krarup, T.; Holst, J.J.; Madsbad, S. Four weeks of near-normalisation of blood glucose improves the insulin response to glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide in patients with type 2 diabetes. Diabetologia 2008, 52, 199–207. [Google Scholar] [CrossRef]
- Vilsbøll, T.; Knop, F.K.; Krarup, T.; Johansen, A.; Madsbad, S.; Larsen, S.; Hansen, T.; Pedersen, O.; Holst, J.J. The Pathophysiology of Diabetes Involves a Defective Amplification of the Late-Phase Insulin Response to Glucose by Glucose-Dependent Insulinotropic Polypeptide—Regardless of Etiology and Phenotype. J. Clin. Endocrinol. Metab. 2003, 88, 4897–4903. [Google Scholar] [CrossRef] [PubMed]
- English, A.; Craig, S.L.; Flatt, P.R.; Irwin, N. Individual and combined effects of GIP and xenin on differentiation, glucose uptake and lipolysis in 3T3-L1 adipocytes. Biol. Chem. 2020, 401, 1293–1303. [Google Scholar] [CrossRef] [PubMed]
- Gasbjerg, L.S.; Gabe, M.B.N.; Hartmann, B.; Christensen, M.B.; Knop, F.K.; Holst, J.J.; Rosenkilde, M.M. Glucose-dependent insulinotropic polypeptide (GIP) receptor antagonists as anti-diabetic agents. Peptides 2018, 100, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Boer, G.A.; Hunt, J.E.; Gabe, M.B.N.; Windeløv, J.A.; Sparre-Ulrich, A.H.; Hartmann, B.; Holst, J.J.; Rosenkilde, M.M. Glucose-dependent insulinotropic polypeptide receptor antagonist treatment causes a reduction in weight gain in ovariectomised high fat diet-fed mice. Br. J. Pharmacol. 2022, 179, 4486–4499. [Google Scholar] [CrossRef] [PubMed]
- Otten, J.; Ryberg, M.; Mellberg, C.; Andersson, T.; Chorell, E.; Lindahl, B.; Larsson, C.; Holst, J.J.; Olsson, T. Postprandial levels of GLP-1, GIP and glucagon after 2 years of weight loss with a Paleolithic diet: A randomised controlled trial in healthy obese women. Eur. J. Endocrinol. 2019, 180, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Stentz, F.B.; Mikhael, A.; Kineish, O.; Christman, J.; Sands, C. High protein diet leads to prediabetes remission and positive changes in incretins and cardiovascular risk factors. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 1227–1237. [Google Scholar] [CrossRef] [PubMed]
- Nbdspqibhf DUP; Kanda, H.; Tateya, S.; Tamori, Y.; Kotani, K.; Hiasa, K.I.; Kitazawa, R.; Kitazawa, S.; Miyachi, H.; Maeda, S.; et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J. Clin. Investig. 2006, 116, 1494–1505. [Google Scholar] [CrossRef]
- Kamei, N.; Tobe, K.; Suzuki, R.; Ohsugi, M.; Watanabe, T.; Kubota, N.; Ohtsuka-Kowatari, N.; Kumagai, K.; Sakamoto, K.; Kobayashi, M.; et al. Overexpression of monocyte chemoattractant protein-1 in adipose tissues causes macrophage recruitment and insulin resistance. J. Biol. Chem. 2006, 281, 26602–26614. [Google Scholar] [CrossRef]
- Herder, C.; Baumert, J.; Thorand, B.; Martin, S.; Lowel, H.; Kolb, H.; Koenig, W. Chemokines and incident coronary heart disease: Results from the MONICA/KORA Augsburg case-cohort study, 1984–2002. Arter. Thromb. Vasc. Biol. 2006, 26, 2147–2152. [Google Scholar] [CrossRef]
- Chavey, C.; Lazennec, G.; Lagarrigue, S.; Clapé, C.; Iankova, I.; Teyssier, J.; Annicotte, J.-S.; Schmidt, J.; Mataki, C.; Yamamoto, H.; et al. CXC Ligand 5 Is an Adipose-Tissue Derived Factor that Links Obesity to Insulin Resistance. Cell Metab. 2009, 9, 339–349. [Google Scholar] [CrossRef]
- Tamura, Y.; Sugimoto, M.; Murayama, T.; Ueda, Y.; Kanamori, H.; Ono, K.; Ariyasu, H.; Akamizu, T.; Kita, T.; Yokode, M.; et al. Inhibition of CCR2 ameliorates insulin resistance and hepatic steatosis in db/db mice. Arter. Thromb. Vasc. Biol. 2008, 28, 2195–2201. [Google Scholar] [CrossRef] [PubMed]
- Jamialahmadi, T.; Abbasifard, M.; Reiner, Ž.; Kesharwani, P.; Sahebkar, A. The Effect of Bariatric Surgery on Circulating Levels of Monocyte Chemoattractant Protein-1: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 7021. [Google Scholar] [CrossRef] [PubMed]
- Ngcobo, S.R.; Nkambule, B.B.; Nyambuya, T.M.; Mokgalaboni, K.; Ntsethe, A.; Mxinwa, V.; Ziqubu, K.; Ntamo, Y.; Nyawo, T.A.; Dludla, P.V. Activated monocytes as a therapeutic target to attenuate vascular inflammation and lower cardiovascular disease-risk in patients with type 2 diabetes: A systematic review of preclinical and clinical studies. Biomed. Pharmacother. 2021, 146, 112579. [Google Scholar] [CrossRef] [PubMed]
- Ruan, W.; Lai, M. Insulin-like growth factor binding protein: A possible marker for the metabolic syndrome? Acta Diabetol. 2009, 47, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.F.; Gu, T.; Hilding, A.; Zhu, Y.; Kärvestedt, L.; Östenson, C.-G.; Lai, M.; Kutsukake, M.; Frystyk, J.; Tamura, K.; et al. Evaluation of IGFBP-7 DNA methylation changes and serum protein variation in Swedish subjects with and without type 2 diabetes. Clin. Epigenet. 2013, 5, 20. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Wen, J.; Wu, F.; Zeng, H.; Guo, B.; Ge, L. Pharmacotherapy weight-loss interventions to prevent type 2 diabetes in overweight or obese adults and older adults: A protocol for systematic review and network meta-analysis. Medicine 2021, 100, e24812. [Google Scholar] [CrossRef]
- Karagiannis, T.; Avgerinos, I.; Liakos, A.; Del Prato, S.; Matthews, D.R.; Tsapas, A.; Bekiari, E. Management of type 2 diabetes with the dual GIP/GLP-1 receptor agonist tirzepatide: A systematic review and meta-analysis. Diabetologia 2022, 65, 1251–1261. [Google Scholar] [CrossRef]
- Watanabe, J.; Takiyama, Y.; Honjyo, J.; Makino, Y.; Fujita, Y.; Tateno, M.; Haneda, M. Role of IGFBP7 in diabetic nephropathy: TGF-β1 induces IGFBP7 via Smad2/4 in human renal proximal tubular epithelial cells. PLoS ONE 2016, 11, e0150897. [Google Scholar] [CrossRef]
- Thiele, K.; Rau, M.; Hartmann, N.U.K.; Möller, M.; Möllmann, J.; Jankowski, J.; Keszei, A.P.; Böhm, M.; Floege, J.; Marx, N.; et al. Empagliflozin reduces markers of acute kidney injury in patients with acute decompensated heart failure. ESC Heart Fail. 2022, 9, 2233–2238. [Google Scholar] [CrossRef]
- Januzzi, J.L.; Packer, M.; Claggett, B.; Liu, J.; Shah, A.M.; Zile, M.R.; Pieske, B.; Voors, A.; Gandhi, P.U.; Prescott, M.F.; et al. IGFBP7 (Insulin-like Growth Factor–Binding Protein-7) and Neprilysin Inhibition in Patients with Heart Failure. Circ. Heart Fail. 2018, 11, e005133. [Google Scholar] [CrossRef]
- Hinney, A.; Körner, A.; Fischer-Posovszky, P. The promise of new anti-obesity therapies arising from knowledge of genetic obesity traits. Nat. Rev. Endocrinol. 2022, 18, 623–637. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.D.; Blüher, M.; Tschöp, M.H.; DiMarchi, R.D. Anti-obesity drug discovery: Advances and challenges. Nat. Rev. Drug Discov. 2022, 21, 201–223. [Google Scholar] [CrossRef] [PubMed]
- Ludvik, B.; Giorgino, F.; Jódar, E.; Frias, J.P.; Landó, L.F.; Brown, K.; Bray, R.; Rodríguez, A. Once-weekly tirzepatide versus once-daily insulin degludec as add-on to metformin with or without SGLT2 inhibitors in patients with type 2 diabetes (SURPASS-3): A randomised, open-label, parallel-group, phase 3 trial. Lancet 2021, 398, 583–598. [Google Scholar] [CrossRef] [PubMed]
- Dahl, D.; Onishi, Y.; Norwood, P.; Huh, R.; Bray, R.; Patel, H.; Rodríguez, Á. Effect of Subcutaneous Tirzepatide vs Placebo Added to Titrated Insulin Glargine on Glycemic Control in Patients with Type 2 Diabetes: The SURPASS-5 Randomized Clinical Trial. JAMA 2022, 327, 534–545. [Google Scholar] [CrossRef] [PubMed]
- Rosenstock, J.; Wysham, C.; Frías, J.P.; Kaneko, S.; Lee, C.J.; Landó, L.F.; Mao, H.; Cui, X.; Karanikas, C.A.; Thieu, V.T. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): A double-blind, randomised, phase 3 trial. Lancet 2021, 398, 143–155. [Google Scholar] [CrossRef]
- Muzurović, E.M.; Volčanšek, Š.; Tomšić, K.Z.; Janež, A.; Mikhailidis, D.P.; Rizzo, M.; Mantzoros, C.S. Glucagon-Like Peptide-1 Receptor Agonists and Dual Glucose-Dependent Insulinotropic Polypeptide/Glucagon-like Peptide-1 Receptor Agonists in the Treatment of Obesity/Metabolic Syndrome, Prediabetes/Diabetes and Non-Alcoholic Fatty Liver Disease—Current Evidence. J. Cardiovasc. Pharmacol. Ther. 2022, 27, 10742484221146371. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Raja, A. Physiology, Gastric Inhibitory Peptide; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Hattersley, A.T.; Patel, K.A. Precision diabetes: Learning from monogenic diabetes. Diabetologia 2017, 60, 769–777. [Google Scholar] [CrossRef]
- Teruya, T.; Sunagawa, S.; Mori, A.; Masuzaki, H.; Yanagida, M. Markers for obese and non-obese Type 2 diabetes identified using whole blood metabolomics. Sci. Rep. 2023, 13, 2460. [Google Scholar] [CrossRef]
- Nauck, M.A.; Vardarli, I.; Deacon, C.F.; Holst, J.J.; Meier, J.J. Secretion of glucagon-like peptide-1 (GLP-1) in type 2 diabetes: What is up, what is down? Diabetologia 2011, 54, 10–18. [Google Scholar] [CrossRef]
- Crockett, S.E.; Mazzaferri, E.L.; Cataland, S. Gastric inhibitory polypeptide (GIP) in maturity-onset diabetes mellitus. Diabetes 1976, 25, 931–935. [Google Scholar] [CrossRef]
- Seshadri, K.G.; Radhakrishnan, P.; Srikanth, P.; Barani, R.; Samanta, M. Serum monocyte chemoattractant protein-1 is a biomarker in patients with diabetes and periodontitis. Indian J. Endocrinol. Metab. 2014, 18, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Szyszkowska, A.; Knapp, M.; Kamiński, K.; Lisowska, A. Insulin-like growth factor-binding protein 7 (IGFBP7): Novel, independent marker of cardiometabolic diseases? Postępy Hig. Med. Doświadczalnej 2019, 73, 735–740. [Google Scholar] [CrossRef]
- Chindarkar, N.S.; Chawla, L.S.; Straseski, J.A.; Jortani, S.A.; Uettwiller-Geiger, D.; Orr, R.R.; Kellum, J.A.; Fitzgerald, R.L. Reference intervals of urinary acute kidney injury (AKI) markers [IGFBP7]∙[TIMP2] in apparently healthy subjects and chronic comorbid subjects without AKI. Clin. Chim. Acta 2016, 452, 32–37. [Google Scholar] [CrossRef] [PubMed]




| Biomarker | Prediction Role | Risk Assessment Role |
|---|---|---|
| GLP-1 | Reduced basal GLP-1 levels or impaired GLP-1 response postprandially may indicate a diminished incretin effect, contributing to type 2 diabetes and obesity. | Elevated fasting serum GLP-1 concentrations could suggest compensatory mechanisms in response to insulin resistance. Monitoring GLP-1 response to glucose challenges provides insights into beta cell function and its role in obesity-associated insulin resistance. |
| GIP | Altered GIP levels, especially post-glucose ingestion, may indicate disruptions in insulin secretion and potential risks for type 2 diabetes and obesity. | Increased fasting serum GIP levels, particularly after glucose ingestion, could signal impaired glucose regulation and contribute to the development of both diabetes and obesity. |
| MCP-1 | Increased levels of MCP-1 in the blood may be linked to inflammatory processes that are connected to obesity, type 2 diabetes, and insulin resistance. | Monitoring MCP-1 levels, especially in the context of periodontal disease, can provide insights into the inflammatory component of diabetes and obesity-related inflammation. |
| IGFBP7 | Changes in IGFBP-7 levels may be associated with insulin resistance, metabolic syndrome, early kidney injury, and obesity-related metabolic dysfunction. | Increased concentrations of IGFBP-7, especially in urine, may indicate a higher risk of metabolic syndrome, insulin resistance, potential kidney injury, and its association with obesity-related metabolic complications. |
| Biomarker | Commonality | Changes across Stages | Quantitative Differences |
|---|---|---|---|
| GLP-1 | GLP-1 is implicated in both diabetes and obesity. In early stages, there might be compensatory increases, while in later stages, reduced levels could contribute to impaired insulin response. | Early stages may show elevated GLP-1 concentrations as a response to insulin resistance. In later stages, a decline might occur, impacting glycaemic control and potentially contributing to the progression of diabetes and obesity. | Quantitative levels can differ, with lower levels in severe illness states and larger concentrations in early compensatory stages. |
| GIP | GIP is linked to both diabetes and obesity. Elevated fasting GIP levels may be observed, especially after glucose ingestion. | Early stages may exhibit increased GIP as a compensatory response. In later stages, this elevation might contribute to insulin resistance, diabetes, and obesity. | In early phases, higher concentrations are observed, and in later stages, there may be changes in the quantitative levels. |
| MCP-1 | MCP-1 is associated with inflammation in diabetes and obesity. Concentrations may increase with the progression of both conditions. | Early stages may show moderate MCP-1 elevations, while in later stages, especially with periodontal disease, a significant increase may occur, exacerbating inflammation in diabetes and obesity. | Quantitative levels may vary, with a more pronounced increase in later stages, especially in the presence of periodontal disease. |
| IGFBP7 | IGFBP-7 is linked to insulin resistance, metabolic syndrome, and obesity-related complications. | Early stages may exhibit changes in IGFBP-7 associated with insulin resistance. In later stages, especially with kidney injury, concentrations may rise, indicating a higher risk of metabolic complications in diabetes and obesity. | Quantitative levels may differ across stages, with potential increases in later stages, particularly in the context of kidney injury and metabolic syndrome. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jędrysik, M.; Wyszomirski, K.; Różańska-Walędziak, A.; Grosicka-Maciąg, E.; Walędziak, M.; Chełstowska, B. The Role of GLP-1, GIP, MCP-1 and IGFBP-7 Biomarkers in the Development of Metabolic Disorders: A Review and Predictive Analysis in the Context of Diabetes and Obesity. Biomedicines 2024, 12, 159. https://doi.org/10.3390/biomedicines12010159
Jędrysik M, Wyszomirski K, Różańska-Walędziak A, Grosicka-Maciąg E, Walędziak M, Chełstowska B. The Role of GLP-1, GIP, MCP-1 and IGFBP-7 Biomarkers in the Development of Metabolic Disorders: A Review and Predictive Analysis in the Context of Diabetes and Obesity. Biomedicines. 2024; 12(1):159. https://doi.org/10.3390/biomedicines12010159
Chicago/Turabian StyleJędrysik, Malwina, Krzysztof Wyszomirski, Anna Różańska-Walędziak, Emilia Grosicka-Maciąg, Maciej Walędziak, and Beata Chełstowska. 2024. "The Role of GLP-1, GIP, MCP-1 and IGFBP-7 Biomarkers in the Development of Metabolic Disorders: A Review and Predictive Analysis in the Context of Diabetes and Obesity" Biomedicines 12, no. 1: 159. https://doi.org/10.3390/biomedicines12010159
APA StyleJędrysik, M., Wyszomirski, K., Różańska-Walędziak, A., Grosicka-Maciąg, E., Walędziak, M., & Chełstowska, B. (2024). The Role of GLP-1, GIP, MCP-1 and IGFBP-7 Biomarkers in the Development of Metabolic Disorders: A Review and Predictive Analysis in the Context of Diabetes and Obesity. Biomedicines, 12(1), 159. https://doi.org/10.3390/biomedicines12010159