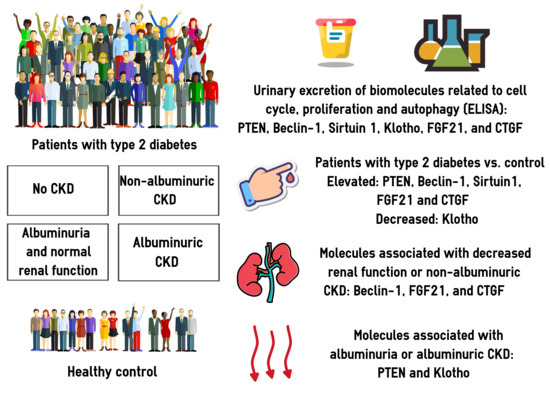
Urinary Excretion of Biomolecules Related to Cell Cycle, Proliferation, and Autophagy in Subjects with Type 2 Diabetes and Chronic Kidney Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design
2.2. Methods
2.3. Statistical Analysis
3. Results
3.1. Clinical Characteristics of the Study Participants
3.2. Urinary Excretion of PTEN, BECN1, SIRT1, Klotho, FGF21, and CTGF
3.3. Associations between the Urinary Biomarkers and CKD: Univariate Models
3.4. Associations between the Urinary Biomarkers and CKD: Multivariate Models
4. Discussion
4.1. PTEN
4.2. BECN1
4.3. SIRT1
4.4. Klotho
4.5. FGF21
4.6. CTGF
4.7. Dysregulation of the PI3K/AKT/mTOR Pathway in the Pathogenesis of Diabetic CKD
4.8. Limitations of the Study and Future Remarks
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lv, J.-C.; Zhang, L.-X. Prevalence and Disease Burden of Chronic Kidney Disease. Adv. Exp. Med. Biol. 2019, 1165, 3–15. [Google Scholar] [CrossRef]
- Magliano, D.J.; Boyko, E.J.; IDF Diabetes Atlas 10th Edition Scientific Committee. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021; ISBN 978-2-930229-98-0. [Google Scholar]
- Zhang, X.-X.; Kong, J.; Yun, K. Prevalence of Diabetic Nephropathy among Patients with Type 2 Diabetes Mellitus in China: A Meta-Analysis of Observational Studies. J. Diabetes Res. 2020, 2020, 2315607. [Google Scholar] [CrossRef]
- Ying, M.; Shao, X.; Qin, H.; Yin, P.; Lin, Y.; Wu, J.; Ren, J.; Zheng, Y. Disease Burden and Epidemiological Trends of Chronic Kidney Disease at the Global, Regional, National Levels from 1990 to 2019. Nephron 2023, 148, 113–123. [Google Scholar] [CrossRef]
- Jayakumari, C.; Gomez, R.; Dipin, S.; Jayakumar, R.V.; Vijayakumar, K.; Sreenath, R.; Ajeesh, T.; Jabbar, P.K.; Das, D.V.; Gopi, G.V.; et al. Prevalence of Normoalbuminuric Chronic Kidney Disease among Individuals with Type 2 Diabetes Mellitus from India. Indian J. Med. Res. 2022, 156, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Scilletta, S.; Di Marco, M.; Miano, N.; Filippello, A.; Di Mauro, S.; Scamporrino, A.; Musmeci, M.; Coppolino, G.; Di Giacomo Barbagallo, F.; Bosco, G.; et al. Update on Diabetic Kidney Disease (DKD): Focus on Non-Albuminuric DKD and Cardiovascular Risk. Biomolecules 2023, 13, 752. [Google Scholar] [CrossRef] [PubMed]
- Klimontov, V.V.; Korbut, A.I. Albuminuric and Non-Albuminuric Patterns of Chronic Kidney Disease in Type 2 Diabetes. Diabetes Metab. Syndr. 2019, 13, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Ni, L.; Gao, L.; Wu, X. Comparison of Nonalbuminuric and Albuminuric Diabetic Kidney Disease Among Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2022, 13, 871272. [Google Scholar] [CrossRef]
- Dai, Q.; Chen, N.; Zeng, L.; Lin, X.-J.; Jiang, F.-X.; Zhuang, X.-J.; Lu, Z.-Y. Clinical Features of and Risk Factors for Normoalbuminuric Diabetic Kidney Disease in Hospitalized Patients with Type 2 Diabetes Mellitus: A Retrospective Cross-Sectional Study. BMC Endocr. Disord. 2021, 21, 104. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Livingston, M.J.; Liu, Z.; Dong, Z. Autophagy in Kidney Homeostasis and Disease. Nat. Rev. Nephrol. 2020, 16, 489–508. [Google Scholar] [CrossRef] [PubMed]
- Sanz, A.B.; Sanchez-Niño, M.D.; Ramos, A.M.; Ortiz, A. Regulated Cell Death Pathways in Kidney Disease. Nat. Rev. Nephrol. 2023, 19, 281–299. [Google Scholar] [CrossRef] [PubMed]
- Mohandes, S.; Doke, T.; Hu, H.; Mukhi, D.; Dhillon, P.; Susztak, K. Molecular Pathways That Drive Diabetic Kidney Disease. J. Clin. Investig. 2023, 133, e165654. [Google Scholar] [CrossRef]
- Klimontov, V.V.; Saik, O.V.; Korbut, A.I. Glucose Variability: How Does It Work? Int. J. Mol. Sci. 2021, 22, 7783. [Google Scholar] [CrossRef]
- Zhang, Y.; Jin, D.; Kang, X.; Zhou, R.; Sun, Y.; Lian, F.; Tong, X. Signaling Pathways Involved in Diabetic Renal Fibrosis. Front. Cell Dev. Biol. 2021, 9, 696542. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Ning, X.; Li, R.; Yang, Z.; Yang, X.; Sun, S.; Qian, Q. Signalling Pathways Involved in Hypoxia-Induced Renal Fibrosis. J. Cell. Mol. Med. 2017, 21, 1248–1259. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, S.; Liu, S.; Li, C.; Wang, J. Role of Smad Signaling in Kidney Disease. Int. Urol. Nephrol. 2015, 47, 1965–1975. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, Y.; Kang, M.; Chang, C.; Wei, H.; Zhang, C.; Chen, Y. Multiple Forms of Cell Death: A Focus on the PI3K/AKT Pathway. J. Cell. Physiol. 2023, 238, 2026–2038. [Google Scholar] [CrossRef] [PubMed]
- Savova, M.S.; Mihaylova, L.V.; Tews, D.; Wabitsch, M.; Georgiev, M.I. Targeting PI3K/AKT Signaling Pathway in Obesity. Biomed. Pharmacother. 2023, 159, 114244. [Google Scholar] [CrossRef] [PubMed]
- Verma, K.; Jaiswal, R.; Paliwal, S.; Dwivedi, J.; Sharma, S. An Insight into PI3k/Akt Pathway and Associated Protein-Protein Interactions in Metabolic Syndrome: A Recent Update. J. Cell. Biochem. 2023, 124, 923–942. [Google Scholar] [CrossRef] [PubMed]
- Maffei, A.; Lembo, G.; Carnevale, D. PI3Kinases in Diabetes Mellitus and Its Related Complications. Int. J. Mol. Sci. 2018, 19, 4098. [Google Scholar] [CrossRef]
- Bilanges, B.; Posor, Y.; Vanhaesebroeck, B. PI3K Isoforms in Cell Signalling and Vesicle Trafficking. Nat. Rev. Mol. Cell Biol. 2019, 20, 515–534. [Google Scholar] [CrossRef]
- Gulluni, F.; De Santis, M.C.; Margaria, J.P.; Martini, M.; Hirsch, E. Class II PI3K Functions in Cell Biology and Disease. Trends Cell Biol. 2019, 29, 339–359. [Google Scholar] [CrossRef] [PubMed]
- Khokhar, M.; Roy, D.; Modi, A.; Agarwal, R.; Yadav, D.; Purohit, P.; Sharma, P. Perspectives on the Role of PTEN in Diabetic Nephropathy: An Update. Crit. Rev. Clin. Lab. Sci. 2020, 57, 470–483. [Google Scholar] [CrossRef] [PubMed]
- Kma, L.; Baruah, T.J. The Interplay of ROS and the PI3K/Akt Pathway in Autophagy Regulation. Biotechnol. Appl. Biochem. 2022, 69, 248–264. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Chen, J.; He, L.; Stiles, B.L. PTEN: Tumor Suppressor and Metabolic Regulator. Front. Endocrinol. 2018, 9, 338. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Pu, S.; Zhou, H.; Guo, Y. Klotho as Potential Autophagy Regulator and Therapeutic Target. Front. Pharmacol. 2021, 12, 755366. [Google Scholar] [CrossRef]
- Menon, M.B.; Dhamija, S. Beclin 1 Phosphorylation—At the Center of Autophagy Regulation. Front. Cell Dev. Biol. 2018, 6, 137. [Google Scholar] [CrossRef]
- Kuro-O, M. The Klotho Proteins in Health and Disease. Nat. Rev. Nephrol. 2019, 15, 27–44. [Google Scholar] [CrossRef]
- Liu, Q.; Huang, J.; Yan, W.; Liu, Z.; Liu, S.; Fang, W. FGFR Families: Biological Functions and Therapeutic Interventions in Tumors. MedComm (2020) 2023, 4, e367. [Google Scholar] [CrossRef]
- Mossahebi-Mohammadi, M.; Quan, M.; Zhang, J.-S.; Li, X. FGF Signaling Pathway: A Key Regulator of Stem Cell Pluripotency. Front. Cell Dev. Biol. 2020, 8, 79. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.; Maity, S.; AlRaawi, Z.; Al-Ameer, M.; Kumar, T.K.S. Targeting Drugs Against Fibroblast Growth Factor(s)-Induced Cell Signaling. Curr. Drug Targets 2021, 22, 214–240. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.-C.; Gao, Y.; Chen, J.; Meng, Q.; Fang, L. EGF Promotes Human Trophoblast Cell Invasion by Downregulating CTGF Expression via PI3K/AKT Signaling. Reproduction 2023, 165, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhou, C.; Yuan, Q.; Zhang, D.; Xie, J.; Zou, S. CTGF Facilitates Cell-Cell Communication in Chondrocytes via PI3K/Akt Signalling Pathway. Cell Prolif. 2021, 54, e13001. [Google Scholar] [CrossRef] [PubMed]
- Pillai, V.B.; Sundaresan, N.R.; Gupta, M.P. Regulation of Akt Signaling by Sirtuins: Its Implication in Cardiac Hypertrophy and Aging. Circ. Res. 2014, 114, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Zhang, Z.; Chen, C.; Liu, Y.; Yuan, D.; Hei, Z.; Luo, G. PI3K/AKT Activation Attenuates Acute Kidney Injury Following Liver Transplantation by Inducing FoxO3a Nuclear Export and Deacetylation. Life Sci. 2021, 272, 119119. [Google Scholar] [CrossRef] [PubMed]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Proteomics. Tissue-Based Map of the Human Proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Gifford, C.C.; Samarakoon, R.; Higgins, P.J. Deregulation of Negative Controls on TGF-Β1 Signaling in Tumor Progression. Cancers 2018, 10, 159. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Shi, Y.; Peng, H.; Shen, X.; Thomas, S.; Wang, Y.; Truong, L.D.; Dryer, S.E.; Hu, Z.; Xu, J. Loss of PTEN Promotes Podocyte Cytoskeletal Rearrangement, Aggravating Diabetic Nephropathy. J. Pathol. 2015, 236, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Feng, Z.; Xie, J.; Wen, F.; Jv, M.; Liang, T.; Li, J.; Wang, Y.; Zuo, Y.; Li, S.; et al. Podocyte-Specific Knockin of PTEN Protects Kidney from Hyperglycemia. Am. J. Physiol. Renal Physiol. 2018, 314, F1096–F1107. [Google Scholar] [CrossRef]
- Audzeyenka, I.; Rachubik, P.; Typiak, M.; Kulesza, T.; Kalkowska, D.; Rogacka, D.; Rychłowski, M.; Angielski, S.; Saleem, M.; Piwkowska, A. PTEN-Induced Kinase 1 Deficiency Alters Albumin Permeability and Insulin Signaling in Podocytes. J. Mol. Med. 2022, 100, 903–915. [Google Scholar] [CrossRef]
- Li, Y.; Hu, Q.; Li, C.; Liang, K.; Xiang, Y.; Hsiao, H.; Nguyen, T.K.; Park, P.K.; Egranov, S.D.; Ambati, C.R.; et al. PTEN-Induced Partial Epithelial-Mesenchymal Transition Drives Diabetic Kidney Disease. J. Clin. Investig. 2019, 129, 1129–1151. [Google Scholar] [CrossRef]
- Klionsky, D.J.; Abdel-Aziz, A.K.; Abdelfatah, S.; Abdellatif, M.; Abdoli, A.; Abel, S.; Abeliovich, H.; Abildgaard, M.H.; Abudu, Y.P.; Acevedo-Arozena, A.; et al. Guidelines for the Use and Interpretation of Assays for Monitoring Autophagy (4th Edition)1. Autophagy 2021, 17, 1–382. [Google Scholar] [CrossRef] [PubMed]
- Korbut, A.I.; Taskaeva, I.S.; Bgatova, N.P.; Muraleva, N.A.; Orlov, N.B.; Dashkin, M.V.; Khotskina, A.S.; Zavyalov, E.L.; Konenkov, V.I.; Klein, T.; et al. SGLT2 Inhibitor Empagliflozin and DPP4 Inhibitor Linagliptin Reactivate Glomerular Autophagy in Db/Db Mice, a Model of Type 2 Diabetes. Int. J. Mol. Sci. 2020, 21, 2987. [Google Scholar] [CrossRef] [PubMed]
- Naguib, M.; Rashed, L.A. Serum Level of the Autophagy Biomarker Beclin-1 in Patients with Diabetic Kidney Disease. Diabetes Res. Clin. Pract. 2018, 143, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Morigi, M.; Perico, L.; Benigni, A. Sirtuins in Renal Health and Disease. J. Am. Soc. Nephrol. 2018, 29, 1799–1809. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Hu, C.; Zhao, D.; Li, X. SIRT1-SIRT7 in Diabetic Kidney Disease: Biological Functions and Molecular Mechanisms. Front. Endocrinol. 2022, 13, 801303. [Google Scholar] [CrossRef] [PubMed]
- Timoshchenko, O.V.; Stakhneva, E.M.; Ragino, Y.I.; Nikitin, Y.P. Klotho Protein in Men with Type 2 Diabetes Mellitus Blood and Its Association with Cardiometabolic Risk Factors. Sib. Nauchnyj Med. Zhurnal 2021, 41, 21–27. [Google Scholar] [CrossRef]
- Nie, F.; Wu, D.; Du, H.; Yang, X.; Yang, M.; Pang, X.; Xu, Y. Serum Klotho Protein Levels and Their Correlations with the Progression of Type 2 Diabetes Mellitus. J. Diabetes Complicat. 2017, 31, 594–598. [Google Scholar] [CrossRef]
- Wang, K.; Mao, Y.; Lu, M.; Liu, X.; Sun, Y.; Li, Z.; Li, Y.; Ding, Y.; Zhang, J.; Hong, J.; et al. Association between Serum Klotho Levels and the Prevalence of Diabetes among Adults in the United States. Front. Endocrinol. 2022, 13, 1005553. [Google Scholar] [CrossRef]
- Typiak, M.; Kulesza, T.; Rachubik, P.; Rogacka, D.; Audzeyenka, I.; Angielski, S.; Saleem, M.A.; Piwkowska, A. Role of Klotho in Hyperglycemia: Its Levels and Effects on Fibroblast Growth Factor Receptors, Glycolysis, and Glomerular Filtration. Int. J. Mol. Sci. 2021, 22, 7867. [Google Scholar] [CrossRef]
- Martín-Vírgala, J.; Fernández-Villabrille, S.; Martín-Carro, B.; Tamargo-Gómez, I.; Navarro-González, J.F.; Mora-Fernández, C.; Calleros, L.; Astudillo-Cortés, E.; Avello-Llano, N.; Mariño, G.; et al. Serum and Urinary Soluble α-Klotho as Markers of Kidney and Vascular Impairment. Nutrients 2023, 15, 1470. [Google Scholar] [CrossRef]
- Xia, J.; Cao, W. Epigenetic Modifications of Klotho Expression in Kidney Diseases. J. Mol. Med. 2021, 99, 581–592. [Google Scholar] [CrossRef]
- Kale, A.; Sankrityayan, H.; Anders, H.-J.; Gaikwad, A.B. Epigenetic and Non-Epigenetic Regulation of Klotho in Kidney Disease. Life Sci. 2021, 264, 118644. [Google Scholar] [CrossRef] [PubMed]
- Sakan, H.; Nakatani, K.; Asai, O.; Imura, A.; Tanaka, T.; Yoshimoto, S.; Iwamoto, N.; Kurumatani, N.; Iwano, M.; Nabeshima, Y.-I.; et al. Reduced Renal α-Klotho Expression in CKD Patients and Its Effect on Renal Phosphate Handling and Vitamin D Metabolism. PLoS ONE 2014, 9, e86301. [Google Scholar] [CrossRef]
- Portales-Castillo, I.; Simic, P. PTH, FGF-23, Klotho and Vitamin D as Regulators of Calcium and Phosphorus: Genetics, Epigenetics and Beyond. Front. Endocrinol. 2022, 13, 992666. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Ren, Q.; Li, L.; Tan, H.; Lu, M.; Tian, Y.; Huang, L.; Zhao, B.; Fu, H.; Hou, F.F.; et al. A Klotho-Derived Peptide Protects against Kidney Fibrosis by Targeting TGF-β Signaling. Nat. Commun. 2022, 13, 438. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Cao, J.; Wei, X.; Ge, Y.; Su, Z.; Yu, D. Klotho Alleviates Contrast-Induced Acute Kidney Injury by Suppressing Oxidative Stress, Inflammation, and NF-KappaB/NLRP3-Mediated Pyroptosis. Int. Immunopharmacol. 2023, 118, 110105. [Google Scholar] [CrossRef]
- Zhao, Y.; Banerjee, S.; Dey, N.; LeJeune, W.S.; Sarkar, P.S.; Brobey, R.; Rosenblatt, K.P.; Tilton, R.G.; Choudhary, S. Klotho Depletion Contributes to Increased Inflammation in Kidney of the Db/Db Mouse Model of Diabetes via RelA (Serine)536 Phosphorylation. Diabetes 2011, 60, 1907–1916. [Google Scholar] [CrossRef]
- Chang, K.; Li, Y.; Qin, Z.; Zhang, Z.; Wang, L.; Yang, Q.; Su, B. Association between Serum Soluble α-Klotho and Urinary Albumin Excretion in Middle-Aged and Older US Adults: NHANES 2007–2016. J. Clin. Med. 2023, 12, 637. [Google Scholar] [CrossRef]
- Fernandez-Fernandez, B.; Izquierdo, M.C.; Valiño-Rivas, L.; Nastou, D.; Sanz, A.B.; Ortiz, A.; Sanchez-Niño, M.D. Albumin Downregulates Klotho in Tubular Cells. Nephrol. Dial. Transplant. 2018, 33, 1712–1722. [Google Scholar] [CrossRef]
- Delitsikou, V.; Jarad, G.; Rajaram, R.D.; Ino, F.; Rutkowski, J.M.; Chen, C.-D.; Santos, C.X.C.; Scherer, P.E.; Abraham, C.R.; Shah, A.M.; et al. Klotho Regulation by Albuminuria Is Dependent on ATF3 and Endoplasmic Reticulum Stress. FASEB J. 2020, 34, 2087–2104. [Google Scholar] [CrossRef]
- Charrin, E.; Dabaghie, D.; Sen, I.; Unnersjö-Jess, D.; Möller-Hackbarth, K.; Burmakin, M.; Mencke, R.; Zambrano, S.; Patrakka, J.; Olauson, H. Soluble Klotho Protects against Glomerular Injury through Regulation of ER Stress Response. Commun. Biol. 2023, 6, 208. [Google Scholar] [CrossRef]
- Kim, J.-H.; Xie, J.; Hwang, K.-H.; Wu, Y.-L.; Oliver, N.; Eom, M.; Park, K.-S.; Barrezueta, N.; Kong, I.-D.; Fracasso, R.P.; et al. Klotho May Ameliorate Proteinuria by Targeting TRPC6 Channels in Podocytes. J. Am. Soc. Nephrol. 2017, 28, 140–151. [Google Scholar] [CrossRef]
- Suassuna, P.G.d.A.; de Paula, R.B.; Sanders-Pinheiro, H.; Moe, O.W.; Hu, M.-C. Fibroblast Growth Factor 21 in Chronic Kidney Disease. J. Nephrol. 2019, 32, 365–377. [Google Scholar] [CrossRef]
- Catalina, M.O.-S.; Redondo, P.C.; Granados, M.P.; Cantonero, C.; Sanchez-Collado, J.; Albarran, L.; Lopez, J.J. New Insights into Adipokines as Potential Biomarkers for Type-2 Diabetes Mellitus. Curr. Med. Chem. 2019, 26, 4119–4144. [Google Scholar] [CrossRef]
- Fisher, F.M.; Maratos-Flier, E. Understanding the Physiology of FGF21. Annu. Rev. Physiol. 2016, 78, 223–241. [Google Scholar] [CrossRef]
- Flippo, K.H.; Potthoff, M.J. Metabolic Messengers: FGF21. Nat. Metab. 2021, 3, 309–317. [Google Scholar] [CrossRef]
- Zhang, C.; Shao, M.; Yang, H.; Chen, L.; Yu, L.; Cong, W.; Tian, H.; Zhang, F.; Cheng, P.; Jin, L.; et al. Attenuation of Hyperlipidemia- and Diabetes-Induced Early-Stage Apoptosis and Late-Stage Renal Dysfunction via Administration of Fibroblast Growth Factor-21 Is Associated with Suppression of Renal Inflammation. PLoS ONE 2013, 8, e82275. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Yu, L.; Ni, Y.; He, L.; Weng, X.; Lu, X.; Zhang, C. Fibroblast Growth Factor 21 Attenuates Diabetes-Induced Renal Fibrosis by Negatively Regulating TGF-β-P53-Smad2/3-Mediated Epithelial-to-Mesenchymal Transition via Activation of AKT. Diabetes Metab. J. 2020, 44, 158–172. [Google Scholar] [CrossRef] [PubMed]
- Weng, W.; Ge, T.; Wang, Y.; He, L.; Liu, T.; Wang, W.; Zheng, Z.; Yu, L.; Zhang, C.; Lu, X. Therapeutic Effects of Fibroblast Growth Factor-21 on Diabetic Nephropathy and the Possible Mechanism in Type 1 Diabetes Mellitus Mice. Diabetes Metab. J. 2020, 44, 566–580. [Google Scholar] [CrossRef] [PubMed]
- Dendooven, A.; Gerritsen, K.G.; Nguyen, T.Q.; Kok, R.J.; Goldschmeding, R. Connective Tissue Growth Factor (CTGF/CCN2) ELISA: A Novel Tool for Monitoring Fibrosis. Biomarkers 2011, 16, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Cai, X.; Yi, B.; Huang, J.; Wang, J.; Sun, J. Correlation of CTGF Gene Promoter Methylation with CTGF Expression in Type 2 Diabetes Mellitus with or without Nephropathy. Mol. Med. Rep. 2014, 9, 2138–2144. [Google Scholar] [CrossRef]
- Tang, S.C.W.; Leung, J.C.K.; Lai, K.N. Diabetic Tubulopathy: An Emerging Entity. Contrib. Nephrol. 2011, 170, 124–134. [Google Scholar] [CrossRef]
- Li, X.; Liu, W.; Wang, Q.; Liu, P.; Deng, Y.; Lan, T.; Zhang, X.; Qiu, B.; Ning, H.; Huang, H. Emodin Suppresses Cell Proliferation and Fibronectin Expression via p38MAPK Pathway in Rat Mesangial Cells Cultured under High Glucose. Mol. Cell. Endocrinol. 2009, 307, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Wang, L.; Liu, X.; Zhou, H.; Fan, Q.; Luo, J.; Yao, L.; Wang, J.; Feng, J.; Ma, J. Janus Kinase 2/Signal Transducers and Activators of Transcription Signal Inhibition Regulates Protective Effects of Probucol on Mesangial Cells Treated with High Glucose. Biol. Pharm. Bull. 2010, 33, 768–772. [Google Scholar] [CrossRef] [PubMed]
- Yi, B.; Zhang, H.; Zhou, H.; Cai, X.; Sun, J.; Liu, Y. High glucose induce the demethylation of CTGF promoter and gene expression. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2011, 27, 747–750. [Google Scholar]
- Gifford, C.C.; Tang, J.; Costello, A.; Khakoo, N.S.; Nguyen, T.Q.; Goldschmeding, R.; Higgins, P.J.; Samarakoon, R. Negative Regulators of TGF-Β1 Signaling in Renal Fibrosis; Pathological Mechanisms and Novel Therapeutic Opportunities. Clin. Sci. 2021, 135, 275–303. [Google Scholar] [CrossRef]
- Lopes, T.G.; de Souza, M.L.; da Silva, V.D.; Dos Santos, M.; da Silva, W.I.C.; Itaquy, T.P.; Garbin, H.I.; Veronese, F.V. Markers of Renal Fibrosis: How Do They Correlate with Podocyte Damage in Glomerular Diseases? PLoS ONE 2019, 14, e0217585. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-M.; Qi, W.; Pollock, C.A. CTGF and Chronic Kidney Fibrosis. Front. Biosci. (Schol. Ed.) 2009, 1, 132–141. [Google Scholar] [CrossRef]
- Zdychová, J.; Kazdová, L.; Pelikanová, T.; Lindsley, J.N.; Anderson, S.; Komers, R. Renal Activity of Akt Kinase in Obese Zucker Rats. Exp. Biol. Med. 2008, 233, 1231–1241. [Google Scholar] [CrossRef]
- Horita, S.; Nakamura, M.; Suzuki, M.; Satoh, N.; Suzuki, A.; Seki, G. Selective Insulin Resistance in the Kidney. BioMed Res. Int. 2016, 2016, 5825170. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Luo, M.; Xu, F.; Lu, Y.; Zhou, X.; Cui, W.; Miao, L. Elabela Protects against Podocyte Injury in Mice with Streptozocin-Induced Diabetes by Associating with the PI3K/Akt/mTOR Pathway. Peptides 2019, 114, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Fei, X.; Wang, M.; Chen, Q.; Zhao, N. Cardamomin Protects from Diabetes-Induced Kidney Damage through Modulating PI3K/AKT and JAK/STAT Signaling Pathways in Rats. Int. Immunopharmacol. 2022, 107, 108610. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Zhang, X.; Liu, Y.; Zhao, T.; Sun, Z.; Liu, P.; Xiang, Q.; Xiong, J.; Du, X.; Yang, X.; et al. Rutin Alleviates EndMT by Restoring Autophagy through Inhibiting HDAC1 via PI3K/AKT/mTOR Pathway in Diabetic Kidney Disease. Phytomedicine 2023, 112, 154700. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Ma, J.; Leng, T.; Yuan, Z.; Hu, T.; Liu, Q.; Shen, T. Advances in Oxidative Stress in Pathogenesis of Diabetic Kidney Disease and Efficacy of TCM Intervention. Ren. Fail. 2023, 45, 2146512. [Google Scholar] [CrossRef]
- Jian, M.; Yunjia, Z.; Zhiying, D.; Yanduo, J.; Guocheng, J. Interleukin 7 Receptor Activates PI3K/Akt/mTOR Signaling Pathway via Downregulation of Beclin-1 in Lung Cancer. Mol. Carcinog. 2019, 58, 358–365. [Google Scholar] [CrossRef]


| Parameter | Groups | |||
|---|---|---|---|---|
| NRF/NA | DRF/NA | NRF/EA | DRF/EA | |
| N | 35 | 35 | 35 | 35 |
| Age, years | 62 (56–66) | 71 (65–75) ^^^ | 63 (58–68) ## | 68 (61–71) |
| Sex (F/M), n | 18/17 | 17/18 | 18/17 | 70/18 |
| BMI, kg/m2 | 31.6 (29.0–35.7) * | 31.4 (27.9–35.7) * | 31.9 (28.7–35.7) | 32.4 (27.5–35.7) |
| WHR | 1.00 (0.94–1.07) | 0.98 (0.93–1.08) | 1.00 (0.95–1.06) | 1.00 (0.98–1.10) |
| Smoking, n (%) | 7 (20.0) | 10 (28.6) | 7 (20.0) | 3 (8.6) |
| Duration of T2D, years | 13 (10–15) | 15 (11–20) | 16 (12–21) | 18 (12–23) ^ |
| HbA1c, % | 8.0 (7.3–9.3) | 7.8 (6.8–8.8) | 9.2 (8.0–10.9) ^## | 8.6 (8.0–9.9) |
| Creatinine, μmol/L | 81 (70–89) | 113 (98–122) ^^^ | 84 (70–97) | 114 (101–149) ^^^ |
| eGFR, mL/min × 1.73 m2 | 81 (70–89) | 113 (98–122) ^^^ | 84 (70–97) | 114 (101–149) ^^^ |
| CKD G1/G2/G3a/G3b/G4 | 7/28/0/0/0 | 0/0/27/8/0 | 7/28/0/0/0 | 0/0/22/11/2 |
| UACR, mg/mmol | 0.3 (0.2–0.4) | 0.4 (0.3–0.6) | 21.1 (7.3–57.7) ^^^### | 43.6 (9.9–99.7) ^^^### |
| Arterial hypertension, n (%) | 35 (100) | 35 (100) | 35 (100) | 35 (100) |
| Coronary artery disease, n (%) | 15 (42.9) | 15 (42.9) | 16 (45.7) | 21 (60) |
| Chronic heart failure (NYHA class III or IV), n (%) | 0 (0) | 1 (2.9) | 0 (0) | 5 (14.3) ^* |
| Myocardial infarction in medical history, n (%) | 6 (17.1) | 7 (20) | 9 (25.7) | 14 (40) ^ |
| Stroke in medical history, n (%) | 2 (5.7) | 4 (11.4) | 4 (11.4) | 4 (11.4) |
| Parameter | Groups | |||
|---|---|---|---|---|
| NRF/NA | DRF/NA | NRF/EA | DRF/EA | |
| N | 35 | 35 | 35 | 35 |
| Antihyperglycemic agents | ||||
| Insulin, n (%) | 21 (60) | 22 (62.9) | 24 (68.6) | 29 (82.9) |
| Metformin, n (%) | 34 (97.1) | 24 (68.6) | 31 (88.6) | 16 (45.7) ^* |
| Sulfonylurea, n (%) | 16 (45.7) | 15 (42.9) | 15 (42.9) | 9 (25.7) |
| DPP4 inhibitors, n (%) | 8 (22.9) | 4 (11.4) | 3 (8.6) | 3 (8.6) |
| GLP-1 analogues, n (%) | 1 (2.86) | 1 (2.86) | 0 (0) | 0 (0) |
| SGLT2 inhibitors, n (%) | 7 (20) | 11 (31.4) | 13 (37.1) | 4 (11.4) |
| Antihypertensive agents | ||||
| ACE inhibitor/ ARB, n (%) | 12/16 (34.3/45.7) | 11/19 (31.4/54.3) | 9/17 (25.7/48.6) | 14/17 (40/48.6) |
| β-blockers, n (%) | 15 (42.9) | 24 (68.6) | 25 (71.4) | 24 (68.6) |
| CCB, n (%) | 8 (22.9) | 16 (45.7) | 14 (40) | 20 (57.1) ^ |
| Diuretics, n (%) | 16 (45.7) | 19 (54.3) | 14 (40) | 24 (68.6) |
| Lipid-lowering agents | ||||
| Statins, n (%) | 23 (65.7) | 22 (62.9) | 21 (60.0) | 24 (68.6) |
| Parameter | Cut-Off Point | AUC ± SE, 95% CI, p-Value | OR, 95% CI, p-Value | Se | Sp |
|---|---|---|---|---|---|
| eGFR < 60 mL/min × 1.73 m2 | |||||
| FGF21 | ≥1.74 ng/mmol | 0.6 ± 0.05 (0.504–0.69), p = 0.04 | 2.12 (1.08–4.16), p = 0.03 | 0.6 | 0.6 |
| CTGF | ≥230 ng/mmol | 0.6 ± 0.05 (0.51–0.7), p = 0.04 | 2.13 (1.08–4.19), p = 0.03 | 0.57 | 0.57 |
| PTEN | ≥1.10 ng/mmol | 0.64 ± 0.05 (0.55–0.73), p = 0.004 | 2.12 (1.08–4.17), p = 0.03 | 0.61 | 0.57 |
| UACR ≥ 3.0 mg/mmol | |||||
| Klotho | ≤24 pg/mmol | 0.6 ± 0.05 (0.504–0.69), p = 0.047 | 1.58 (0.81–3.08), p = 0.18 | 0.56 | 0.56 |
| PTEN | ≥1.10 ng/mmol | 0.61 ± 0.05 (0.51–0.7), p = 0.03 | 2.39 (1.21–4.72), p = 0.01 | 0.63 | 0.59 |
| DRF/NA | |||||
| FGF21 | ≥1.49 ng/mmol | 0.64 ± 0.07 (0.51–0.77), p = 0.046 | 2.86 (1.09–7.55), p = 0.03 | 0.63 | 0.63 |
| BECN1 | ≥10 ng/mmol | 0.73 ± 0.09 (0.55–0.92), p = 0.02 | 4.84 (1.09–21.6), p = 0.04 | 0.69 | 0.69 |
| PTEN | ≥1.03 ng/mmol | 0.64 ± 0.07 (0.51–0.77), p = 0.048 | 1.78 (0.69–4.58), p = 0.23 | 0.57 | 0.57 |
| DRF/EA | |||||
| FGF21 | ≥1.62 ng/mmol | 0.66 ± 0.07 (0.53–0.79), p = 0.02 | 4.18 (1.54–11.4), p = 0.005 | 0.69 | 0.66 |
| CTGF | ≥251 ng/mmol | 0.66 ± 0.07 (0.53–0.79), p = 0.02 | 2.86 (1.09–7.55), p = 0.03 | 0.63 | 0.63 |
| Parameter | Crude OR, 95% CI, p-Value | Adjusted OR, 95% CI, p-Value |
|---|---|---|
| Urinary Klotho, 10 pg/mmol | 0.97 (0.94–1.01), p = 0.1 | 0.96 (0.93–0.9996), p = 0.048 |
| Age, years | 1 (0.98–1.03), p = 0.91 | 0.99 (0.94–1.04), p = 0.61 |
| Male sex | 1.67 (1.31–2.14), p < 0.001 | 1.04 (0.71–1.52), p = 0.83 |
| BMI, kg/m2 | 0.99 (0.94–1.05), p = 0.78 | 1 (0.92–1.09), p = 0.99 |
| Duration of T2D, years | 1.03 (0.99–1.06), p = 0.12 | 1.07 (1.01–1.13), p = 0.01 |
| HbA1c, % | 1.19 (1.05–1.35), p = 0.01 | 1.47 (1.16–1.85), p = 0.001 |
| Parameter | Crude OR, 95% CI, p-Value | Adjusted OR, 95% CI, p-Value |
|---|---|---|
| Urinary PTEN, ng/mmol | 6.33 (1.91–21.0), p = 0.003 | 6.23 (1.43–27.1), p = 0.01 |
| Age, years | 1.08 (1.03–1.13), p < 0.001 | 1.05 (0.97–1.14), p = 0.2 |
| Male sex | 1.31 (0.94–1.84), p = 0.11 | 1.08 (0.58–1.99), p = 0.82 |
| BMI, kg/m2 | 1.0 (0.93–1.08), p = 0.96 | 0.93 (0.81–1.06), p = 0.27 |
| Diabetes duration, years | 1.07 (1.02–1.12), p = 0.003 | 1.12 (1.01–1.25), p = 0.04 |
| HbA1c, % | 1.1 (0.93–1.31), p = 0.26 | 1.33 (0.96–1.85), p = 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korbut, A.I.; Romanov, V.V.; Klimontov, V.V. Urinary Excretion of Biomolecules Related to Cell Cycle, Proliferation, and Autophagy in Subjects with Type 2 Diabetes and Chronic Kidney Disease. Biomedicines 2024, 12, 487. https://doi.org/10.3390/biomedicines12030487
Korbut AI, Romanov VV, Klimontov VV. Urinary Excretion of Biomolecules Related to Cell Cycle, Proliferation, and Autophagy in Subjects with Type 2 Diabetes and Chronic Kidney Disease. Biomedicines. 2024; 12(3):487. https://doi.org/10.3390/biomedicines12030487
Chicago/Turabian StyleKorbut, Anton I., Vyacheslav V. Romanov, and Vadim V. Klimontov. 2024. "Urinary Excretion of Biomolecules Related to Cell Cycle, Proliferation, and Autophagy in Subjects with Type 2 Diabetes and Chronic Kidney Disease" Biomedicines 12, no. 3: 487. https://doi.org/10.3390/biomedicines12030487
APA StyleKorbut, A. I., Romanov, V. V., & Klimontov, V. V. (2024). Urinary Excretion of Biomolecules Related to Cell Cycle, Proliferation, and Autophagy in Subjects with Type 2 Diabetes and Chronic Kidney Disease. Biomedicines, 12(3), 487. https://doi.org/10.3390/biomedicines12030487