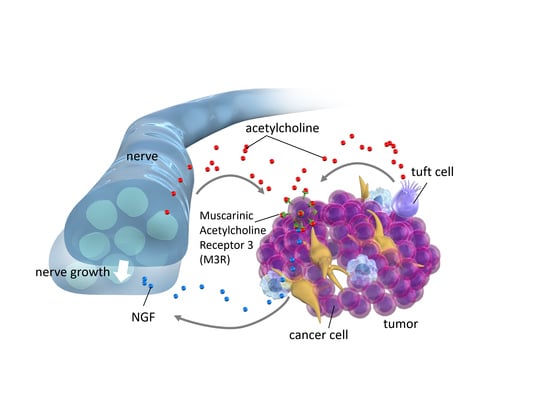
Role of Muscarinic Acetylcholine Signaling in Gastrointestinal Cancers
Abstract
:1. Interaction between Nerves and Cancers
2. Muscarinic Acetylcholine (ACh) Receptors
3. ACh Signaling and Gastric Cancer
4. Tuft Cells as a Source of ACh
5. ACh Signaling and Colon Cancer
6. Conclusions
Funding
Conflicts of Interest
References
- Brownell, I.; Guevara, E.; Bai, C.B.; Loomis, C.A.; Joyner, A.L. Nerve-derived sonic hedgehog defines a niche for hair follicle stem cells capable of becoming epidermal stem cells. Cell Stem Cell 2011, 8, 552–565. [Google Scholar] [CrossRef] [PubMed]
- Hanoun, M.; Zhang, D.; Mizoguchi, T.; Pinho, S.; Pierce, H.; Kunisaki, Y.; Lacombe, J.; Armstrong, S.A.; Duhrsen, U.; Frenette, P.S. Acute myelogenous leukemia-induced sympathetic neuropathy promotes malignancy in an altered hematopoietic stem cell niche. Cell Stem Cell 2014, 15, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Katayama, Y.; Battista, M.; Kao, W.M.; Hidalgo, A.; Peired, A.J.; Thomas, S.A.; Frenette, P.S. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell 2006, 124, 407–421. [Google Scholar] [CrossRef] [PubMed]
- Magnon, C.; Hall, S.J.; Lin, J.; Xue, X.; Gerber, L.; Freedland, S.J.; Frenette, P.S. Autonomic nerve development contributes to prostate cancer progression. Science 2013, 341, 1236361. [Google Scholar] [CrossRef] [PubMed]
- Mendez-Ferrer, S.; Michurina, T.V.; Ferraro, F.; Mazloom, A.R.; Macarthur, B.D.; Lira, S.A.; Scadden, D.T.; Ma’ayan, A.; Enikolopov, G.N.; Frenette, P.S. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 2010, 466, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Peterson, S.C.; Eberl, M.; Vagnozzi, A.N.; Belkadi, A.; Veniaminova, N.A.; Verhaegen, M.E.; Bichakjian, C.K.; Ward, N.L.; Dlugosz, A.A.; Wong, S.Y. Basal cell carcinoma preferentially arises from stem cells within hair follicle and mechanosensory niches. Cell Stem Cell 2015, 16, 400–412. [Google Scholar] [CrossRef]
- Stopczynski, R.E.; Normolle, D.P.; Hartman, D.J.; Ying, H.; DeBerry, J.J.; Bielefeldt, K.; Rhim, A.D.; DePinho, R.A.; Albers, K.M.; Davis, B.M. Neuroplastic changes occur early in the development of pancreatic ductal adenocarcinoma. Cancer Res. 2014, 74, 1718–1727. [Google Scholar] [CrossRef]
- Venkatesh, H.S.; Johung, T.B.; Caretti, V.; Noll, A.; Tang, Y.; Nagaraja, S.; Gibson, E.M.; Mount, C.W.; Polepalli, J.; Mitra, S.S.; et al. Neuronal Activity Promotes Glioma Growth through Neuroligin-3 Secretion. Cell 2015, 161, 803–816. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.M.; Hayakawa, Y.; Kodama, Y.; Muthupalani, S.; Westphalen, C.B.; Andersen, G.T.; Flatberg, A.; Johannessen, H.; Friedman, R.A.; Renz, B.W.; et al. Denervation suppresses gastric tumorigenesis. Sci. Transl. Med. 2014, 6, 250ra115. [Google Scholar] [CrossRef]
- Zahalka, A.H.; Arnal-Estape, A.; Maryanovich, M.; Nakahara, F.; Cruz, C.D.; Finley, L.W.S.; Frenette, P.S. Adrenergic nerves activate an angio-metabolic switch in prostate cancer. Science 2017, 358, 321–326. [Google Scholar] [CrossRef] [Green Version]
- Hayakawa, Y.; Wang, T.C. Nerves switch on angiogenic metabolism. Science 2017, 358, 305–306. [Google Scholar] [CrossRef] [PubMed]
- Renz, B.W.; Takahashi, R.; Tanaka, T.; Macchini, M.; Hayakawa, Y.; Dantes, Z.; Maurer, H.C.; Chen, X.; Jiang, Z.; Westphalen, C.B.; et al. β2 Adrenergic-Neurotrophin Feedforward Loop Promotes Pancreatic Cancer. Cancer Cell 2018, 33, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Renz, B.W.; Tanaka, T.; Sunagawa, M.; Takahashi, R.; Jiang, Z.; Macchini, M.; Dantes, Z.; Valenti, G.; White, R.A.; Middelhoff, M.A.; et al. Cholinergic Signaling via Muscarinic Receptors Directly and Indirectly Suppresses Pancreatic Tumorigenesis and Cancer Stemness. Cancer Discov. 2018, 8, 1458–1473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faulkner, S.; Jobling, P.; March, B.; Jiang, C.C.; Hondermarck, H. Tumor Neurobiology and the War of Nerves in Cancer. Cancer Discov. 2019, 9, 702–710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilman, A.G. G proteins and dual control of adenylate cyclase. Cell 1984, 36, 577–579. [Google Scholar] [CrossRef]
- Malbon, C.C. G proteins in development. Nat. Rev. Mol. Cell Biol. 2005, 6, 689–701. [Google Scholar] [CrossRef] [PubMed]
- Von Rosenvinge, E.C.; Raufman, J.P. Muscarinic receptor signaling in colon cancer. Cancers 2011, 3, 971–981. [Google Scholar] [CrossRef] [PubMed]
- Bonner, T.I.; Buckley, N.J.; Young, A.C.; Brann, M.R. Identification of a family of muscarinic acetylcholine receptor genes. Science 1987, 237, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Kruse, A.C.; Li, J.; Hu, J.; Kobilka, B.K.; Wess, J. Novel insights into M3 muscarinic acetylcholine receptor physiology and structure. J. Mol. Neurosci. 2014, 53, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Abrams, P.; Andersson, K.E.; Buccafusco, J.J.; Chapple, C.; De Groat, W.C.; Fryer, A.D.; Kay, G.; Laties, A.; Nathanson, N.M.; Pasricha, P.J.; et al. Muscarinic receptors: Their distribution and function in body systems, and the implications for treating overactive bladder. Br. J. Pharm. 2006, 148, 565–578. [Google Scholar] [CrossRef]
- Pronin, A.N.; Wang, Q.; Slepak, V.Z. Teaching an Old Drug New Tricks: Agonism, Antagonism, and Biased Signaling of Pilocarpine through M3 Muscarinic Acetylcholine Receptor. Mol. Pharm. 2017, 92, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Shi, H.; Wang, H. Functional M3 muscarinic acetylcholine receptors in mammalian hearts. Br. J. Pharm. 2004, 142, 395–408. [Google Scholar] [CrossRef] [PubMed]
- Kovacevic, I.; Muller, M.; Kojonazarov, B.; Ehrke, A.; Randriamboavonjy, V.; Kohlstedt, K.; Hindemith, T.; Schermuly, R.T.; Fleming, I.; Hoffmeister, M.; et al. The F-BAR Protein NOSTRIN Dictates the Localization of the Muscarinic M3 Receptor and Regulates Cardiovascular Function. Circ. Res. 2015, 117, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Radu, B.M.; Osculati, A.M.M.; Suku, E.; Banciu, A.; Tsenov, G.; Merigo, F.; Di Chio, M.; Banciu, D.D.; Tognoli, C.; Kacer, P.; et al. All muscarinic acetylcholine receptors (M1–M5) are expressed in murine brain microvascular endothelium. Sci. Rep. 2017, 7, 5083. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Lin, G.; Han, D.; Shi, D.; Liu, T.; Gao, Y.; Guan, W.; Cheng, G. Aclidinium Bromide holds promising inhibitory effects in A549 lung cancer cells potentials by regulating PI3K/AKT signaling pathway. J. Buon. 2019, 24, 560–565. [Google Scholar]
- Fryer, A.D.; Jacoby, D.B. Muscarinic receptors and control of airway smooth muscle. Am. J. Respir. Crit. Care Med. 1998, 158, S154–S160. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Wang, M.; Li, Y.; Meng, Q.; Tang, Y.; Lu, H.; Yu, W.; Cheng, Q.; Xu, L.; Jian, S.; et al. Muscarinic cholinergic signaling and overactive bladder-like symptoms associated with invasive bladder cancer. Oncol. Lett. 2018, 16, 775–784. [Google Scholar] [CrossRef]
- Kitazawa, T.; Hirama, R.; Masunaga, K.; Nakamura, T.; Asakawa, K.; Cao, J.; Teraoka, H.; Unno, T.; Komori, S.; Yamada, M.; et al. Muscarinic receptor subtypes involved in carbachol-induced contraction of mouse uterine smooth muscle. Naunyn. Schmiedebergs Arch. Pharm. 2008, 377, 503–513. [Google Scholar] [CrossRef]
- Ecknauer, R.; Dial, E.; Thompson, W.J.; Johnson, L.R.; Rosenfeld, G.C. Isolated rat gastric parietal cells: Cholinergic response and pharmacology. Life Sci. 1981, 28, 609–621. [Google Scholar] [CrossRef]
- Soll, A.H. Specific inhibition by prostaglandins E2 and I2 of histamine-stimulated [14C] aminopyrine accumulation and cyclic adenosine monophosphate generation by isolated canine parietal cells. J. Clin. Investig. 1980, 65, 1222–1229. [Google Scholar] [CrossRef]
- Aihara, T.; Fujishita, T.; Kanatani, K.; Furutani, K.; Nakamura, E.; Taketo, M.M.; Matsui, M.; Chen, D.; Okabe, S. Impaired gastric secretion and lack of trophic responses to hypergastrinemia in M3 muscarinic receptor knockout mice. Gastroenterology 2003, 125, 1774–1784. [Google Scholar] [CrossRef]
- Kajimura, M.; Reuben, M.A.; Sachs, G. The muscarinic receptor gene expressed in rabbit parietal cells is the m3 subtype. Gastroenterology 1992, 103, 870–875. [Google Scholar] [CrossRef]
- Raufman, J.P.; Sutliff, V.E.; Kasbekar, D.K.; Jensen, R.T.; Gardner, J.D. Pepsinogen secretion from dispersed chief cells from guinea pig stomach. Am. J. Physiol. 1984, 247, G95–G104. [Google Scholar] [CrossRef]
- Sutliff, V.E.; Rattan, S.; Gardner, J.D.; Jensen, R.T. Characterization of cholinergic receptors mediating pepsinogen secretion from chief cells. Am. J. Physiol. 1989, 257, G226–G234. [Google Scholar] [CrossRef]
- Xie, G.; Drachenberg, C.; Yamada, M.; Wess, J.; Raufman, J.P. Cholinergic agonist-induced pepsinogen secretion from murine gastric chief cells is mediated by M1 and M3 muscarinic receptors. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 289, G521–G529. [Google Scholar] [CrossRef]
- Tobin, G.; Giglio, D.; Lundgren, O. Muscarinic receptor subtypes in the alimentary tract. J. Physiol. Pharm. 2009, 60, 3–21. [Google Scholar]
- Aihara, T.; Nakamura, Y.; Taketo, M.M.; Matsui, M.; Okabe, S. Cholinergically stimulated gastric acid secretion is mediated by M3 and M5 but not M1 muscarinic acetylcholine receptors in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 288, G1199–G1207. [Google Scholar] [CrossRef]
- Raufman, J.P.; Samimi, R.; Shah, N.; Khurana, S.; Shant, J.; Drachenberg, C.; Xie, G.; Wess, J.; Cheng, K. Genetic ablation of M3 muscarinic receptors attenuates murine colon epithelial cell proliferation and neoplasia. Cancer Res. 2008, 68, 3573–3578. [Google Scholar] [CrossRef]
- Ockenga, W.; Kuhne, S.; Bocksberger, S.; Banning, A.; Tikkanen, R. Non-neuronal functions of the m2 muscarinic acetylcholine receptor. Genes 2013, 4, 171–197. [Google Scholar] [CrossRef]
- Rabben, H.L.; Zhao, C.M.; Hayakawa, Y.; Wang, T.C.; Chen, D. Vagotomy and Gastric Tumorigenesis. Curr. Neuropharmacol. 2016, 14, 967–972. [Google Scholar] [CrossRef] [Green Version]
- Kodaira, M.; Kajimura, M.; Takeuchi, K.; Lin, S.; Hanai, H.; Kaneko, E. Functional muscarinic m3 receptor expressed in gastric cancer cells stimulates tyrosine phosphorylation and MAP kinase. J. Gastroenterol. 1999, 34, 163–171. [Google Scholar] [CrossRef]
- Said, A.H.; Hu, S.; Abutaleb, A.; Watkins, T.; Cheng, K.; Chahdi, A.; Kuppusamy, P.; Saxena, N.; Xie, G.; Raufman, J.P. Interacting post-muscarinic receptor signaling pathways potentiate matrix metalloproteinase-1 expression and invasion of human colon cancer cells. Biochem. J. 2017, 474, 647–665. [Google Scholar] [CrossRef] [Green Version]
- Sales, M.E.; Espanol, A.J.; Salem, A.R.; Martinez, P.P.; Sanchez, Y.; Sanchez, F. Role of muscarinic acetylcholine receptors in Breast Cancer. Design of metronomic chemotherapy. Curr. Clin. Pharm. 2018. [Google Scholar] [CrossRef]
- Felton, J.; Hu, S.; Raufman, J.P. Targeting M3 Muscarinic Receptors for Colon Cancer Therapy. Curr. Mol. Pharm. 2018, 11, 184–190. [Google Scholar] [CrossRef]
- Song, P.; Sekhon, H.S.; Lu, A.; Arredondo, J.; Sauer, D.; Gravett, C.; Mark, G.P.; Grando, S.A.; Spindel, E.R. M3 muscarinic receptor antagonists inhibit small cell lung carcinoma growth and mitogen-activated protein kinase phosphorylation induced by acetylcholine secretion. Cancer Res. 2007, 67, 3936–3944. [Google Scholar] [CrossRef]
- Belo, A.; Cheng, K.; Chahdi, A.; Shant, J.; Xie, G.; Khurana, S.; Raufman, J.P. Muscarinic receptor agonists stimulate human colon cancer cell migration and invasion. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 300, G749–G760. [Google Scholar] [CrossRef]
- Kuol, N.; Stojanovska, L.; Apostolopoulos, V.; Nurgali, K. Role of the nervous system in cancer metastasis. J. Exp. Clin. Cancer Res. 2018, 37, 5. [Google Scholar] [CrossRef]
- Hayakawa, Y.; Sakitani, K.; Konishi, M.; Asfaha, S.; Niikura, R.; Tomita, H.; Renz, B.W.; Tailor, Y.; Macchini, M.; Middelhoff, M.; et al. Nerve Growth Factor Promotes Gastric Tumorigenesis through Aberrant Cholinergic Signaling. Cancer Cell 2017, 31, 21–34. [Google Scholar] [CrossRef]
- Wang, L.; Zhi, X.; Zhang, Q.; Wei, S.; Li, Z.; Zhou, J.; Jiang, J.; Zhu, Y.; Yang, L.; Xu, H.; et al. Muscarinic receptor M3 mediates cell proliferation induced by acetylcholine and contributes to apoptosis in gastric cancer. Tumour. Biol. 2016, 37, 2105–2117. [Google Scholar] [CrossRef]
- Yu, H.; Xia, H.; Tang, Q.; Xu, H.; Wei, G.; Chen, Y.; Dai, X.; Gong, Q.; Bi, F. Acetylcholine acts through M3 muscarinic receptor to activate the EGFR signaling and promotes gastric cancer cell proliferation. Sci. Rep. 2017, 7, 40802. [Google Scholar] [CrossRef]
- Calses, P.C.; Crawford, J.J.; Lill, J.R.; Dey, A. Hippo Pathway in Cancer: Aberrant Regulation and Therapeutic Opportunities. Trends Cancer 2019, 5, 297–307. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.A.; Lu, C.Y.; Cheng, T.Y.; Pan, S.H.; Chen, H.F.; Chang, N.S. WW Domain-Containing Proteins YAP and TAZ in the Hippo Pathway as Key Regulators in Stemness Maintenance, Tissue Homeostasis, and Tumorigenesis. Front Oncol. 2019, 9, 60. [Google Scholar] [CrossRef] [Green Version]
- Azzolin, L.; Panciera, T.; Soligo, S.; Enzo, E.; Bicciato, S.; Dupont, S.; Bresolin, S.; Frasson, C.; Basso, G.; Guzzardo, V.; et al. YAP/TAZ incorporation in the β-catenin destruction complex orchestrates the Wnt response. Cell 2014, 158, 157–170. [Google Scholar] [CrossRef]
- Cai, J.; Maitra, A.; Anders, R.A.; Taketo, M.M.; Pan, D. β-Catenin destruction complex-independent regulation of Hippo-YAP signaling by APC in intestinal tumorigenesis. Genes Dev. 2015, 29, 1493–1506. [Google Scholar] [CrossRef]
- Rosenbluh, J.; Nijhawan, D.; Cox, A.G.; Li, X.; Neal, J.T.; Schafer, E.J.; Zack, T.I.; Wang, X.; Tsherniak, A.; Schinzel, A.C.; et al. β-Catenin-driven cancers require a YAP1 transcriptional complex for survival and tumorigenesis. Cell 2012, 151, 1457–1473. [Google Scholar] [CrossRef]
- Gregorieff, A.; Liu, Y.; Inanlou, M.R.; Khomchuk, Y.; Wrana, J.L. Yap-dependent reprogramming of Lgr5+ stem cells drives intestinal regeneration and cancer. Nature 2015, 526, 715–718. [Google Scholar] [CrossRef]
- Imajo, M.; Ebisuya, M.; Nishida, E. Dual role of YAP and TAZ in renewal of the intestinal epithelium. Nat. Cell Biol. 2015, 17, 7–19. [Google Scholar] [CrossRef]
- Jiao, S.; Wang, H.; Shi, Z.; Dong, A.; Zhang, W.; Song, X.; He, F.; Wang, Y.; Zhang, Z.; Wang, W.; et al. A peptide mimicking VGLL4 function acts as a YAP antagonist therapy against gastric cancer. Cancer Cell 2014, 25, 166–180. [Google Scholar] [CrossRef]
- Yu, F.X.; Zhao, B.; Panupinthu, N.; Jewell, J.L.; Lian, I.; Wang, L.H.; Zhao, J.; Yuan, H.; Tumaneng, K.; Li, H.; et al. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell 2012, 150, 780–791. [Google Scholar] [CrossRef]
- Serizawa, T.; Hirata, Y.; Hayakawa, Y.; Suzuki, N.; Sakitani, K.; Hikiba, Y.; Ihara, S.; Kinoshita, H.; Nakagawa, H.; Tateishi, K.; et al. Gastric Metaplasia Induced by Helicobacter pylori is Associated with Enhanced SOX9 Expression via Interleukin-1 Signaling. Infect. Immun. 2015, 84, 562–572. [Google Scholar] [CrossRef]
- Zhou, H.; Li, G.; Huang, S.; Feng, Y.; Zhou, A. SOX9 promotes epithelial-mesenchymal transition via the Hippo-YAP signaling pathway in gastric carcinoma cells. Oncol. Lett. 2019, 18, 599–608. [Google Scholar] [CrossRef]
- Barry, E.R.; Morikawa, T.; Butler, B.L.; Shrestha, K.; Rosa, R.D.L.; Yan, K.S.; Fuchs, C.S.; Magness, S.T.; Smits, R.; Ogino, S.; et al. Restriction of intestinal stem cell expansion and the regenerative response by YAP. Nature 2013, 493, 106–110. [Google Scholar] [CrossRef]
- Schutz, B.; Jurastow, I.; Bader, S.; Ringer, C.; Von Engelhardt, J.; Chubanov, V.; Gudermann, T.; Diener, M.; Kummer, W.; Krasteva-Christ, G.; et al. Chemical coding and chemosensory properties of cholinergic brush cells in the mouse gastrointestinal and biliary tract. Front Physiol. 2015, 6, 87. [Google Scholar] [CrossRef]
- Cheng, K.; Xie, G.; Khurana, S.; Heath, J.; Drachenberg, C.B.; Timmons, J.; Shah, N.; Raufman, J.P. Divergent effects of muscarinic receptor subtype gene ablation on murine colon tumorigenesis reveals association of M3R and zinc finger protein 277 expression in colon neoplasia. Mol. Cancer 2014, 13, 77. [Google Scholar] [CrossRef]
- Raufman, J.P.; Shant, J.; Xie, G.; Cheng, K.; Gao, X.M.; Shiu, B.; Shah, N.; Drachenberg, C.B.; Heath, J.; Wess, J.; et al. Muscarinic receptor subtype-3 gene ablation and scopolamine butylbromide treatment attenuate small intestinal neoplasia in Apcmin+ mice. Carcinogenesis 2011, 32, 1396–1402. [Google Scholar] [CrossRef]
- Peng, Z.; Heath, J.; Drachenberg, C.; Raufman, J.P.; Xie, G. Cholinergic muscarinic receptor activation augments murine intestinal epithelial cell proliferation and tumorigenesis. BMC Cancer 2013, 13, 204. [Google Scholar] [CrossRef]
- Cheng, K.; Shang, A.C.; Drachenberg, C.B.; Zhan, M.; Raufman, J.P. Differential expression of M3 muscarinic receptors in progressive colon neoplasia and metastasis. Oncotarget 2017, 8, 21106–21114. [Google Scholar] [CrossRef] [Green Version]
- Tolaymat, M.; Larabee, S.M.; Hu, S.; Xie, G.; Raufman, J.P. The Role of M3 Muscarinic Receptor Ligand-Induced Kinase Signaling in Colon Cancer Progression. Cancers 2019, 11, 308. [Google Scholar] [CrossRef]
- Hayakawa, Y.; Tsuboi, M.; Asfaha, S.; Kinoshita, H.; Niikura, R.; Konishi, M.; Hata, M.; Oya, Y.; Kim, W.; Middelhoff, M.; et al. BHLHA15-Positive Secretory Precursor Cells Can Give Rise to Tumors in Intestine and Colon in Mice. Gastroenterology 2019, 156, 1066–1081. [Google Scholar] [CrossRef]
- Yui, S.; Azzolin, L.; Maimets, M.; Pedersen, M.T.; Fordham, R.P.; Hansen, S.L.; Larsen, H.L.; Guiu, J.; Alves, M.R.P.; Rundsten, C.F.; et al. YAP/TAZ-Dependent Reprogramming of Colonic Epithelium Links ECM Remodeling to Tissue Regeneration. Cell Stem Cell 2018, 22, 35–49. [Google Scholar] [CrossRef]
- Blanchard, T.G.; Lapidus, R.; Banerjee, V.; Bafford, A.C.; Czinn, S.J.; Ahmed, H.; Banerjee, A. Upregulation of RASSF1A in Colon Cancer by Suppression of Angiogenesis Signaling and Akt Activation. Cell. Physiol. Biochem. 2018, 48, 1259–1273. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Shi, S.; Guo, Z.; Zhang, X.; Han, S.; Yang, A.; Wen, W.; Zhu, Q. Overexpression of YAP and TAZ is an independent predictor of prognosis in colorectal cancer and related to the proliferation and metastasis of colon cancer cells. PLoS ONE 2013, 8, e65539. [Google Scholar] [CrossRef] [PubMed]
- Ou, C.; Sun, Z.; Li, S.; Li, G.; Li, X.; Ma, J. Dual roles of yes-associated protein (YAP) in colorectal cancer. Oncotarget 2017, 8, 75727–75741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubeykovskaya, Z.; Si, Y.; Chen, X.; Worthley, D.L.; Renz, B.W.; Urbanska, A.M.; Hayakawa, Y.; Xu, T.; Westphalen, C.B.; Dubeykovskiy, A.; et al. Neural innervation stimulates splenic TFF2 to arrest myeloid cell expansion and cancer. Nat. Commun. 2016, 7, 10517. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Aranda, E.; Hayakawa, Y.; Bhanja, P.; Atay, S.; Brodin, N.P.; Li, J.; Asfaha, S.; Liu, L.; Tailor, Y.; et al. Macrophage-derived extracellular vesicle-packaged WNTs rescue intestinal stem cells and enhance survival after radiation injury. Nat. Commun. 2016, 7, 13096. [Google Scholar] [CrossRef] [PubMed]
- Westphalen, C.B.; Asfaha, S.; Hayakawa, Y.; Takemoto, Y.; Lukin, D.J.; Nuber, A.H.; Brandtner, A.; Setlik, W.; Remotti, H.; Muley, A.; et al. Long-lived intestinal tuft cells serve as colon cancer-initiating cells. J. Clin. Investig. 2014, 124, 1283–1295. [Google Scholar] [CrossRef] [PubMed]
- Goto, N.; Fukuda, A.; Yamaga, Y.; Yoshikawa, T.; Maruno, T.; Maekawa, H.; Inamoto, S.; Kawada, K.; Sakai, Y.; Miyoshi, H.; et al. Lineage tracing and targeting of IL17RB+ tuft cell-like human colorectal cancer stem cells. Proc. Natl. Acad. Sci. USA 2019, 116, 12996–13005. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konishi, M.; Hayakawa, Y.; Koike, K. Role of Muscarinic Acetylcholine Signaling in Gastrointestinal Cancers. Biomedicines 2019, 7, 58. https://doi.org/10.3390/biomedicines7030058
Konishi M, Hayakawa Y, Koike K. Role of Muscarinic Acetylcholine Signaling in Gastrointestinal Cancers. Biomedicines. 2019; 7(3):58. https://doi.org/10.3390/biomedicines7030058
Chicago/Turabian StyleKonishi, Mitsuru, Yoku Hayakawa, and Kazuhiko Koike. 2019. "Role of Muscarinic Acetylcholine Signaling in Gastrointestinal Cancers" Biomedicines 7, no. 3: 58. https://doi.org/10.3390/biomedicines7030058
APA StyleKonishi, M., Hayakawa, Y., & Koike, K. (2019). Role of Muscarinic Acetylcholine Signaling in Gastrointestinal Cancers. Biomedicines, 7(3), 58. https://doi.org/10.3390/biomedicines7030058