Vindoline—A Natural Product from Catharanthus Roseus Reduces Hyperlipidemia and Renal Pathophysiology in Experimental Type 2 Diabetes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Bioactive Compound
2.3. Animal Handling and Ethics Statement
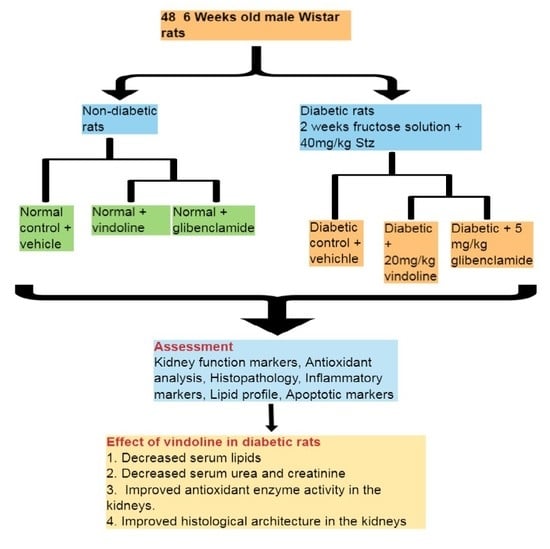
2.4. Animal Grouping
2.5. Induction of Type 2 Diabetes Mellitus (T2DM)
2.6. Treatment
2.7. Oral Glucose Tolerance Test (OGTT)
2.8. Collection of Heart, Kidney and Blood Samples
2.9. Relative Kidney and Heart Weights
2.10. Serum Lipid Profile Measurement
Atherogenic Indices
2.11. Endogenous Antioxidant Analysis
2.12. Lipid Peroxidation
2.13. The Oxygen Radical Absorbance Capacity (ORAC)
2.14. Ferric Reducing Antioxidant Power (FRAP)
2.15. Inflammatory Cytokines Measurement
2.16. Histological Assessment of the Kidney Using Haematoxylin and Eosin Stain
2.17. Immunohistochemistry Analysis
2.18. Statistical Analysis
3. Results
3.1. Effect of Vindoline on the 2 h OGTT in Nondiabetic and Diabetic Rats
3.2. Effect of Vindoline Administration on the Kidney and Heart Weights and Kidney Function Parameters in Nondiabetic and Diabetic Rats
3.3. Serum Lipid Levels in Normal and T2DM-Induced Rats after Receiving Respective Treatments for 6 Weeks
3.4. Effect of Vindoline on Levels of Inflammatory Cytokines in the Heart and Kidney Tissues
3.5. Effect of Vindoline on Oxidative Stress Markers in the Cardiac and Nephron Tissues
3.6. Histopathology
3.7. Effect of Vindoline on Apoptotic Markers in the Kidney Tissues
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shu, X.S.; Lv, J.H.; Tao, J.; Li, G.M.; den Li, H.; Ma, N. Antihyperglycemic effects of total flavonoids from Polygonatum odoratum in STZ and alloxan-induced diabetic rats. J. Ethnopharmacol. 2009, 124, 539–543. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Report on Diabetes; World Health Organization: Geneva, Switzerland, 2016; Volume 978, p. 88. [Google Scholar]
- Sa’adah, N.N.; Purwani, K.I.; Nurhayati, A.P.D.; Ashuri, N.M. Analysis of lipid profile and atherogenic index in hyperlipidemic rat (Rattus norvegicus Berkenhout, 1769 that given the methanolic extract of Parijoto (Medinilla speciosa). In AIP Conference Proceedings; AIP Publishing: New York, NY, USA, 2017. [Google Scholar]
- Patel, Y.; Vadgama, V.; Baxi, S.; Tripathi, C.B. Evaluation of hypolipidemic activity of leaf juice of Catharanthus roseus (Linn.) G. Donn. in guinea pigs. Acta Pol. Pharm. Drug Res. 2011, 68, 927–935. [Google Scholar]
- Sani, M.F.; Kouhsari, S.M.; Moradabadi, L. Effects of three medicinal plants extracts in experimental diabetes: Antioxidant enzymes activities and plasma lipids profiles in comparison with metformin. Iran. J. Pharm. Res. 2012, 11, 897–903. [Google Scholar]
- Subramaniam, S.; Subramaniam, R.; Rajapandian, S.; Uthrapathi, S.; Gnanamanickam, V.R.; Dubey, G.P. Anti-atherogenic activity of ethanolic fraction of terminalia arjuna bark on hypercholesterolemic rabbits. Evid. Based Complement. Altern. Med. 2011, 2011, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Oguntibeju, O.O.; Meyer, S.; Aboua, Y.G.; Goboza, M. Hypoxis hemerocallidea Significantly Reduced Hyperglycaemia and Hyperglycaemic-Induced Oxidative Stress in the Liver and Kidney Tissues of Streptozotocin-Induced Diabetic Male Wistar Rats. Evid. Based Complement. Altern. Med. 2016, 2016, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Oyenihi, A.B.; Chegou, N.N.; Oguntibeju, O.O.; Masola, B. Centella asiatica enhances hepatic antioxidant status and regulates hepatic inflammatory cytokines in type 2 diabetic rats. Pharm. Biol. 2017, 55, 1671–1678. [Google Scholar] [CrossRef]
- Siti, H.N.; Kamisah, Y.; Kamsiah, J. The role of oxidative stress, antioxidants and vascular inflammation in cardiovascular disease (a review). Vascul. Pharmacol. 2015, 71, 40–56. [Google Scholar] [CrossRef]
- Bobryshev, Y.V.; Ivanova, E.A.; Chistiakov, D.A.; Nikiforov, N.G.; Orekhov, A.N. Macrophages and Their Role in Atherosclerosis: Pathophysiology and Transcriptome Analysis. Biomed. Res. Int. 2016, 2016, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Kawanami, D.; Matoba, K.; Utsunomiya, K. Dyslipidemia in diabetic nephropathy. Ren. Replace. Ther. 2016, 2, 1–9. [Google Scholar] [CrossRef]
- Suchitra, M.M.; Sheshu, K.M.; Bitla, A.R.; Madhusudhana, R.A.; Alok, S. Atherogenic dyslipidemia in diabetic nephropathy: Lipoprotein (a), lipid ratios and atherogenic index. Int. J. Res. Med. Sci. 2013, 1, 455–459. [Google Scholar]
- Chen, S.; Jiang, H.; Wu, X.; Fang, J. Therapeutic Effects of Quercetin on Inflammation, Obesity, and Type 2 Diabetes. Mediat. Inflamm. 2016, 2016, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Matthaei, S.; Bierwirth, R.; Fritsche, A.; Gallwitz, B.; Häring, H.U.; Joost, H.G.; Kellerer, M.; Kloos, C.; Kunt, T.; Nauck, M.; et al. Medical antihyperglycaemic treatment of type 2 diabetes mellitus: Update of the evidence-based guideline of the German diabetes association. Exp. Clin. Endocrinol. Diabetes 2009, 117, 522–557. [Google Scholar] [CrossRef] [PubMed]
- Society of Endocrinology, Society for Endocrinology, Metabolism and Diabetes of South Africa 2017. J. Endocrinol. Metab. Diabetes S. Afr. 2017, 22, 64–67.
- Das, S.; Sharangi, A.B. Madagascar periwinkle (Catharanthus roseus L.): Diverse medicinal and therapeutic benefits to humankind. J. Pharmacogn. Phytochem. 2017, 6, 1695–1701. [Google Scholar]
- Mohammed, A.; Islam, M.S. Antioxidant potential of Xylopia aethiopica fruit acetone fraction in a type 2 diabetes model of rats. Biomed. Pharmacother. 2017, 96, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Kotakadi, V.S.; Rao, Y.S.; Gaddam, S.A.; Prasad, T.N.V.K.V.; Reddy, A.V.; Gopal, S.V.R.S. Simple and rapid biosynthesis of stable silver nanoparticles using dried leaves of Catharanthus roseus. Linn. G. Donn and its anti microbial activity. Coll. Surf. B Biointerfaces 2013, 105, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.G.; Chen, F.; Li, P.; Quan, L.; Chen, J.; Yu, L.; Ding, H.; Li, C.; Chen, L.; Gao, Z.; et al. Natural product vindoline stimulates insulin secretion and efficiently ameliorates glucose homeostasis in diabetic murine models. J. Ethnopharmacol. 2013, 150, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Khan, M.R.I.; Hossain, M.S.; Alam, A.H.M.K.; Wahed, M.I.I.; Rahman, B.M.; Anisuzzaman, A.; Shaheen, S.M.; Maruf, A. Antidiabetic and hypolipidemic effects of different fractions of Catharanthus roseus (Linn). on normal and streptozotocin-induced diabetic rats. J. Sci. Res. 2009, 1, 334–344. [Google Scholar] [CrossRef]
- Bobadoye, M.F.; Bamisi, O.O.; Enujiugha, V.N. Hypolipidemic and Antioxidative Effects of African Star Apple Juice (Chrysophylum albidum) on Rats Fed on Diets High in Cholesterol and Oil. Food Nutr. Sci. 2016, 7, 825–843. [Google Scholar] [CrossRef] [Green Version]
- Lafta, M.A. A Comparative Study for Some Atherogenic Indices in Sera of Myocardial infarction, Ischemic Heart Disease Patients and Control. J. Nat. Sci. Res. 2014, 4, 2225–2921. [Google Scholar]
- Ellerby, L.M.; Bredesen, D.E. Measurement of Cellular Oxidation, Reactive Oxygen Species, and Antioxidant Enzymes during Apoptosis. Methods Enzymol. 2000, 322, 413–421. [Google Scholar] [PubMed]
- Tug, T.; Karatas, F.; Terzi, S.M. Antioxidant vitamins (A, C and E) and malondialdehyde levels in acute exacerbation and stable periods of patients with chronic obstructive pulmonary disease. Clin. Investig. Med. 2004, 27, 123–128. [Google Scholar]
- Cao, G.; Prior, R.L. Comparison of different analytical methods for assessing total antioxidant capacity of human serum. Clin. Chem. 1998, 44, 1309–1315. [Google Scholar] [PubMed]
- Benzie, I.F.F.; Strain, J.J. Ferric reducing (antioxidant) power as a measure of antioxidant capacity: The FRAP assay. Methods Enzym. 1999, 299, 15–36. [Google Scholar]
- Pálsson, R.; Patel, U.D. Cardiovascular Complications of Diabetic Kidney Disease. Adv. Chronic Kidney Dis. 2014, 21, 273–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sornalakshmi, V.; Soris, P.T.; Paulpriya, K.; Lincy, M.P.; Mohan, V.R. Oral glucose tolerance test (OGTT) in normal control and glucose induced hyperglycemic rats with hedyotis leschenaultiana DC. Int. J. Toxicol. Pharmacol. Res. 2016, 8, 59–62. [Google Scholar]
- Mestry, S.N.; Dhodi, J.B.; Kumbhar, S.B.; Juvekar, A.R. Attenuation of diabetic nephropathy in streptozotocin-induced diabetic rats by Punica granatum Linn. leaves extract. J. Tradit. Complement. Med. 2016, 7, 273–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hostetter, T.H. Prevention of End-Stage Renal Disease Due to Type 2 Diabetes. N. Engl. J. Med. 2001, 345, 910–912. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Saxena, N.; You, A.X.; Wong, R.C.C.; Lim, C.P.; Loh, S.Y.; George, P.P. Effect of multimorbidity on survival of patients diagnosed with heart failure: A retrospective cohort study in Singapore. BMJ Open 2018, 8, 1–7. [Google Scholar] [CrossRef]
- Zhang, J.J.; Yang, L.; Huang, J.W.; Liu, Y.J.; Wang, J.W.; Zhang, L.X.; Zhao, M.H.; Liu, Z.S. Characteristics and comparison between diabetes mellitus and non-diabetes mellitus among chronic kidney disease patients: A cross-sectional study of the Chinese Cohort Study of Chronic Kidney Disease (C-STRIDE). Oncotarget 2017, 8, 106324–106332. [Google Scholar] [CrossRef] [Green Version]
- Adisa, R.A.; Choudhary, M.I.; Olorunsogo, O.O. Hypoglycemic activity of Buchholzia coriacea (Capparaceae) seeds in streptozotocin-induced diabetic rats and mice. Exp. Toxicol. Pathol. 2011, 63, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elkader, M.Y.; Farag, E.A.; Omar, A.I. Histological Study on the Potential Effect of Sildenafil on the Kidney and Testosterone Level in Experimentally Induced Diabetes in Male Rats. J. Cytol. Histol. 2016, 7, 1–8. [Google Scholar]
- Li, W.; Wang, G.; Lu, X.; Jiang, Y.; Xu, L.; Zhao, X. Lycopene ameliorates renal function in rats with streptozotocin-induced diabetes. Int. J. Clin. Exp. Pathol. 2014, 7, 5008–5015. [Google Scholar] [PubMed]
- Dash, A.K.; Mishra, J.; Dash, D.K. Antidiabetic along with antihyperlipidemic and antioxidant activity of aqueous extract of Platycladus orientalis in streptozotocin-induced diabetic rats. Curr. Med. Res. Pract. 2014, 4, 255–262. [Google Scholar] [CrossRef]
- Islam, M.A.; Akhtar, M.A.; Khan, M.R.; Hossain, M.S.; Alam, A.H.; Ibne-Wahed, M.I.; Amran, M.S.; Rahman, B.M.; Ahmed, M. Oral Glucose Tolerance Test (Ogtt ) in Normal Control and Glucose Induced Hyperglycemic Rats with Coccinia Cordifolia L. and Catharanthus Roseus L. Pak. J. Pharm. Sci. 2009, 22, 402–404. [Google Scholar] [PubMed]
- Donath, M.Y. Targeting inflammation in the treatment of type 2 diabetes: Time to start. Nat. Rev. Drug Discov. 2014, 13, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Domingueti, C.P.; Dusse, L.M.S.; Carvalho, M.D.G.; de Sousa, L.P.; Gomes, K.B.; Fernandes, A.P. Diabetes mellitus: The linkage between oxidative stress, inflammation, hypercoagulability and vascular complications. J. Diabetes Complicat. 2016, 30, 738–745. [Google Scholar] [CrossRef] [PubMed]
- Alexandru, N.; Badila, E.; Weiss, E.; Cochior, D.; Stępień, E.; Georgescu, A. Vascular complications in diabetes: Microparticles and microparticle associated microRNAs as active players Dedicated to the 150th anniversary of the Romanian Academy., Biochem. Biophys. Res. Commun. 2016, 472, 1–10. [Google Scholar]
- Omodanisi, E.I.; Aboua, Y.G.; Oguntibeju, O.O.; Lamuela-Raventós, R.M. Assessment of the anti-hyperglycaemic, anti-inflammatory and antioxidant activities of the methanol extract of moringa oleifera in diabetes-induced nephrotoxic male wistar rats. Molecules 2017, 22, 439. [Google Scholar] [CrossRef] [PubMed]
- Nowotny, K.; Jung, T.; Höhn, A.; Weber, D.; Grune, T. Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules 2015, 5, 194–222. [Google Scholar] [CrossRef]
- Anjum, K.M.; Sayyed, U.; Ullah, A.; Mughal, M.S.; Yaqub, A.; Rashid, M.A.; Yousaf, M.Z. Anti-hypercholesterolemic and anti-atherogenic activity of terminalia chebula fruit in normal and cholesterol fed rabbits. J. Anim. Plant Sci. 2014, 24, 1618–1622. [Google Scholar]
- Ali, M.Y.; Paul, S.; Tanvir, E.M.; Hossen, M.S.; Rumpa, N.E.N.; Saha, M.; Bhoumik, N.C.; Islam, M.A.; Hossain, M.S.; Alam, N.; et al. Antihyperglycemic, Antidiabetic, and Antioxidant Effects of Garcinia pedunculata in Rats. Evid. Based Complement. Altern. Med. 2017, 2017, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kedziora-Kornatowska, K.Z.; Luciak, M.; Paszkowski, J. Lipid peroxidation and activities of antioxidant enzymes in the diabetic kidney: Effect of treatment with angiotensin convertase inhibitors. IUBMB Life 2000, 49, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, R.; Kalra, J.; Mantha, S.V.; Prasad, K. Lipid peroxidation and activity of antioxidant enzymes in diabetic rats. Mol. Cell. Biochem. 1995, 151, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Ayeleso, A.; Brooks, N.; Oguntibeju, O. Modulation of antioxidant status in streptozotocin-induced diabetic male wistar rats following intake of red palm oil and/or rooibos. Asian Pac. J. Trop. Med. 2014, 7, 536–544. [Google Scholar] [CrossRef] [Green Version]
- Benammar, C.; Baghdad, C. Antidiabetic and Antioxidant Activities of Zizyphus lotus L Aqueous Extracts in Wistar Rats. J. Nutr. Food Sci. 2014, 2014, 2–6. [Google Scholar] [CrossRef]
- Kim, B.H.; Lee, E.S.; Choi, R.; Nawaboot, J.; Lee, M.Y.; Lee, E.Y.; Kim, H.S.; Chung, C.H. Protective effects of curcumin on renal oxidative stress and lipid metabolism in a rat model of type 2 diabetic nephropathy. Yonsei Med. J. 2016, 57, 664–673. [Google Scholar] [CrossRef]
- Reddy, S.; Ramakrishna, C.; Mallikarjuna, K.; Shanmugam, K. Perturbation in kidney lipid metabolic profiles in diabetic rats with reference to alcoholic oxidative stress. Indian J. Nephrol. 2009, 19, 101–106. [Google Scholar] [CrossRef]
- Pourghasem, M.; Shafi, H.; Babazadeh, Z. Histological changes of kidney in diabetic nephropath. Casp. J. Intern. Med. 2015, 6, 120–127. [Google Scholar]
- Simsek, S.; van den Oever, I.A.M.; Raterman, H.G.; Nurmohamed, M.T. Endothelial dysfunction, inflammation, and apoptosis in diabetes mellitus. Mediat. Inflamm. 2010, 2010, 1–15. [Google Scholar]
- Teijido, O.; Dejean, L. Upregulation of Bcl2 inhibits apoptosis-driven BAX insertion but favors BAX relocalization in mitochondria. FEBS Lett. 2010, 584, 3305–3310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habib, S.L. Diabetes and renal tubular cell apoptosis. World J. Diabetes 2013, 4, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Loreto, C.; la Rocca, G.; Anzalone, R.; Caltabiano, R.; Vespasiani, G.; Castorina, S.; Ralph, D.J.; Cellek, S.; Musumeci, G.; Giunta, S.; et al. The role of intrinsic pathway in apoptosis activation and progression in Peyronie’s disease. Biomed. Res. Int. 2014, 2014, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.; Emancipator, S.N.; Kern, T.; Simonson, M.S. High glucose evokes an intrinsic proapoptotic signaling pathway in mesangial cells. Kidney Int. 2005, 67, 82–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]












| Groups | T = 0 h | T = 0.5 h | T = 1 h | T = 1.5 h | T = 2 h | AUC |
|---|---|---|---|---|---|---|
| NC | 5.21 ± 0.14 | 5.31 ± 0.19 | 5.25 ± 0.13 | 5.16 ± 0.19 | 4.76 ± 0.07 | 621.4 |
| NV | 5.28 ± 0.11 b | 5.56 ± 0.22 b | 5.09 ± 0.19 b | 5.06 ± 0.11 b | 4.74 ± 0.18 b | 622.3 |
| NG | 5.68 ± 0.17 b | 5.775 ± 0.39 b | 4.74 ± 0.36 b | 3.86 ± 0.45 b | 3.99 ± 0.31 b | 576.2 |
| DC | 31.89 ± 0.75 acd | 31.95 ± 0.72 a | 30.31 ± 0.75 a | 30.96 ± 0.81 a | 27.98 ± 1.30 a | 3695 |
| DV | 29.05 ± 2.41 acd | 28.11 ± 2.19 a | 26.48 ± 2.42 a | 23.39 ± 2.4 ab | 26.08 ± 2.27 a | 3166 |
| DG | 27.59 ± 1.65 acd | 31.48 ± 0.8 a | 27.98 ± 1.26 a | 26.66 ± 1.97 a | 25.39 ± 1.62 a | 3375 |
| Groups | RHW (g) | RKW (g) | Urea (g/L) | Creatinine (mg/dl) |
|---|---|---|---|---|
| NC | 0.28 ± 0.009 | 0.64 ± 0.02 | 7.471 ± 0.34 | 33.72 ± 1.5 |
| NV | 0.25 ± 0.005 b | 0.61 ± 0.01 b | 7.97 ± 0.5 b | 37.47 ± 2.5 b |
| NG | 0.25 ± 0.004 b | 0.66 ± 0.02 b | 6.974 ± 0.5 b | 31.14 ± 1.5 b |
| DC | 0.34 ± 0.008 a | 1.19 ± 0.03 a | 13.66 ± 0.9 a | 47.59 ± 4.3 a |
| DV | 0.30 ± 0.005 a | 1.07 ± 0.04 a | 10.62 ± 0.6 ab | 37.24 ± 1.6 b |
| DG | 0.30 ± 0.006 a | 1.07 ± 0.04 a | 10.82 ± 0.8 ab | 37.49 ± 2.3 b |
| Groups | TC (g/L) | HDL (%) | TG (g/L) | LDL (g/L) | VLDL (g/L) |
|---|---|---|---|---|---|
| NC | 0.93 ± 0.07 | 68.72 ± 2.0 | 0.54 ± 0.08 | 0.17 ± 0.04 | 0.13 ± 0.02 |
| NV | 1.3 ± 0.08 b | 61.04 ± 1.11 bd | 1 ± 0.16 b | 0.32 ± 0.04 b | 0.18 ± 0.03 b |
| NG | 0.83 ± 0.08 b | 74.11 ± 3.54 b | 0.54 ± 0.05 b | 0.13 ± 0.05 b | 0.11 ± 0.01 b |
| DC | 2.83 ± 0.48 a | 51.85 ± 3.86 a | 2.87 ± 0.6 a | 0.75 ± 0.13 a | 0.78 ± 0.15 a |
| DV | 1.43 ± 0.12 b | 65.58 ± 1.02 b | 1 ± 0.2 b | 0.3 ± 0.03 b | 0.2 ± 0.04 b |
| DG | 1.48 ± 0.18 b | 63.10 ± 2.2 b | 1.24 ± 0.2 b | 0.25 ± 0.04 b | 0.24 ± 0.05 b |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oguntibeju, O.O.; Aboua, Y.; Goboza, M. Vindoline—A Natural Product from Catharanthus Roseus Reduces Hyperlipidemia and Renal Pathophysiology in Experimental Type 2 Diabetes. Biomedicines 2019, 7, 59. https://doi.org/10.3390/biomedicines7030059
Oguntibeju OO, Aboua Y, Goboza M. Vindoline—A Natural Product from Catharanthus Roseus Reduces Hyperlipidemia and Renal Pathophysiology in Experimental Type 2 Diabetes. Biomedicines. 2019; 7(3):59. https://doi.org/10.3390/biomedicines7030059
Chicago/Turabian StyleOguntibeju, Oluwafemi Omoniyi, Yapo Aboua, and Mediline Goboza. 2019. "Vindoline—A Natural Product from Catharanthus Roseus Reduces Hyperlipidemia and Renal Pathophysiology in Experimental Type 2 Diabetes" Biomedicines 7, no. 3: 59. https://doi.org/10.3390/biomedicines7030059
APA StyleOguntibeju, O. O., Aboua, Y., & Goboza, M. (2019). Vindoline—A Natural Product from Catharanthus Roseus Reduces Hyperlipidemia and Renal Pathophysiology in Experimental Type 2 Diabetes. Biomedicines, 7(3), 59. https://doi.org/10.3390/biomedicines7030059