Cholesterol Efflux Efficiency of Reconstituted HDL Is Affected by Nanoparticle Lipid Composition
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Human ApoA-I Purification
2.3. Human ApoA-I HDL Reconstitution and Purification
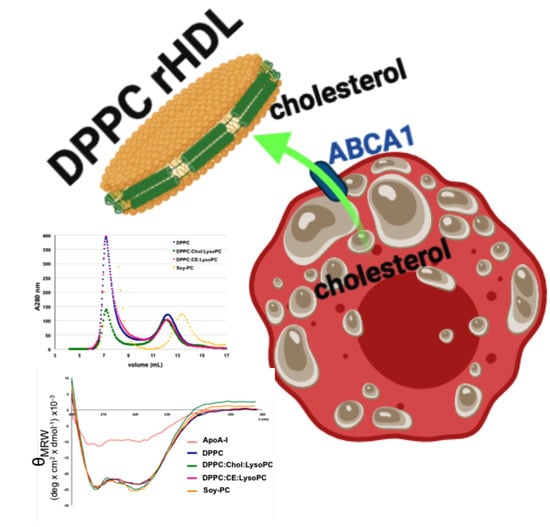
2.4. rHDL Biophysical Characterization
2.4.1. Circular Dichroism
2.4.2. Dynamic Light Scattering (DLS)
2.4.3. Negative Stain Electron Microscopy (NS-EM)
2.4.4. rHDLs Transition Temperature: Steady State Fluorescence Measurements
2.5. Isolation of Human Plasma HDL and LDL
2.6. LDL Acetylation
2.7. Cell Cultures
2.8. Cholesterol Efflux Assay
2.9. Statistical Analysis
3. Results
3.1. Development and Biophysical Characterization of rHDL
3.2. Effect of rHDL Lipid Composition on Cholesterol Efflux In Vitro
3.2.1. Cholesterol Efflux Promoted in Human and Murine Macrophages
3.2.2. Cholesterol Efflux Promoted in Human VSMC-Foam Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Cardiovascular Diseases (CVDs). 2020. Available online: https://www.who.int/health-topics/cardiovascular-diseases (accessed on 23 September 2020).
- Tabas, I.; Williams, K.J.; Boren, J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: Update and therapeutic implications. Circulation 2007, 116, 1832–1844. [Google Scholar] [PubMed]
- Benito-Vicente, A.; Uribe, K.B.; Jebari, S.; Galicia-Garcia, U.; Ostolaza, H.; Martin, C. Familial Hypercholesterolemia: The Most Frequent Cholesterol Metabolism Disorder Caused Disease. Int. J. Mol. Sci. 2018, 19, 3426. [Google Scholar]
- Boren, J.; Chapman, M.J.; Krauss, R.M.; Packard, C.J.; Bentzon, J.F.; Binder, C.J.; Daemen, M.J.; Demer, L.L.; Hegele, R.A.; Nicholls, S.J.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: Pathophysiological, genetic, and therapeutic insights: A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2020, 41, 2313–2330. [Google Scholar] [PubMed]
- Camejo, G.; Hurt-Camejo, E. Macrophages, extracellular matrix, and lipoproteins in arterial cholesterol balance. J. Lipid Res. 2014, 55, 1–3. [Google Scholar]
- Hurt-Camejo, E.; Camejo, G. ApoB-100 Lipoprotein Complex Formation with Intima Proteoglycans as a Cause of Atherosclerosis and Its Possible Ex Vivo Evaluation as a Disease Biomarker. J. Cardiovasc. Dev. Dis. 2018, 5, 36. [Google Scholar]
- Ference, B.A.; Ginsberg, H.N.; Graham, I.; Ray, K.K.; Packard, C.J.; Bruckert, E.; Hegele, R.A.; Krauss, R.M.; Raal, F.J.; Schunkert, H.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2017, 38, 2459–2472. [Google Scholar]
- Miller, Y.I.; Choi, S.H.; Wiesner, P.; Fang, L.; Harkewicz, R.; Hartvigsen, K.; Boullier, A.; Gonen, A.; Diehl, C.J.; Que, X.; et al. Oxidation-specific epitopes are danger-associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circ. Res. 2011, 108, 235–248. [Google Scholar]
- Steinbrecher, U.P. Role of superoxide in endothelial-cell modification of low-density lipoproteins. Biochim. Biophys. Acta 1988, 959, 20–30. [Google Scholar]
- Moore, K.J.; Freeman, M.W. Scavenger receptors in atherosclerosis: Beyond lipid uptake. Arter. Thromb. Vasc. Biol. 2006, 26, 1702–1711. [Google Scholar]
- Galkina, E.; Ley, K. Immune and inflammatory mechanisms of atherosclerosis (*). Annu. Rev. Immunol. 2009, 27, 165–197. [Google Scholar] [CrossRef] [Green Version]
- Tontonoz, P.; Mangelsdorf, D.J. Liver X receptor signaling pathways in cardiovascular disease. Mol. Endocrinol. 2003, 17, 985–993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leaf, D.A. The effect of physical exercise on reverse cholesterol transport. Metabolism 2003, 52, 950–957. [Google Scholar] [CrossRef]
- Phillips, M.C. Is ABCA1 a lipid transfer protein? J. Lipid Res. 2018, 59, 749–763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pourmousa, M.; Song, H.D.; He, Y.; Heinecke, J.W.; Segrest, J.P.; Pastor, R.W. Tertiary structure of apolipoprotein A-I in nascent high-density lipoproteins. Proc. Natl. Acad. Sci. USA 2018, 115, 5163–5168. [Google Scholar] [CrossRef] [Green Version]
- Oram, J.F.; Lawn, R.M.; Garvin, M.R.; Wade, D.P. ABCA1 is the cAMP-inducible apolipoprotein receptor that mediates cholesterol secretion from macrophages. J. Biol. Chem. 2000, 275, 34508–34511. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Silver, D.L.; Costet, P.; Tall, A.R. Specific binding of ApoA-I, enhanced cholesterol efflux, and altered plasma membrane morphology in cells expressing ABC1. J. Biol. Chem. 2000, 275, 33053–33058. [Google Scholar] [CrossRef] [Green Version]
- Vedhachalam, C.; Duong, P.T.; Nickel, M.; Nguyen, D.; Dhanasekaran, P.; Saito, H.; Rothblat, G.H.; Lund-Katz, S.; Phillips, M.C. Mechanism of ATP-binding cassette transporter A1-mediated cellular lipid efflux to apolipoprotein A-I and formation of high density lipoprotein particles. J. Biol. Chem. 2007, 282, 25123–25130. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Mei, X.; Herscovitz, H.; Atkinson, D. N-terminal mutation of apoA-I and interaction with ABCA1 reveal mechanisms of nascent HDL biogenesis. J. Lipid Res. 2019, 60, 44–57. [Google Scholar] [CrossRef] [Green Version]
- Sorci-Thomas, M.G.; Owen, J.S.; Fulp, B.; Bhat, S.; Zhu, X.; Parks, J.S.; Shah, D.; Jerome, W.G.; Gerelus, M.; Zabalawi, M.; et al. Nascent high density lipoproteins formed by ABCA1 resemble lipid rafts and are structurally organized by three apoA-I monomers. J. Lipid Res. 2012, 53, 1890–1909. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, M.A.; Barrera, G.C.; Nakamura, K.; Baldan, A.; Tarr, P.; Fishbein, M.C.; Frank, J.; Francone, O.L.; Edwards, P.A. ABCG1 has a critical role in mediating cholesterol efflux to HDL and preventing cellular lipid accumulation. Cell Metab. 2005, 1, 121–131. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, K.; Kennedy, M.A.; Baldan, A.; Bojanic, D.D.; Lyons, K.; Edwards, P.A. Expression and regulation of multiple murine ATP-binding cassette transporter G1 mRNAs/isoforms that stimulate cellular cholesterol efflux to high density lipoprotein. J. Biol. Chem. 2004, 279, 45980–45989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sankaranarayanan, S.; Oram, J.F.; Asztalos, B.F.; Vaughan, A.M.; Lund-Katz, S.; Adorni, M.P.; Phillips, M.C.; Rothblat, G.H. Effects of acceptor composition and mechanism of ABCG1-mediated cellular free cholesterol efflux. J. Lipid Res. 2009, 50, 275–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kontush, A.; Lhomme, M.; Chapman, M.J. Unraveling the complexities of the HDL lipidome. J. Lipid Res. 2013, 54, 2950–2963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Libby, P. Changing concepts of atherogenesis. J. Intern. Med. 2000, 247, 349–358. [Google Scholar] [CrossRef] [PubMed]
- De Nardo, D.; Labzin, L.I.; Kono, H.; Seki, R.; Schmidt, S.V.; Beyer, M.; Xu, D.; Zimmer, S.; Lahrmann, C.; Schildberg, F.A.; et al. High-density lipoprotein mediates anti-inflammatory reprogramming of macrophages via the transcriptional regulator ATF3. Nat. Immunol. 2014, 15, 152–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pirillo, A.; Catapano, A.L.; Norata, G.D. Biological Consequences of Dysfunctional HDL. Curr. Med. Chem. 2019, 26, 1644–1664. [Google Scholar] [CrossRef]
- Gordon, D.J.; Probstfield, J.L.; Garrison, R.J.; Neaton, J.D.; Castelli, W.P.; Knoke, J.D.; Jacobs, D.R., Jr.; Bangdiwala, S.; Tyroler, H.A. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation 1989, 79, 8–15. [Google Scholar] [CrossRef] [Green Version]
- Barter, P.; Gotto, A.M.; LaRosa, J.C.; Maroni, J.; Szarek, M.; Grundy, S.M.; Kastelein, J.J.; Bittner, V.; Fruchart, J.C.; Treating to New Targets, I. HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. N. Engl. J. Med. 2007, 357, 1301–1310. [Google Scholar] [CrossRef] [Green Version]
- Chyu, K.Y.; Shah, P.K. HDL/ApoA-1 infusion and ApoA-1 gene therapy in atherosclerosis. Front. Pharmacol. 2015, 6, 187. [Google Scholar] [CrossRef] [Green Version]
- Michael Gibson, C.; Korjian, S.; Tricoci, P.; Daaboul, Y.; Yee, M.; Jain, P.; Alexander, J.H.; Steg, P.G.; Lincoff, A.M.; Kastelein, J.J.; et al. Safety and Tolerability of CSL112, a Reconstituted, Infusible, Plasma-Derived Apolipoprotein A-I, After Acute Myocardial Infarction: The AEGIS-I Trial (ApoA-I Event Reducing in Ischemic Syndromes I). Circulation 2016, 134, 1918–1930. [Google Scholar] [CrossRef]
- Sirtori, C.R.; Calabresi, L.; Franceschini, G.; Baldassarre, D.; Amato, M.; Johansson, J.; Salvetti, M.; Monteduro, C.; Zulli, R.; Muiesan, M.L.; et al. Cardiovascular status of carriers of the apolipoprotein A-I(Milano) mutant: The Limone sul Garda study. Circulation 2001, 103, 1949–1954. [Google Scholar] [CrossRef] [Green Version]
- Franceschini, G.; Sirtori, C.R.; Bosisio, E.; Gualandri, V.; Orsini, G.B.; Mogavero, A.M.; Capurso, A. Relationship of the phenotypic expression of the A-IMilano apoprotein with plasma lipid and lipoprotein patterns. Atherosclerosis 1985, 58, 159–174. [Google Scholar] [CrossRef]
- Nissen, S.E.; Tsunoda, T.; Tuzcu, E.M.; Schoenhagen, P.; Cooper, C.J.; Yasin, M.; Eaton, G.M.; Lauer, M.A.; Sheldon, W.S.; Grines, C.L.; et al. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: A randomized controlled trial. JAMA 2003, 290, 2292–2300. [Google Scholar] [CrossRef]
- Nicholls, S.J.; Puri, R.; Ballantyne, C.M.; Jukema, J.W.; Kastelein, J.J.P.; Koenig, W.; Wright, R.S.; Kallend, D.; Wijngaard, P.; Borgman, M.; et al. Effect of Infusion of High-Density Lipoprotein Mimetic Containing Recombinant Apolipoprotein A-I Milano on Coronary Disease in Patients With an Acute Coronary Syndrome in the MILANO-PILOT Trial: A Randomized Clinical Trial. JAMA Cardiol. 2018, 3, 806–814. [Google Scholar] [CrossRef] [Green Version]
- Tardif, J.C.; Ballantyne, C.M.; Barter, P.; Dasseux, J.L.; Fayad, Z.A.; Guertin, M.C.; Kastelein, J.J.; Keyserling, C.; Klepp, H.; Koenig, W.; et al. Effects of the high-density lipoprotein mimetic agent CER-001 on coronary atherosclerosis in patients with acute coronary syndromes: A randomized trial. Eur. Heart J. 2014, 35, 3277–3286. [Google Scholar] [CrossRef]
- Tardy, C.; Goffinet, M.; Boubekeur, N.; Cholez, G.; Ackermann, R.; Sy, G.; Keyserling, C.; Lalwani, N.; Paolini, J.F.; Dasseux, J.L.; et al. HDL and CER-001 Inverse-Dose Dependent Inhibition of Atherosclerotic Plaque Formation in apoE-/-Mice: Evidence of ABCA1 Down-Regulation. PLoS ONE 2015, 10, e0137584. [Google Scholar] [CrossRef] [Green Version]
- Tardif, J.C.; Gregoire, J.; L’Allier, P.L.; Ibrahim, R.; Lesperance, J.; Heinonen, T.M.; Kouz, S.; Berry, C.; Basser, R.; Lavoie, M.A.; et al. Effects of reconstituted high-density lipoprotein infusions on coronary atherosclerosis: A randomized controlled trial. JAMA 2007, 297, 1675–1682. [Google Scholar] [CrossRef] [Green Version]
- Newton, R.S.; Krause, B.R. HDL therapy for the acute treatment of atherosclerosis. Atheroscler. Suppl. 2002, 3, 31–38. [Google Scholar] [CrossRef]
- Badimon, J.J.; Badimon, L.; Fuster, V. Regression of atherosclerotic lesions by high density lipoprotein plasma fraction in the cholesterol-fed rabbit. J. Clin. Investig. 1990, 85, 1234–1241. [Google Scholar] [CrossRef] [Green Version]
- Eriksson, M.; Carlson, L.A.; Miettinen, T.A.; Angelin, B. Stimulation of fecal steroid excretion after infusion of recombinant proapolipoprotein A-I. Potential reverse cholesterol transport in humans. Circulation 1999, 100, 594–598. [Google Scholar] [CrossRef] [Green Version]
- Nanjee, M.N.; Doran, J.E.; Lerch, P.G.; Miller, N.E. Acute effects of intravenous infusion of ApoA1/phosphatidylcholine discs on plasma lipoproteins in humans. Arter. Thromb. Vasc. Biol. 1999, 19, 979–989. [Google Scholar]
- Marchesi, M.; Booth, E.A.; Davis, T.; Bisgaier, C.L.; Lucchesi, B.R. Apolipoprotein A-IMilano and 1-palmitoyl-2-oleoyl phosphatidylcholine complex (ETC-216) protects the in vivo rabbit heart from regional ischemia-reperfusion injury. J. Pharmacol. Exp. Ther. 2004, 311, 1023–1031. [Google Scholar] [PubMed] [Green Version]
- Hippalgaonkar, K.; Majumdar, S.; Kansara, V. Injectable lipid emulsions-advancements, opportunities and challenges. AAPS Pharm. Sci. Tech. 2010, 11, 1526–1540. [Google Scholar]
- Cavigiolio, G.; Shao, B.; Geier, E.G.; Ren, G.; Heinecke, J.W.; Oda, M.N. The interplay between size, morphology, stability, and functionality of high-density lipoprotein subclasses. Biochemistry 2008, 47, 4770–4779. [Google Scholar] [PubMed] [Green Version]
- Greenfield, N.; Fasman, G.D. Computed circular dichroism spectra for the evaluation of protein conformation. Biochemistry 1969, 8, 4108–4116. [Google Scholar]
- Klemetsrud, T.; Jonassen, H.; Hiorth, M.; Kjøniksen, A.L.; Smistad, G. Studies on pectin-coated liposomes and their interaction with mucin. Colloids Surf. B Biointerfaces 2013, 103, 158–165. [Google Scholar]
- Fernandez-Higuero, J.A.; Etxebarria, A.; Benito-Vicente, A.; Alves, A.C.; Arrondo, J.L.; Ostolaza, H.; Bourbon, M.; Martin, C. Structural analysis of APOB variants, p.(Arg3527Gln), p.(Arg1164Thr) and p.(Gln4494del), causing Familial Hypercholesterolaemia provides novel insights into variant pathogenicity. Sci. Rep. 2015, 5, 18184. [Google Scholar]
- Van Blitterswijk, W.J.; Van Hoeven, R.P.; Van der Meer, B.W. Lipid structural order parameters (reciprocal of fluidity) in biomembranes derived from steady-state fluorescence polarization measurements. Biochim. Biophys. Acta 1981, 644, 323–332. [Google Scholar]
- van der Meer, B.W.; van Hoeven, R.P.; van Blitterswijk, W.J. Steady-state fluorescence polarization data in membranes. Resolution into physical parameters by an extended Perrin equation for restricted rotation of fluorophores. Biochim. Biophys. Acta 1986, 854, 38–44. [Google Scholar]
- Jahnig, F. Structural order of lipids and proteins in membranes: Evaluation of fluorescence anisotropy data. Proc. Natl. Acad. Sci. USA 1979, 76, 6361–6365. [Google Scholar]
- Etxebarria, A.; Benito-Vicente, A.; Palacios, L.; Stef, M.; Cenarro, A.; Civeira, F.; Ostolaza, H.; Martin, C. Functional characterization and classification of frequent low-density lipoprotein receptor variants. Hum. Mutat. 2015, 36, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.K.; Goldstein, J.L.; Anderson, G.W.; Brown, M.S. Degradation of cationized low density lipoprotein and regulation of cholesterol metabolism in homozygous familial hypercholesterolemia fibroblasts. Proc. Natl. Acad. Sci. USA 1976, 73, 3178–3182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sankaranarayanan, S.; Kellner-Weibel, G.; de la Llera-Moya, M.; Phillips, M.C.; Asztalos, B.F.; Bittman, R.; Rothblat, G.H. A sensitive assay for ABCA1-mediated cholesterol efflux using BODIPY-cholesterol. J. Lipid Res. 2011, 52, 2332–2340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramirez, C.M.; Lin, C.S.; Abdelmohsen, K.; Goedeke, L.; Yoon, J.H.; Madrigal-Matute, J.; Martin-Ventura, J.L.; Vo, D.T.; Uren, P.J.; Penalva, L.O.; et al. RNA binding protein HuR regulates the expression of ABCA1. J. Lipid Res. 2014, 55, 1066–1076. [Google Scholar] [CrossRef] [Green Version]
- Lakowicz, J.R.; Prendergast, F.G.; Hogen, D. Fluorescence anisotropy measurements under oxygen quenching conditions as a method to quantify the depolarizing rotations of fluorophores. Application to diphenylhexatriene in isotropic solvents and in lipid bilayers. Biochemistry 1979, 18, 520–527. [Google Scholar]
- Halling, K.K.; Ramstedt, B.; Nystrom, J.H.; Slotte, J.P.; Nyholm, T.K. Cholesterol interactions with fluid-phase phospholipids: Effect on the lateral organization of the bilayer. Biophys. J. 2008, 95, 3861–3871. [Google Scholar]
- Shaw, A.W.; McLean, M.A.; Sligar, S.G. Phospholipid phase transitions in homogeneous nanometer scale bilayer discs. FEBS Lett. 2004, 556, 260–264. [Google Scholar] [CrossRef]
- Prassl, R.; Pregetter, M.; Amenitsch, H.; Kriechbaum, M.; Schwarzenbacher, R.; Chapman, J.M.; Laggner, P. Low density lipoproteins as circulating fast temperature sensors. PLoS ONE 2008, 3, e4079. [Google Scholar]
- Goikuria, H.; Freijo, M.D.M.; Vega Manrique, R.; Sastre, M.; Elizagaray, E.; Lorenzo, A.; Vandenbroeck, K.; Alloza, I. Characterization of Carotid Smooth Muscle Cells during Phenotypic Transition. Cells 2018, 7, 23. [Google Scholar] [CrossRef] [Green Version]
- Dergunov, A.D.; Garaeva, E.A.; Savushkin, E.V.; Litvinov, D.Y. Significance of Lipid-Free and Lipid-Associated ApoA-I in Cellular Cho-lesterol Efflux. Curr. Protein Pept. Sci. 2017, 18, 92–99. [Google Scholar]
- Litvinov, D.Y.; Savushkin, E.V.; Garaeva, E.A.; Dergunov, A.D. Cholesterol Efflux and Reverse Cholesterol Transport: Experimental Approaches. Curr. Med. Chem. 2016, 23, 3883–3908. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.C. Molecular mechanisms of cellular cholesterol efflux. J. Biol. Chem. 2014, 289, 24020–24029. [Google Scholar] [CrossRef] [Green Version]
- Yvan-Charvet, L.; Wang, N.; Tall, A.R. Role of HDL, ABCA1, and ABCG1 transporters in cholesterol efflux and immune responses. Arter. Thromb. Vasc. Biol. 2010, 30, 139–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vedhachalam, C.; Chetty, P.S.; Nickel, M.; Dhanasekaran, P.; Lund-Katz, S.; Rothblat, G.H.; Phillips, M.C. Influence of apolipoprotein (Apo) A-I structure on nascent high density lipoprotein (HDL) particle size distribution. J. Biol. Chem. 2010, 285, 31965–31973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anantharamaiah, G.M.; Jones, J.L.; Brouillette, C.G.; Schmidt, C.F.; Chung, B.H.; Hughes, T.A.; Bhown, A.S.; Segrest, J.P. Studies of synthetic peptide analogs of the amphipathic helix. Structure of complexes with dimyristoyl phosphatidylcholine. J. Biol. Chem. 1985, 260, 10248–10255. [Google Scholar]
- Bielicki, J.K.; Zhang, H.; Cortez, Y.; Zheng, Y.; Narayanaswami, V.; Patel, A.; Johansson, J.; Azhar, S. A new HDL mimetic peptide that stimulates cellular cholesterol efflux with high efficiency greatly reduces atherosclerosis in mice. J. Lipid Res. 2010, 51, 1496–1503. [Google Scholar] [CrossRef] [Green Version]
- Bloedon, L.T.; Dunbar, R.; Duffy, D.; Pinell-Salles, P.; Norris, R.; DeGroot, B.J.; Movva, R.; Navab, M.; Fogelman, A.M.; Rader, D.J. Safety, pharmacokinetics, and pharmacodynamics of oral apoA-I mimetic peptide D-4F in high-risk cardiovascular patients. J. Lipid Res. 2008, 49, 1344–1352. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.L.N.; Drake, S.L.; Crockatt, J.G.; Dasseux, J.L.H. Single-dose intravenous infusion of ETC-642, a 22-Mer ApoA-I analogue and phospholipids complex, elevates HDL-C in atherosclerosis patients. Circulation 2003, 108, 2. [Google Scholar]
- Schwendeman, A.; Sviridov, D.O.; Yuan, W.; Guo, Y.; Morin, E.E.; Yuan, Y.; Stonik, J.; Freeman, L.; Ossoli, A.; Thacker, S.; et al. The effect of phospholipid composition of reconstituted HDL on its cholesterol efflux and anti-inflammatory properties. J. Lipid Res. 2015, 56, 1727–1737. [Google Scholar] [CrossRef] [Green Version]
- Yancey, P.G.; de la Llera-Moya, M.; Swarnakar, S.; Monzo, P.; Klein, S.M.; Connelly, M.A.; Johnson, W.J.; Williams, D.L.; Rothblat, G.H. High density lipoprotein phospholipid composition is a major determinant of the bi-directional flux and net movement of cellular free cholesterol mediated by scavenger receptor BI. J. Biol. Chem. 2000, 275, 36596–36604. [Google Scholar] [CrossRef] [Green Version]
- Yancey, P.G.; Kawashiri, M.A.; Moore, R.; Glick, J.M.; Williams, D.L.; Connelly, M.A.; Rader, D.J.; Rothblat, G.H. In vivo modulation of HDL phospholipid has opposing effects on SR-BI- and ABCA1-mediated cholesterol efflux. J. Lipid Res. 2004, 45, 337–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, C.I.; Beckstead, J.A.; Thompson, A.; Hafiane, A.; Wang, R.H.; Ryan, R.O.; Kiss, R.S. Tweaking the cholesterol efflux capacity of reconstituted HDL. Biochem. Cell Biol. 2012, 90, 636–645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marmillot, P.; Patel, S.; Lakshman, M.R. Reverse cholesterol transport is regulated by varying fatty acyl chain saturation and sphingomyelin content in reconstituted high-density lipoproteins. Metabolism 2007, 56, 251–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramstedt, B.; Slotte, J.P. Interaction of cholesterol with sphingomyelins and acyl-chain-matched phosphatidylcholines: A comparative study of the effect of the chain length. Biophys. J. 1999, 76, 908–915. [Google Scholar] [CrossRef] [Green Version]
- Ohvo-Rekila, H.; Ramstedt, B.; Leppimaki, P.; Slotte, J.P. Cholesterol interactions with phospholipids in membranes. Prog. Lipid Res. 2002, 41, 66–97. [Google Scholar] [CrossRef]
- Di Bartolo, B.A.; Nicholls, S.J.; Bao, S.; Rye, K.A.; Heather, A.K.; Barter, P.J.; Bursill, C. The apolipoprotein A-I mimetic peptide ETC-642 exhibits anti-inflammatory properties that are comparable to high density lipoproteins. Atherosclerosis 2011, 217, 395–400. [Google Scholar] [CrossRef]
- Montalvo, G.; Pons, R.; Zhang, G.; Diaz, M.; Valiente, M. Structure and phase equilibria of the soybean lecithin/PEG 40 monostearate/water system. Langmuir 2013, 29, 14369–14379. [Google Scholar] [CrossRef] [Green Version]
- Nakagawa, Y.; Inoue, K.; Nojima, S. Transfer of cholesterol between liposomal membranes. Biochim. Biophys. Acta 1979, 553, 307–319. [Google Scholar] [CrossRef]
- Fugler, L.; Clejan, S.; Bittman, R. Movement of cholesterol between vesicles prepared with different phospholipids or sizes. J. Biol. Chem. 1985, 260, 4098–4102. [Google Scholar]
- Yeagle, P.L. Cholesterol and the cell membrane. Biochim. Biophys. Acta 1985, 822, 267–287. [Google Scholar] [CrossRef]
- McLean, L.R.; Phillips, M.C. Cholesterol transfer from small and large unilamellar vesicles. Biochim. Biophys. Acta 1984, 776, 21–26. [Google Scholar] [CrossRef]
- Frolov, V.A.; Shnyrova, A.V.; Zimmerberg, J. Lipid polymorphisms and membrane shape. Cold Spring Harb. Perspect. Biol. 2011, 3, a004747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanni, S.; Hirose, H.; Barelli, H.; Antonny, B.; Gautier, R. A sub-nanometre view of how membrane curvature and composition modulate lipid packing and protein recruitment. Nat. Commun. 2014, 5, 4916. [Google Scholar] [CrossRef] [Green Version]
- Pinot, M.; Vanni, S.; Pagnotta, S.; Lacas-Gervais, S.; Payet, L.A.; Ferreira, T.; Gautier, R.; Goud, B.; Antonny, B.; Barelli, H. Lipid cell biology. Polyunsaturated phospholipids facilitate membrane deformation and fission by endocytic proteins. Science 2014, 345, 693–697. [Google Scholar] [CrossRef] [PubMed]
- Chernomordik, L.V.; Kozlov, M.M. Protein-lipid interplay in fusion and fission of biological membranes. Annu. Rev. Biochem. 2003, 72, 175–207. [Google Scholar] [CrossRef] [PubMed]
- Ikonen, E. Cellular cholesterol trafficking and compartmentalization. Nat. Rev. Mol. Cell Biol. 2008, 9, 125–138. [Google Scholar] [CrossRef]
- Churchward, M.A.; Rogasevskaia, T.; Brandman, D.M.; Khosravani, H.; Nava, P.; Atkinson, J.K.; Coorssen, J.R. Specific lipids supply critical negative spontaneous curvature-an essential component of native Ca2+-triggered membrane fusion. Biophys. J. 2008, 94, 3976–3986. [Google Scholar] [CrossRef] [Green Version]
- McMahon, H.T.; Gallop, J.L. Membrane curvature and mechanisms of dynamic cell membrane remodelling. Nature 2005, 438, 590–596. [Google Scholar] [CrossRef]
- McLean, L.R.; Phillips, M.C. Mechanism of cholesterol and phosphatidylcholine exchange or transfer between unilamellar vesicles. Biochemistry 1981, 20, 2893–2900. [Google Scholar] [CrossRef]
- Thomas, P.D.; Poznansky, M.J. Effect of surface curvature on the rate of cholesterol transfer between lipid vesicles. Biochem. J. 1988, 254, 155–160. [Google Scholar] [CrossRef] [Green Version]





| α-Helical Content | α-Helicity Ratio rHDL/apoA-I | |
|---|---|---|
| ApoA-I | 30.7 ± 2.3 | - |
| DPPC | 70.0 ± 2.8 * | 2.3 ± 0.3 * |
| DPPC:Chol:LysoPC | 77.4 ± 6.6 * | 2.5 ± 0.02 * |
| DPPC:CE:LysoPC | 64.7 ± 3.8 * | 2.2 ± 0.04 * |
| Soy-PC | 70.3 ± 3.5 * | 2.5 ± 0.3 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jebari-Benslaiman, S.; Uribe, K.B.; Benito-Vicente, A.; Galicia-Garcia, U.; Larrea-Sebal, A.; Alloza, I.; Vandenbroeck, K.; Ostolaza, H.; Martín, C. Cholesterol Efflux Efficiency of Reconstituted HDL Is Affected by Nanoparticle Lipid Composition. Biomedicines 2020, 8, 373. https://doi.org/10.3390/biomedicines8100373
Jebari-Benslaiman S, Uribe KB, Benito-Vicente A, Galicia-Garcia U, Larrea-Sebal A, Alloza I, Vandenbroeck K, Ostolaza H, Martín C. Cholesterol Efflux Efficiency of Reconstituted HDL Is Affected by Nanoparticle Lipid Composition. Biomedicines. 2020; 8(10):373. https://doi.org/10.3390/biomedicines8100373
Chicago/Turabian StyleJebari-Benslaiman, Shifa, Kepa B. Uribe, Asier Benito-Vicente, Unai Galicia-Garcia, Asier Larrea-Sebal, Iraide Alloza, Koen Vandenbroeck, Helena Ostolaza, and César Martín. 2020. "Cholesterol Efflux Efficiency of Reconstituted HDL Is Affected by Nanoparticle Lipid Composition" Biomedicines 8, no. 10: 373. https://doi.org/10.3390/biomedicines8100373
APA StyleJebari-Benslaiman, S., Uribe, K. B., Benito-Vicente, A., Galicia-Garcia, U., Larrea-Sebal, A., Alloza, I., Vandenbroeck, K., Ostolaza, H., & Martín, C. (2020). Cholesterol Efflux Efficiency of Reconstituted HDL Is Affected by Nanoparticle Lipid Composition. Biomedicines, 8(10), 373. https://doi.org/10.3390/biomedicines8100373