Modulation of NLRP3 Inflammasome Attenuated Inflammatory Response Associated to Diarrhea-Predominant Irritable Bowel Syndrome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Materials
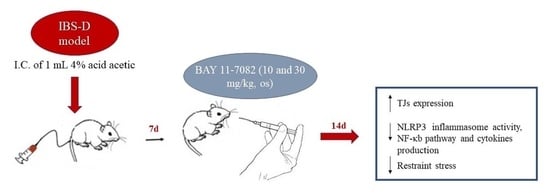
2.3. IBS-D Model
2.4. Experimental Groups
- Group 1.
- Sham + vehicle (saline). Rats received saline for 2 weeks (N= 10).
- Group 2.
- Sham + BAY 11-7082 10 mg/kg. Rats received BAY 11-7082 10 mg/kg for 2 weeks (N = 10).
- Group 3.
- Sham + BAY 11-7082 30 mg/kg. Rats received BAY 11-7082 30 mg/kg for 2 weeks (N = 10).
- Group 4.
- IBS-D + vehicle (saline). Rats received saline for 2 weeks after IBS-D induction (N = 10).
- Group 5.
- IBS-D + BAY 11-7082 10 mg/kg. Rats received BAY 11-7082 10 mg/kg for 2 weeks after IBS-D induction (N = 10).
- Group 6.
- IBS-D + BAY 11-7082 30 mg/kg. Rats received BAY 11-7082 30 mg/kg for 2 weeks after IBS-D induction (N = 10).
2.5. Restraint Stress Procedure
2.6. Histological Analysis
2.7. Myeloperoxidase (MPO) Activity
2.8. Malondialdehyde (MDA) Assay
2.9. Immunohistochemical Localization of ZO-1, Occludin, IL-1β, IL-18, TNF-α, iNOS, and COX-2
2.10. Western Blot Analysis
2.11. ELISA Assay for IL-1β, TNF-α, and IL-18
2.12. Statistical Analysis
3. Results
3.1. Effect of BAY 11-7082 on Restraint Stress
3.2. Effect of BAY 11-7082 Treatment on TJs Expression
3.3. Effect of BAY 11-7082 Treatment on Histological Damage
3.4. Effect of BAY 11-7082 Treatment on NLRP3 Inflammasome Pathway
3.5. Effect of BAY 11-7082 Treatment on Pro-Inflammatory Cytokines, Nitrosative Stress, and Lipid Peroxidation
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gracie, D.J.; Ford, A.C. Irritable Bowel Syndrome-Type Symptoms Are Associated with Psychological Comorbidity, Reduced Quality of Life, and Health Care Use in Patients With Inflammatory Bowel Disease. Gastroenterology 2017, 153, 324–325. [Google Scholar] [CrossRef] [PubMed]
- Longstreth, G.F.; Thompson, W.G.; Chey, W.D.; Houghton, L.A.; Mearin, F.; Spiller, R.C. Functional bowel disorders. Gastroenterology 2006, 130, 1480–1491. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, M.I.; Sandler, R.S.; Kappelman, M.D.; Martin, C.F.; Chen, W.; Anton, K.; Long, M.D. Prevalence and Impact of Inflammatory Bowel Disease-Irritable Bowel Syndrome on Patient-reported Outcomes in CCFA Partners. Inflamm. Bowel Dis. 2017, 23, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.C.; Lacy, B.E.; Talley, N.J. Irritable Bowel Syndrome. N. Engl. J. Med. 2017, 376, 2566–2578. [Google Scholar] [CrossRef] [Green Version]
- Lacy, B.E. Diagnosis and treatment of diarrhea-predominant irritable bowel syndrome. Int. J. Gen. Med. 2016, 9, 7–17. [Google Scholar] [CrossRef] [Green Version]
- Ghoshal, U.C.; Shukla, R.; Ghoshal, U.; Gwee, K.A.; Ng, S.C.; Quigley, E.M. The gut microbiota and irritable bowel syndrome: Friend or foe? Int. J. Inflam. 2012, 2012, 151085. [Google Scholar] [CrossRef]
- Ortiz-Lucas, M.; Saz-Peiro, P.; Sebastian-Domingo, J.J. Irritable bowel syndrome immune hypothesis. Part two: The role of cytokines. Rev. Esp. Enferm. Dig. 2010, 102, 711–717. [Google Scholar] [CrossRef] [Green Version]
- Corridoni, D.; Arseneau, K.O.; Cifone, M.G.; Cominelli, F. The dual role of nod-like receptors in mucosal innate immunity and chronic intestinal inflammation. Front. Immunol. 2014, 5, 317. [Google Scholar] [CrossRef] [Green Version]
- Awad, F.; Assrawi, E.; Louvrier, C.; Jumeau, C.; Georgin-Lavialle, S.; Grateau, G.; Amselem, S.; Giurgea, I.; Karabina, S.A. Inflammasome biology, molecular pathology and therapeutic implications. Pharmacol. Ther. 2018, 187, 133–149. [Google Scholar] [CrossRef]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef] [Green Version]
- Schroder, K.; Tschopp, J. The inflammasomes. Cell 2010, 140, 821–832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Latz, E. The inflammasomes: Mechanisms of activation and function. Curr. Opin. Immunol. 2010, 22, 28–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pellegrini, C.; Antonioli, L.; Lopez-Castejon, G.; Blandizzi, C.; Fornai, M. Canonical and Non-Canonical Activation of NLRP3 Inflammasome at the Crossroad between Immune Tolerance and Intestinal Inflammation. Front. Immunol. 2017, 8, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juliana, C.; Fernandes-Alnemri, T.; Wu, J.; Datta, P.; Solorzano, L.; Yu, J.W.; Meng, R.; Quong, A.A.; Latz, E.; Scott, C.P.; et al. Anti-inflammatory compounds parthenolide and Bay 11-7082 are direct inhibitors of the inflammasome. J. Biol. Chem. 2010, 285, 9792–9802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, W.; Li, M.; He, F.; Zhou, S.; Zhu, L. Targeting the NLRP3 inflammasome to attenuate spinal cord injury in mice. J. Neuroinflamm. 2017, 14, 207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiazza, F.; Couturier-Maillard, A.; Benetti, E.; Mastrocola, R.; Nigro, D.; Cutrin, J.C.; Serpe, L.; Aragno, M.; Fantozzi, R.; Ryffel, B.; et al. Targeting the NLRP3 Inflammasome to Reduce Diet-Induced Metabolic Abnormalities in Mice. Mol. Med. 2016, 21, 1025–1037. [Google Scholar] [CrossRef]
- Ghashghaeinia, M.; Cluitmans, J.C.; Toulany, M.; Saki, M.; Koberle, M.; Lang, E.; Dreischer, P.; Biedermann, T.; Duszenko, M.; Lang, F.; et al. Age sensitivity of NFkappaB abundance and programmed cell death in erythrocytes induced by NFkappaB inhibitors. Cell Physiol. Biochem. 2013, 32, 801–813. [Google Scholar] [CrossRef] [Green Version]
- Rauert-Wunderlich, H.; Siegmund, D.; Maier, E.; Giner, T.; Bargou, R.C.; Wajant, H.; Stuhmer, T. The IKK inhibitor Bay 11-7082 induces cell death independent from inhibition of activation of NFkappaB transcription factors. PLoS ONE 2013, 8, e59292. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.L.; Sun, C.M. BAY-11-7082 induces apoptosis of multiple myeloma U266 cells through inhibiting NF-kappaB pathway. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 2564–2571. [Google Scholar] [CrossRef]
- Sasaki, C.T.; Doukas, S.G.; Vageli, D.P. In Vivo Short-Term Topical Application of BAY 11-7082 Prevents the Acidic Bile-Induced mRNA and miRNA Oncogenic Phenotypes in Exposed Murine Hypopharyngeal Mucosa. Neoplasia 2018, 20, 374–386. [Google Scholar] [CrossRef]
- Xu, J.R.; Luo, J.Y.; Shang, L.; Kong, W.M. Effect of change in an inhibitory neurotransmitter of the myenteric plexus on the pathogenetic mechanism of irritable bowel syndrome subgroups in rat models. Chin. J. Dig. Dis. 2006, 7, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Esposito, E.; Campolo, M.; Casili, G.; Lanza, M.; Franco, D.; Filippone, A.; Peritore, A.F.; Cuzzocrea, S. Protective Effects of Xyloglucan in Association with the Polysaccharide Gelose in an Experimental Model of Gastroenteritis and Urinary Tract Infections. Int. J. Mol. Sci. 2018, 19, 1844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asseman, C.; Mauze, S.; Leach, M.W.; Coffman, R.L.; Powrie, F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J. Exp. Med. 1999, 190, 995–1004. [Google Scholar] [CrossRef] [PubMed]
- Casili, G.; Cordaro, M.; Impellizzeri, D.; Bruschetta, G.; Paterniti, I.; Cuzzocrea, S.; Esposito, E. Dimethyl Fumarate Reduces Inflammatory Responses in Experimental Colitis. J. Crohns Colitis 2016, 10, 472–483. [Google Scholar] [CrossRef] [PubMed]
- Al-Henhena, N.; Ying, R.P.; Ismail, S.; Najm, W.; Khalifa, S.A.; El-Seedi, H.; Ameen Abdulla, M. Chemopreventive efficacy of Andrographis paniculata on azoxymethane-induced aberrant colon crypt foci in vivo. PLoS ONE 2014, 9, e111118. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Zhang, W.; Hui, F.M.; Zhang, Y.H.; Zhang, F.F.; Li, X.M.; Shi, F.X. Cell-specific expression and immunolocalization of nitric oxide synthase isoforms and soluble guanylyl cyclase alpha1 and beta1 subunits in the ovary of fetal, neonatal and immature pigs. Anim. Reprod. Sci. 2012, 131, 172–180. [Google Scholar] [CrossRef]
- Impellizzeri, D.; Bruschetta, G.; Di Paola, R.; Ahmad, A.; Campolo, M.; Cuzzocrea, S.; Esposito, E.; Navarra, M. The anti-inflammatory and antioxidant effects of bergamot juice extract (BJe) in an experimental model of inflammatory bowel disease. Clin. Nutr. 2015, 34, 1146–1154. [Google Scholar] [CrossRef]
- Hwang, I.; An, B.S.; Yang, H.; Kang, H.S.; Jung, E.M.; Jeung, E.B. Tissue-specific expression of occludin, zona occludens-1, and junction adhesion molecule A in the duodenum, ileum, colon, kidney, liver, lung, brain, and skeletal muscle of C57BL mice. J. Physiol. Pharmacol. 2013, 64, 11–18. [Google Scholar]
- Kolodziej, L.E.; Lodolce, J.P.; Chang, J.E.; Schneider, J.R.; Grimm, W.A.; Bartulis, S.J.; Zhu, X.; Messer, J.S.; Murphy, S.F.; Reddy, N.; et al. TNFAIP3 maintains intestinal barrier function and supports epithelial cell tight junctions. PLoS ONE 2011, 6, e26352. [Google Scholar] [CrossRef] [Green Version]
- Ziade, F.; Rungoe, C.; Kallemose, T.; Paerregaard, A.; Wewer, A.V.; Jakobsen, C. Biochemical Markers, Genotype, and Inflammation in Pediatric Inflammatory Bowel Disease: A Danish Population-Based Study. Dig. Dis. 2019, 37, 140–146. [Google Scholar] [CrossRef]
- Nanini, H.F.; Bernardazzi, C.; Castro, F.; de Souza, H.S.P. Damage-associated molecular patterns in inflammatory bowel disease: From biomarkers to therapeutic targets. World J. Gastroenterol. 2018, 24, 4622–4634. [Google Scholar] [CrossRef] [PubMed]
- Ringel-Scaia, V.M.; McDaniel, D.K.; Allen, I.C. The Goldilocks Conundrum: NLR Inflammasome Modulation of Gastrointestinal Inflammation during Inflammatory Bowel Disease. Crit. Rev. Immunol. 2016, 36, 283–314. [Google Scholar] [CrossRef] [PubMed]
- Rashidian, A.; Muhammadnejad, A.; Dehpour, A.R.; Mehr, S.E.; Akhavan, M.M.; Shirkoohi, R.; Chamanara, M.; Mousavi, S.E.; Rezayat, S.M. Atorvastatin attenuates TNBS-induced rat colitis: The involvement of the TLR4/NF-kB signaling pathway. Inflammopharmacology 2016, 24, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Li, M.C.; He, S.H. IL-10 and its related cytokines for treatment of inflammatory bowel disease. World J. Gastroenterol. 2004, 10, 620–625. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, J.S.; Kim, J.M.; Kim, N.; Jung, H.C.; Song, I.S. Simvastatin inhibits NF-kappaB signaling in intestinal epithelial cells and ameliorates acute murine colitis. Int. Immunopharmacol. 2007, 7, 241–248. [Google Scholar] [CrossRef]
- Sands, B.E. Biomarkers of Inflammation in Inflammatory Bowel Disease. Gastroenterology 2015, 149, 1275–1285. [Google Scholar] [CrossRef]
- Xu, Y.; Li, Y.; Scott, K.; Lindh, C.H.; Jakobsson, K.; Fletcher, T.; Ohlsson, B.; Andersson, E.M. Inflammatory bowel disease and biomarkers of gut inflammation and permeability in a community with high exposure to perfluoroalkyl substances through drinking water. Environ. Res. 2020, 181, 108923. [Google Scholar] [CrossRef]
- Wallace, J.L.; Miller, M.J. Nitric oxide in mucosal defense: A little goes a long way. Gastroenterology 2000, 119, 512–520. [Google Scholar] [CrossRef] [Green Version]
- Alzoghaibi, M.A.; Al Mofleh, I.A.; Al-Jebreen, A.M. Lipid peroxides in patients with inflammatory bowel disease. Saudi J. Gastroenterol. 2007, 13, 187–190. [Google Scholar] [CrossRef]
- Miller, Y.I.; Choi, S.H.; Wiesner, P.; Fang, L.; Harkewicz, R.; Hartvigsen, K.; Boullier, A.; Gonen, A.; Diehl, C.J.; Que, X.; et al. Oxidation-specific epitopes are danger-associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circ. Res. 2011, 108, 235–248. [Google Scholar] [CrossRef] [Green Version]
- Dudzinska, E.; Gryzinska, M.; Ognik, K.; Gil-Kulik, P.; Kocki, J. Oxidative Stress and Effect of Treatment on the Oxidation Product Decomposition Processes in IBD. Oxid. Med. Cell. Longev. 2018, 2018, 7918261. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.L.; Devchand, P.R. Emerging roles for cyclooxygenase-2 in gastrointestinal mucosal defense. Br. J. Pharmacol. 2005, 145, 275–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbara, G.; Zecchi, L.; Barbaro, R.; Cremon, C.; Bellacosa, L.; Marcellini, M.; De Giorgio, R.; Corinaldesi, R.; Stanghellini, V. Mucosal permeability and immune activation as potential therapeutic targets of probiotics in irritable bowel syndrome. J. Clin. Gastroenterol. 2012, 46, S52–S55. [Google Scholar] [CrossRef] [PubMed]
- Strober, W.; Fuss, I.J.; Blumberg, R.S. The immunology of mucosal models of inflammation. Annu. Rev. Immunol. 2002, 20, 495–549. [Google Scholar] [CrossRef]
- Atreya, I.; Atreya, R.; Neurath, M.F. NF-kappaB in inflammatory bowel disease. J. Intern. Med. 2008, 263, 591–596. [Google Scholar] [CrossRef]
- Lamkanfi, M.; Dixit, V.M. Mechanisms and functions of inflammasomes. Cell 2014, 157, 1013–1022. [Google Scholar] [CrossRef]
- Lazaridis, N.; Germanidis, G. Current insights into the innate immune system dysfunction in irritable bowel syndrome. Ann. Gastroenterol. 2018, 31, 171–187. [Google Scholar] [CrossRef]
- Liu, J.J.; Kay, T.M.; Davis, E.M.; Lou, Y.; Kao, D.; Claggett, B.; Fedorak, R.N.; Irvin, R.T. Epithelial Cell Extrusion Zones Observed on Confocal Laser Endomicroscopy Correlates with Immunohistochemical Staining of Mucosal Biopsy Samples. Dig. Dis. Sci. 2016, 61, 1895–1902. [Google Scholar] [CrossRef]
- Mayer, E.A.; Naliboff, B.D.; Chang, L.; Coutinho, S.V.V. Stress and irritable bowel syndrome. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 280, G519–G524. [Google Scholar] [CrossRef]
- Blake, M.R.; Raker, J.M.; Whelan, K. Validity and reliability of the Bristol Stool Form Scale in healthy adults and patients with diarrhoea-predominant irritable bowel syndrome. Aliment. Pharmacol. Ther. 2016, 44, 693–703. [Google Scholar] [CrossRef] [Green Version]
- Walker, J.R.; Ediger, J.P.; Graff, L.A.; Greenfeld, J.M.; Clara, I.; Lix, L.; Rawsthorne, P.; Miller, N.; Rogala, L.; McPhail, C.M.; et al. The Manitoba IBD cohort study: A population-based study of the prevalence of lifetime and 12-month anxiety and mood disorders. Am. J. Gastroenterol. 2008, 103, 1989–1997. [Google Scholar] [CrossRef] [PubMed]
- Hou, Q.; Zhu, S.; Zhang, C.; Huang, Y.; Guo, Y.; Li, P.; Chen, X.; Wen, Y.; Han, Q.; Liu, F. Berberine improves intestinal epithelial tight junctions by upregulating A20 expression in IBS-D mice. Biomed. Pharmacother. 2019, 118, 109206. [Google Scholar] [CrossRef] [PubMed]
- Sanchez de Medina, F.; Romero-Calvo, I.; Mascaraque, C.; Martinez-Augustin, O. Intestinal inflammation and mucosal barrier function. Inflamm. Bowel Dis. 2014, 20, 2394–2404. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, S.P.; Hebden, J.; Campbell, E.; Naesdal, J.; Olbe, L.; Perkins, A.C.; Spiller, R.C. Abnormal intestinal permeability in subgroups of diarrhea-predominant irritable bowel syndromes. Am. J. Gastroenterol. 2006, 101, 1288–1294. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.Y.; Zhang, J.; Feng, Y.C. Role of NLRP3 inflammasome in Bifidobacterium longum-regulated visceral hypersensitivity of postinfectious irritable bowel syndrome. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1933–1937. [Google Scholar] [CrossRef] [Green Version]
- Zhen, Y.; Zhang, H. NLRP3 Inflammasome and Inflammatory Bowel Disease. Front. Immunol. 2019, 10, 276. [Google Scholar] [CrossRef] [Green Version]
- Hausmann, M.; Obermeier, F.; Schreiter, K.; Spottl, T.; Falk, W.; Scholmerich, J.; Herfarth, H.; Saftig, P.; Rogler, G. Cathepsin D is up-regulated in inflammatory bowel disease macrophages. Clin. Exp. Immunol. 2004, 136, 157–167. [Google Scholar] [CrossRef]
- Vallabhapurapu, S.; Karin, M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu. Rev. Immunol. 2009, 27, 693–733. [Google Scholar] [CrossRef]
- Neurath, M.F. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2014, 14, 329–342. [Google Scholar] [CrossRef]
- Wang, L.H.; Fang, X.C.; Pan, G.Z. Bacillary dysentery as a causative factor of irritable bowel syndrome and its pathogenesis. Gut 2004, 53, 1096–1101. [Google Scholar] [CrossRef] [Green Version]
- Takagi, H.; Kanai, T.; Okazawa, A.; Kishi, Y.; Sato, T.; Takaishi, H.; Inoue, N.; Ogata, H.; Iwao, Y.; Hoshino, K.; et al. Contrasting action of IL-12 and IL-18 in the development of dextran sodium sulphate colitis in mice. Scand. J. Gastroenterol. 2003, 38, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Singer, I.I.; Kawka, D.W.; Scott, S.; Weidner, J.R.; Mumford, R.A.; Riehl, T.E.; Stenson, W.F. Expression of inducible nitric oxide synthase and nitrotyrosine in colonic epithelium in inflammatory bowel disease. Gastroenterology 1996, 111, 871–885. [Google Scholar] [CrossRef]
- Lih-Brody, L.; Powell, S.R.; Collier, K.P.; Reddy, G.M.; Cerchia, R.; Kahn, E.; Weissman, G.S.; Katz, S.; Floyd, R.A.; McKinley, M.J.; et al. Increased oxidative stress and decreased antioxidant defenses in mucosa of inflammatory bowel disease. Dig. Dis. Sci. 1996, 41, 2078–2086. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Moon, S.H.; Jenkins, C.M.; Sims, H.F.; Gross, R.W. Cyclooxygenase-2 Mediated Oxidation of 2-Arachidonoyl-Lysophospholipids Identifies Unknown Lipid Signaling Pathways. Cell Chem. Biol. 2016, 23, 1217–1227. [Google Scholar] [CrossRef] [Green Version]
- Parks, D.A.; Bulkley, G.B.; Granger, D.N. Role of oxygen-derived free radicals in digestive tract diseases. Surgery 1983, 94, 415–422. [Google Scholar]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scuderi, S.A.; Casili, G.; Lanza, M.; Filippone, A.; Paterniti, I.; Esposito, E.; Campolo, M. Modulation of NLRP3 Inflammasome Attenuated Inflammatory Response Associated to Diarrhea-Predominant Irritable Bowel Syndrome. Biomedicines 2020, 8, 519. https://doi.org/10.3390/biomedicines8110519
Scuderi SA, Casili G, Lanza M, Filippone A, Paterniti I, Esposito E, Campolo M. Modulation of NLRP3 Inflammasome Attenuated Inflammatory Response Associated to Diarrhea-Predominant Irritable Bowel Syndrome. Biomedicines. 2020; 8(11):519. https://doi.org/10.3390/biomedicines8110519
Chicago/Turabian StyleScuderi, Sarah Adriana, Giovanna Casili, Marika Lanza, Alessia Filippone, Irene Paterniti, Emanuela Esposito, and Michela Campolo. 2020. "Modulation of NLRP3 Inflammasome Attenuated Inflammatory Response Associated to Diarrhea-Predominant Irritable Bowel Syndrome" Biomedicines 8, no. 11: 519. https://doi.org/10.3390/biomedicines8110519
APA StyleScuderi, S. A., Casili, G., Lanza, M., Filippone, A., Paterniti, I., Esposito, E., & Campolo, M. (2020). Modulation of NLRP3 Inflammasome Attenuated Inflammatory Response Associated to Diarrhea-Predominant Irritable Bowel Syndrome. Biomedicines, 8(11), 519. https://doi.org/10.3390/biomedicines8110519