Ovariopexy—Before and after Endometriosis Surgery
Abstract
:1. Introduction
2. Technique of Ovariopexy
3. Counseling
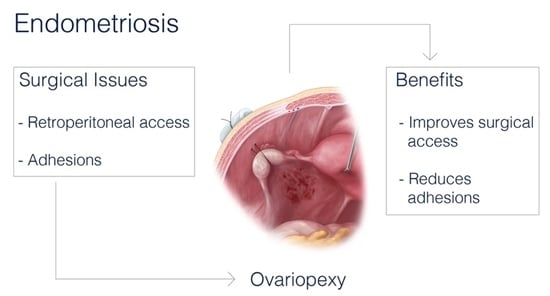
4. Advantages of Ovariopexy: Surgeon’s Way to Maximize Surgical Outcome
4.1. Access to Surgical Field
4.2. Reduction of Postoperative Adhesion
5. Effect of Ovariopexyon Fertility
6. Effect of Ovariopexyon Pain
7. Complications
8. Conclusions
9. Future Perspective
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Parazzini, F.; Roncella, E.; Cipriani, S.; Trojano, G.; Barbera, V.; Herranz, B.; Colli, E. The frequency of endometriosis in the general and selected populations: A systematic review. J. Endometr. Pelvic Pain Disord. 2020, 12, 176–189. [Google Scholar] [CrossRef]
- Alkatout, I.; Mettler, L.; Beteta, C.; Hedderich, J.; Jonat, W.; Schollmeyer, T.; Salmassi, A. Combined Surgical and Hormone Therapy for Endometriosis is the Most Effective Treatment: Prospective, Randomized, Controlled Trial. J. Minim. Invasive Gynecol. 2013, 20, 473–481. [Google Scholar] [CrossRef]
- Hirsch, M.; Dhillon-Smith, R.; Cutner, A.; Yap, M.; Creighton, S.M. The prevalence of endometriosis in adolescents with pelvic pain: A systematic review. J. Pediatric Adolesc. Gynecol. 2020. [Google Scholar] [CrossRef]
- Giampaolino, P.; Della Corte, L.; Saccone, G.; Vitagliano, A.; Bifulco, G.; Calagna, G.; Carugno, J.; Sardo, A.D.S. Role of Ovarian Suspension in Preventing Postsurgical Ovarian Adhesions in Patients with Stage III-IV Pelvic Endometriosis: A Systematic Review. J. Minim. Invasive Gynecol. 2019, 26, 53–62. [Google Scholar] [CrossRef]
- Agarwal, S.K.; Chapron, C.; Giudice, L.C.; Laufer, M.R.; Leyland, N.; Missmer, S.A.; Singh, S.; Taylor, H.S. Clinical diagnosis of endometriosis: A call to action. Am. J. Obs. Gynecol. 2019, 220, 354.e1–354.e12. [Google Scholar] [CrossRef] [Green Version]
- Andres, M.P.; Borrelli, G.M.; Abrão, M.S. Endometriosis classification according to pain symptoms: Can the ASRM classification be improved? Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 51, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, M.; Akira, S.; Kaseki, H.; Watanabe, K.; Ono, S.; Takeshita, T. Accuracy and clinical value of an adhesion scoring system: A preoperative diagnostic method using transvaginal ultrasonography for endometriotic adhesion. J. Obstet. Gynaecol. Res. 2020, 46, 466–478. [Google Scholar] [CrossRef] [PubMed]
- Canis, M.; Donnez, J.G.; Guzick, D.S.; Halme, J.K.; Rock, J.A.; Schenken, R.S.; Vernon, M.W. Revised american society for reproductive medicine classification of endometriosis: 1996. Fertil. Steril. 1997, 67, 817–821. [Google Scholar]
- Johnson, N.P.; Hummelshoj, L.; Adamson, G.D.; Keckstein, J.; Taylor, H.S.; Abrao, M.S.; Bush, D.; Kiesel, L.; Tamimi, R.; Sharpe-Timms, K.L.; et al. World Endometriosis Society consensus on the classification of endometriosis. Hum. Reprod. 2017, 32, 315–324. [Google Scholar] [CrossRef]
- Maul, L.V.; Morrision, J.E.; Schollmeyer, T.; Alkatout, I.; Mettler, L. Surgical Therapy of Ovarian Endometrioma: Recurrence and Pregnancy Rates. JSLS J. Soc. Laparoendosc. Surg. 2014, 18, 18. [Google Scholar] [CrossRef] [Green Version]
- Johnson, N.P.; Hummelshoj, L.; World Endometriosis Society Montpellier Consortium; Abrao, M.S.; Adamson, G.D.; Allaire, C.; Amelung, V.; Andersson, E.; Becker, C.; Árdal, K.B.B.; et al. Consensus on current management of endometriosis. Hum. Reprod. 2013, 28, 1552–1568. [Google Scholar] [CrossRef]
- Buţureanu, S.; Buţureanu, T. Pathophysiology of adhesions. Chirurgia (Bucharest Romania 1990) 2014, 109, 293–298. [Google Scholar]
- Mettler, L.; Schollmeyer, T.; Alkatout, I. Adhesions during and after Surgical Procedures, Their Prevention and Impact on Women’s Health; SAGE Publications Sage UK: London, UK, 2012. [Google Scholar]
- Koninckx, P.R.; Ussia, A.; Tahlak, M.; Adamyan, L.; Wattiez, A.; Martin, D.C.; Gomel, V. Infection as a potential cofactor in the genetic-epigenetic pathophysiology of endometriosis: a systematic review. Factsviews Vis. Obgyn 2019, 11, 209–216. [Google Scholar]
- Abd El-Kader, A.I.; Gonied, A.S.; Mohamed, M.L.; Mohamed, S.L. Impact of endometriosis-related adhesions on quality of life among infertile women. Int. J. Fertil. Steril. 2019, 13, 72. [Google Scholar]
- Menzies, D.; Ellis, H. Intestinal obstruction from adhesions–How big is the problem? Ann. R. Coll. Surg. Engl. 1990, 72, 60. [Google Scholar]
- Turkgeldi, L.; Cutner, A.; Turkgeldi, E.; Al Chami, A.; Cassoni, A.; Macdonald, N.; Mould, T.; Nichol, A.; Olaitan, A.; Saridogan, E. Laparoscopic Ovarian Transposition and Ovariopexy for Fertility Preservation in Patients Treated with Pelvic Radiotherapy with or without Chemotherapy. Factsviews Vis. Obgyn 2019, 11, 235–242. [Google Scholar]
- Ray, G.R.; Trueblood, H.W.; Enright, L.P.; Kaplan, H.S.; Nelsen, T.S. Oophoropexy: A means of preserving ovarian function following pelvic megavoltage radiotherapy for Hodgkin’s disease. Radiology 1970, 96, 175–180. [Google Scholar] [CrossRef]
- McCall, M.L.; Keaty, E.C.; Thompson, J.D. Conservation of ovarian tissue in the treatment of carcinoma of the cervix with radical surgery. Am. J. Obstet. Gynecol. 1958, 75, 590–600. [Google Scholar] [CrossRef]
- Sasaki, K.J.; Miller, C.E. Adnexal torsion: Review of the literature. J. Minim. Invasive Gynecol. 2014, 21, 196–202. [Google Scholar] [CrossRef]
- Seracchioli, R.; Di Donato, N.; Bertoldo, V.; La Marca, A.; Vicenzi, C.; Zannoni, L.; Villa, G.; Monti, G.; Leonardi, D.; Giovanardi, G.; et al. The Role of Ovarian Suspension in Endometriosis Surgery: A Randomized Controlled Trial. J. Minim. Invasive Gynecol. 2014, 21, 1029–1035. [Google Scholar] [CrossRef]
- Abuzeid, O.M.; Hebert, J.; Ashraf, M.; Mitwally, M.; Diamond, M.P.; Abuzeid, M.I. Safety and efficacy of two techniques of temporary ovarian suspension to the anterior abdominal wall after operative laparoscopy. Factsviews Vis. Obgyn 2018, 10, 71. [Google Scholar] [CrossRef]
- Chapman, L.; Sharma, M.; Papalampros, P.; Gambadauro, P.; Polyzos, D.; Papadopoulos, N.; Magos, A. A new technique for temporary ovarian suspension. Am. J. Obs. Gynecol. 2007, 196, 494.e1–494.e3. [Google Scholar] [CrossRef]
- Cutner, A.S.; Lazanakis, M.S.; Saridogan, E. Laparoscopic ovarian suspension to facilitate surgery for advanced endometriosis. Fertil. Steril. 2004, 82, 702–704. [Google Scholar] [CrossRef]
- Dehbashi, Z.; Khazali, S.; Tanha, F.D.; Mottahedian, F.; Ghajarzadeh, M.; Sadeh, S.S.; Kamali, K. Effectiveness of ovarian suspension in preventing postoperative ovarian adhesions in patients with severe pelvic endometriosis—A case-control study. Gynecol. Surg. 2019, 16, 1–7. [Google Scholar] [CrossRef]
- Poncelet, C.; Ducarme, G.; Yazbeck, C.; Madelenat, P.; Carbonnel, M. Safety of transient abdominal ovariopexy in patients with severe endometriosis. Int. J. Gynecol. Obstet. 2012, 118, 120–2. [Google Scholar] [CrossRef]
- Carbonnel, M.; Ducarme, G.; Dessapt, A.-L.; Yazbeck, C.; Hugues, J.-N.; Madelenat, P.; Poncelet, C. Efficacy of transient abdominal ovariopexy in patients with severe endometriosis. Eur. J. Obs. Gynecol. Reprod. Biol. 2011, 155, 183–187. [Google Scholar] [CrossRef]
- Ouahba, J.; Madelenat, P.; Poncelet, C. Transient abdominal ovariopexy for adhesion prevention in patients who underwent surgery for severe pelvic endometriosis. Fertil. Steril. 2004, 82, 1407–1411. [Google Scholar] [CrossRef]
- Alkatout, I.; Mettler, L.; Maass, N.; Noé, G.-K.; Elessawy, M. Abdominal anatomy in the context of port placement and trocars. J. Turk. Ger. Gynecol. Assoc. 2015, 16, 241. [Google Scholar] [CrossRef]
- Hoo, W.-L.; Saridogan, E.; Cutner, A.; Pandis, G.; Jurkovic, D. Effectiveness of ovarian suspension in preventing post-operative ovarian adhesions in women with pelvic endometriosis: A randomised controlled trial. Bmc Women’s Health 2011, 11, 14. [Google Scholar] [CrossRef] [Green Version]
- Pellicano, M.; Giampaolino, P.; Tommaselli, G.A.; Catena, U.; Nappi, C.; Bifulco, G. Efficacy of ovarian suspension to round ligament with a resorbable suture to prevent postoperative adhesions in women with ovarian endometrioma: Follow-up by transvaginal hydrolaparoscopy. Gynecol. Surg. 2014, 11, 261–266. [Google Scholar] [CrossRef] [Green Version]
- Redwine, D. Laparoscopic ovarian suspension (LOS). Fertil. Steril. 2001, 76, S105. [Google Scholar] [CrossRef]
- Abuzeid, M.I.; Ashraf, M.; Shamma, F.N. Temporary ovarian suspension at laparoscopy for prevention of adhesions. J. Am. Assoc. Gynecol. Laparosc. 2002, 9, 98–102. [Google Scholar] [CrossRef]
- Abuzeid, O.M.; Raju, R.; Hebert, J.; Ashraf, M.; Abuzeid, M.I. A modified technique of temporary suspension of the ovary to the anterior abdominal wall. J. Minim. Invasive Gynecol. 2018, 25, 26–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trehan, A.; Trehan, A.K. Ovarian suspension for longer than 36 h is necessary for temporary ovarian suspension to fulfil its remit. Hum. Reprod. 2014, 29, 1831–1832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Wilde, R.; Alvarez, J.; Brölmann, H.; Campo, R.; Cheong, Y. Prevention of Adhesions in Gynecological Surgery: The 2016 Experts Recommendations on Adhesion Prophylaxis. Gynecol Obs. (Sunnyvale) 2017, 7, 2161. [Google Scholar]
- De Wilde, R.L.; Brölmann, H.; Koninckx, P.R.; Lundorff, P.; Lower, A.M.; Wattiez, A.; Mara, M.; Wallwiener, M.; The Anti-Adhesions in Gynecology Expert Panel (ANGEL). Prevention of adhesions in gynaecological surgery: The 2012 European field guideline. Gynecol. Surg. 2012, 9, 365–368. [Google Scholar] [CrossRef] [Green Version]
- Delara, R.; Wasson, M. Temporary oophoropexy and uteropexy during gynecologic surgery. Am. J. Obs. Gynecol. 2020. [Google Scholar] [CrossRef]
- Surgeons PCotASfRMicwtSoR. Pathogenesis, consequences, and control of peritoneal adhesions in gynecologic surgery: a committee opinion. Fertil. Steril. 2013, 99, 1550–1555. [Google Scholar] [CrossRef]
- Wallwiener, M.; Koninckx, P.R.; Hackethal, A.; Brölmann, H.; Lundorff, P.; Mara, M.; Wattiez, A.; De Wilde, R.L.; The Anti-Adhesions in Gynecology Expert Panel (ANGEL). A European survey on awareness of post-surgical adhesions among gynaecological surgeons. Gynecol. Surg. 2014, 11, 105–112. [Google Scholar] [CrossRef] [Green Version]
- Schreinemacher, M.H.; Richard, P.; Bakkum, E.A.; van Goor, H.; Bouvy, N.D. Adhesion awareness: A national survey of surgeons. World J. Surg. 2010, 34, 2805–2812. [Google Scholar] [CrossRef] [Green Version]
- Alruwaili, A.; Alosaimi, M.; Alnahas, T.M.; Alferayan, T.A.; Alsulami, G.O.; Alhulaybi, A.; Alsulaiman, A.A.; Habtar, H.S.; Alqarni, S.A.; Alqhtani, H.M.; et al. Adhesion Awareness among Saudi Surgeons: A National Survey. Int. J. Pharm. Res. Allied Sci. 2020, 9, 75–83. [Google Scholar]
- La Roche, L.A.T.-D.; Devassy, R.; De Wilde, M.S.; Cezar, C.; Krentel, H.; Korell, M.; De Wilde, R.L. A new approach to avoid ovarian failure as well function-impairing adhesion formation in endometrioma infertility surgery. Arch. Gynecol. Obs. 2020, 301, 1113–1115. [Google Scholar] [CrossRef]
- Dizerega, G.S.; Campeau, J.D. Peritoneal repair and post-surgical adhesion formation. Hum. Reprod. Update 2001, 7, 547–555. [Google Scholar] [CrossRef]
- Pergialiotis, V.; Prodromidou, A.; Karampetsou, N.; Diamantopoulos, M.; Perrea, D.; Nikiteas, N. Ovarian suspension for adhesion prevention during laparoscopic excision of severe pelvic endometriosis and endometrioma excision: A systematic review. Gynecol. Surg. 2016, 13, 445–450. [Google Scholar] [CrossRef]
- Okaro, E.; Condous, G.; Khalid, A.; Timmerman, D.; Ameye, L.; Van Huffel, S.; Bourne, T. The use of ultrasound-based ‘soft markers’ for the prediction of pelvic pathology in women with chronic pelvic pain—can we reduce the need for laparoscopy? Bjog: Int. J. Obstet. Gynaecol. 2006, 113, 251–256. [Google Scholar] [CrossRef]
- Hoo, W.L.; Stavroulis, A.; Pateman, K.; Saridogan, E.; Cutner, A.; Pandis, G.; Tong, E.N.; Jurkovic, D. Does ovarian suspension following laparoscopic surgery for endometriosis reduce postoperative adhesions? An RCT. Hum. Reprod. 2014, 29, 670–676. [Google Scholar] [CrossRef] [Green Version]




| Author | Year | Ovariopexy Site | Suture | Day of Suspension Removal |
|---|---|---|---|---|
| Redwine | 2001 | ipsilateral round ligament | vicryl 3-0 | N/A |
| Abuzeid | 2002 | anterior abdominal wall * | polypropylene 3-0 | 5th–7th |
| Oubha | 2004 | anterior abdominal wall * | prolene 3-0 | 4th |
| Chapman | 2007 | Both ovaries together, in front of the uterus, on the anterior abdominal wall | Vicryl 2-0 | Immediately ater surgery |
| Carbonnel | 2011 | anterior abdominal wall * | prolene 0,mersuture | 5th |
| Hoo | 2011 | anterior abdominal wall * | prolene | 36–48 h |
| Poncelet | 2012 | anterior abdominal wall * | prolene-0 | 5th |
| Seracchioli | 2014 | anterolateral abdominal wall * | vicryl 2-0 | no |
| Pellicano | 2014 | ipsilateral round ligament | vicryl rapid 2-0 | N/A |
| Abuzeid | 2018 | group1- fascia of anterior abdominal wall *; group 2-anterior abdominal wall * | group 1-plain catgut 3-0; group 2-nylon 3-0 | group 2–5th–7th |
| Dehbashi | 2019 | anterior abdominal wall * | prolene | 7th |
| Author | Year | Postoperative Adhesions Formation | Pain at Surgical Site | Fertility (Clinical Pregnancy Rate) | Complications |
|---|---|---|---|---|---|
| Redwine | 2001 | 0%(3 patients) | None | ||
| Abuzeid | 2002 | 20% (1/5)(minimal adhesions) | 45%(9/20) | None | |
| Oubha | 2004 | 58.3%(7/12)(flimsy+mild adhseions) | 53.3%(8/15) | None | |
| Carbonnel | 2011 | 50% | 55%(58/105) | 0.60% | |
| Hoo | 2011 | 68.8%(11/16) | None | ||
| Poncelet | 2012 | No differencein oocyte retrieval rate, pulsatility index, and antral follicle count between suspended and non-suspended ovaries. | 0.70% | ||
| Seracchioli | 2014 | 37.7% vs. 77.2%(17/45 vs. 34/44)(moderate and severe adhesion)(suspended vs. non-suspended) | No difference between the suspended and non suspended site | None | |
| Pellicano | 2014 | 33.3%(8/24)vs. 80.8%(21/26) (suspended vs. non-suspended) | No difference between the suspended and non suspended site | None | |
| Abuzeid | 2018 | 0%(both groups) | 41.00% | None | |
| Dehbashi | 2019 | 18%vs. 76%(9/50 vs. 38/50)(moderate adhesions; suspended vs. non-suspended) | 19% vs. 22%(suspended vs. non-suspended) | None |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhanawat, J.; Pape, J.; Freytag, D.; Maass, N.; Alkatout, I. Ovariopexy—Before and after Endometriosis Surgery. Biomedicines 2020, 8, 533. https://doi.org/10.3390/biomedicines8120533
Dhanawat J, Pape J, Freytag D, Maass N, Alkatout I. Ovariopexy—Before and after Endometriosis Surgery. Biomedicines. 2020; 8(12):533. https://doi.org/10.3390/biomedicines8120533
Chicago/Turabian StyleDhanawat, Juhi, Julian Pape, Damaris Freytag, Nicolai Maass, and Ibrahim Alkatout. 2020. "Ovariopexy—Before and after Endometriosis Surgery" Biomedicines 8, no. 12: 533. https://doi.org/10.3390/biomedicines8120533
APA StyleDhanawat, J., Pape, J., Freytag, D., Maass, N., & Alkatout, I. (2020). Ovariopexy—Before and after Endometriosis Surgery. Biomedicines, 8(12), 533. https://doi.org/10.3390/biomedicines8120533