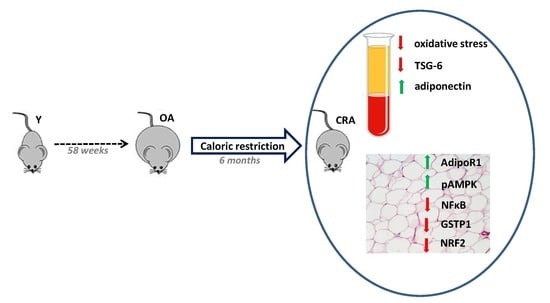
Antioxidant/Anti-Inflammatory Effects of Caloric Restriction in an Aged and Obese Rat Model: The Role of Adiponectin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Measurement of Plasma Oxidative Status
2.3. Western Blot and Densitometric Analysis
2.4. Statistical Analysis
3. Result
3.1. Effects of CR on Obesity and Plasmatic Oxidative Balance
3.2. Effects of CR on Plasmatic Adiponectin Levels and Inflammation Markers
3.3. Effects of CR on AdipoR1–pAMPK–NFκB Pathway in Adipose Tissue
3.4. Effects of CR on Antioxidant Enzymes in Adipose Tissue
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Jones, A.R.; Tovee, M.J.; Cutler, L.R.; Parkinson, K.N.; Ells, L.J.; Araujo-Soares, V.; Pearce, M.S.; Mann, K.D.; Scott, D.; Harris, J.M.; et al. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. Yearb. Paediatr. Endocrinol. 2018. [Google Scholar] [CrossRef]
- Cattaneo, A.; Monasta, L.; Stamatakis, E.; Lioret, S.; Castetbon, K.; Frenken, F.; Manios, Y.; Moschonis, G.; Savva, S.; Zaborskis, A.; et al. Overweight and obesity in infants and pre-school children in the European Union: A review of existing data. Obes. Rev. 2010, 11, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Salvestrini, V.; Sell, C.; Lorenzini, A. Obesity may accelerate the aging process. Front. Endocrinol. (Lausanne) 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Jura, M.; Kozak, L.P. Obesity and related consequences to ageing. Age (Omaha) 2016, 38. [Google Scholar] [CrossRef] [Green Version]
- Harman, D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef] [Green Version]
- Remigante, A.; Morabito, R.; Marino, A. Natural antioxidants beneficial effects on anion exchange through band 3 protein in human erythrocytes. Antioxidants 2020, 9, 25. [Google Scholar] [CrossRef] [Green Version]
- Barja, G. Free radicals and aging. Trends Neurosci. 2004, 27, 595–600. [Google Scholar] [CrossRef]
- Gutteridge, J.M. Free radicals and aging. Rev. Clin. Gerontol. 1994, 4, 279–288. [Google Scholar] [CrossRef] [Green Version]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [Green Version]
- Pérez, V.I.; Van Remmen, H.; Bokov, A.; Epstein, C.J.; Vijg, J.; Richardson, A. The overexpression of major antioxidant enzymes does not extend the lifespan of mice. Aging Cell 2009, 8, 73–75. [Google Scholar] [CrossRef] [Green Version]
- Mele, J.; Van Remmen, H.; Vijg, J.; Richardson, A. Characterization of transgenic mice that overexpress both copper zinc superoxide dismutase and catalase. Antioxidants Redox Signal. 2006, 8, 628–638. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Ottaviani, E.; Olivieri, F.; De Benedictis, G.; Bonafè, M.; De Luca, M.; Valensin, S. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Vasto, S.; Candore, G.; Balistreri, C.R.; Caruso, M.; Colonna-Romano, G.; Grimaldi, M.P.; Listi, F.; Nuzzo, D.; Lio, D.; Caruso, C. Inflammatory networks in ageing, age-related diseases and longevity. Mech. Ageing Dev. 2007, 128, 83–91. [Google Scholar] [CrossRef]
- Fuente, M.; Miquel, J. An Update of the Oxidation-Inflammation Theory of Aging: The Involvement of the Immune System in Oxi-Inflamm-Aging. Curr. Pharm. Des. 2009, 15, 3003–3026. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente, M. Editorial crosstalk between the nervous and the immune systems in health and sickness. Curr. Pharm. Des. 2014, 20, 4605–4607. [Google Scholar] [CrossRef]
- Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity: Implications for metabolic syndrome, diabetes, hypertension, dyslipidemia, atherosclerosis, and cancer. Obes. Res. Clin. Pract. 2013, 7. [Google Scholar] [CrossRef]
- Manna, P.; Jain, S.K. Obesity, Oxidative Stress, Adipose Tissue Dysfunction, and the Associated Health Risks: Causes and Therapeutic Strategies. Metab. Syndr. Relat. Disord. 2015, 13, 423–444. [Google Scholar] [CrossRef] [Green Version]
- Marseglia, L.; Manti, S.; D’Angelo, G.; Nicotera, A.; Parisi, E.; Di Rosa, G.; Gitto, E.; Arrigo, T. Oxidative stress in obesity: A critical component in human diseases. Int. J. Mol. Sci. 2015, 16, 378–400. [Google Scholar] [CrossRef] [Green Version]
- Furukawa, S.; Matsuda, M.; Shimomura, I.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 2017, 114, 1752–1761. [Google Scholar] [CrossRef]
- Włodarczyk, M.; Nowicka, G. Obesity, DNA damage, and development of obesity-related diseases. Int. J. Mol. Sci. 2019, 20, 1146. [Google Scholar] [CrossRef]
- Dludla, P.V.; Nkambule, B.B.; Jack, B.; Mkandla, Z.; Mutize, T.; Silvestri, S.; Orlando, P.; Tiano, L.; Louw, J.; Mazibuko-Mbeje, S.E. Inflammation and oxidative stress in an obese state and the protective effects of gallic acid. Nutrients 2019, 11, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, H.; Judd, R.L. Adiponectin regulation and function. Compr. Physiol. 2018, 8, 1031–1063. [Google Scholar] [CrossRef] [PubMed]
- Naimo, G.D.; Gelsomino, L.; Catalano, S.; Mauro, L.; Andò, S. Interfering Role of ERα on Adiponectin Action in Breast Cancer. Front. Endocrinol. (Lausanne) 2020, 11, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andò, S.; Naimo, G.D.; Gelsomino, L.; Catalano, S.; Mauro, L. Novel insights into adiponectin action in breast cancer: Evidence of its mechanistic effects mediated by ERα expression. Obes. Rev. 2020, 21. [Google Scholar] [CrossRef]
- Li, J.-B.; Nishida, M.; Kaimoto, K.; Asakawa, A.; Chaolu, H.; Cheng, K.-C.; Li, Y.-X.; Terashi, M.; Koyama, K.I.; Amitani, H.; et al. Effects of aging on the plasma levels of nesfatin-1 and adiponectin. Biomed. Rep. 2014, 2, 152–156. [Google Scholar] [CrossRef]
- Ukkola, O.; Santaniemi, M. Adiponectin: A link between excess adiposity and associated comorbidities? J. Mol. Med. 2002, 80, 696–702. [Google Scholar] [CrossRef]
- Chung, K.W.; Kim, D.H.; Park, M.H.; Choi, Y.J.; Kim, N.D.; Lee, J.; Yu, B.P.; Chung, H.Y. Recent advances in calorie restriction research on aging. Exp. Gerontol. 2013, 48, 1049–1053. [Google Scholar] [CrossRef]
- Chujo, Y.; Fujii, N.; Okita, N.; Konishi, T.; Narita, T.; Yamada, A.; Haruyama, Y.; Tashiro, K.; Chiba, T.; Shimokawa, I.; et al. Caloric restriction-Associated remodeling of rat white adipose tissue: Effects on the growth hormone/insulin-like growth factor-1 axis, sterol regulatory element binding protein-1, and macrophage infiltration. Age (Omaha) 2013, 35, 1143–1156. [Google Scholar] [CrossRef]
- Fujii, N.; Narita, T.; Okita, N.; Kobayashi, M.; Furuta, Y.; Chujo, Y.; Sakai, M.; Yamada, A.; Takeda, K.; Konishi, T.; et al. Sterol regulatory element-binding protein-1c orchestrates metabolic remodeling of white adipose tissue by caloric restriction. Aging Cell 2017, 16, 508–517. [Google Scholar] [CrossRef]
- Redman, L.M.; Smith, S.R.; Burton, J.H.; Martin, C.K.; Il’yasova, D.; Ravussin, E. Metabolic Slowing and Reduced Oxidative Damage with Sustained Caloric Restriction Support the Rate of Living and Oxidative Damage Theories of Aging. Cell Metab. 2018, 27, 805–815.e4. [Google Scholar] [CrossRef] [Green Version]
- Calment, J.L. Aging, Adiposity, and Calorie Restriction. JAMA 2015, 297, 986–994. [Google Scholar]
- Savas, H.B. Positive effects of meal frequency and calorie restriction on antioxidant systems in rats. Nothern Clin. Istanbul 2017. [Google Scholar] [CrossRef] [PubMed]
- Brunelli, E.; Domanico, F.; La Russa, D.; Pellegrino, D. Sex differences in oxidative stress biomarkers. Curr. Drug Targets 2014, 15. [Google Scholar] [CrossRef] [PubMed]
- La Russa, D.; Brunelli, E.; Pellegrino, D. Oxidative imbalance and kidney damage in spontaneously hypertensive rats: Activation of extrinsic apoptotic pathways. Clin. Sci. 2017, 131, 1419–1428. [Google Scholar] [CrossRef] [PubMed]
- Brunelli, E.; La Russa, D.; Pellegrino, D. Impaired oxidative status is strongly associated with cardiovascular risk factors. Oxid. Med. Cell. Longev. 2017, 2017. [Google Scholar] [CrossRef] [Green Version]
- La Russa, D.; Giordano, F.; Marrone, A.; Parafati, M.; Janda, E.; Pellegrino, D. Oxidative Imbalance and Kidney Damage in Cafeteria Diet-Induced Rat Model of Metabolic Syndrome: Effect of Bergamot Polyphenolic Fraction. Antioxidants 2019, 8, 66. [Google Scholar] [CrossRef] [Green Version]
- Russa, D.L.; Pellegrino, D.; Montesanto, A.; Gigliotti, P.; Perri, A.; Russa, A.L.; Bonofiglio, R. Oxidative Balance and Inflammation in Hemodialysis Patients: Biomarkers of Cardiovascular Risk? Oxid. Med. Cell. Longev. 2019, 2019, 1–7. [Google Scholar] [CrossRef]
- Pellegrino, D.; La Russa, D.; Marrone, A. Oxidative imbalance and kidney damage: New study perspectives from animal models to hospitalized patients. Antioxidants 2019, 8, 594. [Google Scholar] [CrossRef] [Green Version]
- Novelli, E.L.B.; Diniz, Y.S.; Galhardi, C.M.; Ebaid, G.M.X.; Rodrigues, H.G.; Mani, F.; Fernandes, A.A.H.; Cicogna, A.C.; Novelli Filho, J.L.V.B. Anthropometrical parameters and markers of obesity in rats. Lab. Anim. 2007, 41, 111–119. [Google Scholar] [CrossRef] [Green Version]
- Kuk, J.L.; Saunders, T.J.; Davidson, L.E.; Ross, R. Age-related changes in total and regional fat distribution. Ageing Res. Rev. 2009, 8, 339–348. [Google Scholar] [CrossRef]
- Huffman, D.M.; Barzilai, N. Role of visceral adipose tissue in aging. Biochim. Biophys. Acta Gen. Subj. 2009, 1790, 1117–1123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milner, C.M.; Day, A.J. TSG-6: A multifunctional protein associated with inflammation. J. Cell Sci. 2003, 116, 1863–1873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engin, A. Adiponectin-resistance in obesity. Adv. Exp. Med. Biol. 2017, 960, 415–441. [Google Scholar] [CrossRef] [PubMed]
- Koh, S.J.; Hyun, Y.J.; Choi, S.Y.; Chae, J.S.; Kim, J.Y.; Park, S.; Ahn, C.M.; Jang, Y.; Lee, J.H. Influence of age and visceral fat area on plasma adiponectin concentrations in women with normal glucose tolerance. Clin. Chim. Acta 2008, 389, 45–50. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R. Adipocytokines: Mediators linking adipose tissue, inflammation and immunity. Nat. Rev. Immunol. 2006, 6, 772–783. [Google Scholar] [CrossRef]
- Ouchi, N.; Walsh, K. Adiponectin as an anti-inflammatory factor. Clin. Chim. Acta 2007, 380, 24–30. [Google Scholar] [CrossRef] [Green Version]
- Piao, C.; Park, J.H.; Lee, M. Anti-Inflammatory Therapeutic Effect of Adiponectin Gene Delivery Using a Polymeric Carrier in an Acute Lung Injury Model. Pharm. Res. 2017, 34, 1517–1526. [Google Scholar] [CrossRef]
- Lettieri-Barbato, D.; Giovannetti, E.; Aquilano, K. Effects of dietary restriction on adipose mass and biomarkers of healthy aging in human. Aging (Albany N.Y.) 2016, 8, 3341–3355. [Google Scholar] [CrossRef] [Green Version]
- Most, J.; Tosti, V.; Redman, L.M.; Fontana, L. Calorie restriction in humans: An update. Ageing Res. Rev. 2017, 39, 36–45. [Google Scholar] [CrossRef]
- Frühbeck, G.; Catalán, V.; Rodríguez, A.; Ramírez, B.; Becerril, S.; Salvador, J.; Portincasa, P.; Colina, I.; Gómez-Ambrosi, J. Involvement of the leptin-adiponectin axis in inflammation and oxidative stress in the metabolic syndrome. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Gómez-Ambrosi, J.; Catalán, V.; Rodríguez, A.; Andrada, P.; Ramírez, B.; Ibáñez, P.; Vila, N.; Romero, S.; Margall, M.A.; Gil, M.J.; et al. Increased cardiometabolic risk factors and inflammation in adipose tissue in obese subjects classified as metabolically healthy. Diabetes Care 2014, 37, 2813–2821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandrasekar, B.; Boylston, W.H.; Venkatachalam, K.; Webster, N.J.G.; Prabhu, S.D.; Valente, A.J. Adiponectin blocks interleukin-18-mediated endothelial cell death via APPL1-dependent AMP-activated protein kinase (AMPK) activation and IKK/NF-κB/PTEN suppression. J. Biol. Chem. 2008, 283, 24889–24898. [Google Scholar] [CrossRef] [Green Version]
- Steinberg, G.R.; Kemp, B.E. AMPK in health and disease. Physiol. Rev. 2009, 89, 1025–1078. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liu, Y.; Yang, W.; Yuan, J.; Mo, Z. Adiponectin inhibits NLRP3 inflammasome by modulating the AMPK-ROS pathway. Int. J. Clin. Exp. Pathol. 2018, 11, 3338–3347. [Google Scholar] [PubMed]
- Yanar, K.; Simsek, B.; Çaylı, N.; Övül Bozkır, H.; Mengi, M.; Belce, A.; Aydin, S.; Çakatay, U. Caloric restriction and redox homeostasis in various regions of aging male rat brain: Is caloric restriction still worth trying even after early-adulthood?: Redox homeostasis and caloric restriction in brain. J. Food Biochem. 2019, 43. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
La Russa, D.; Marrone, A.; Mandalà, M.; Macirella, R.; Pellegrino, D. Antioxidant/Anti-Inflammatory Effects of Caloric Restriction in an Aged and Obese Rat Model: The Role of Adiponectin. Biomedicines 2020, 8, 532. https://doi.org/10.3390/biomedicines8120532
La Russa D, Marrone A, Mandalà M, Macirella R, Pellegrino D. Antioxidant/Anti-Inflammatory Effects of Caloric Restriction in an Aged and Obese Rat Model: The Role of Adiponectin. Biomedicines. 2020; 8(12):532. https://doi.org/10.3390/biomedicines8120532
Chicago/Turabian StyleLa Russa, Daniele, Alessandro Marrone, Maurizio Mandalà, Rachele Macirella, and Daniela Pellegrino. 2020. "Antioxidant/Anti-Inflammatory Effects of Caloric Restriction in an Aged and Obese Rat Model: The Role of Adiponectin" Biomedicines 8, no. 12: 532. https://doi.org/10.3390/biomedicines8120532
APA StyleLa Russa, D., Marrone, A., Mandalà, M., Macirella, R., & Pellegrino, D. (2020). Antioxidant/Anti-Inflammatory Effects of Caloric Restriction in an Aged and Obese Rat Model: The Role of Adiponectin. Biomedicines, 8(12), 532. https://doi.org/10.3390/biomedicines8120532