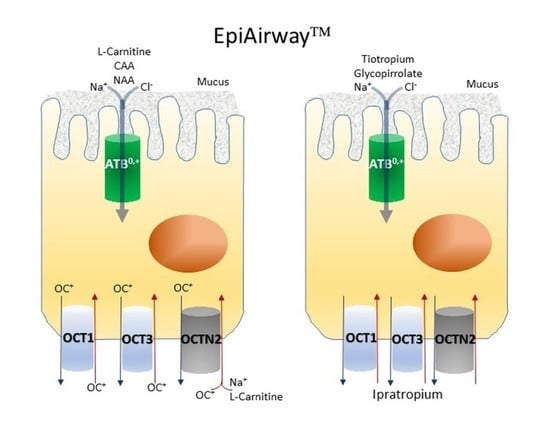
Organic Cation Transporters (OCTs) in EpiAirway™, a Cellular Model of Normal Human Bronchial Epithelium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Cultures
2.2. MPP+ Uptake
2.3. RT-qPCR Analysis
2.4. Western Blot Analysis
2.5. Statistical Analysis
2.6. Materials
3. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Ciarimboli, G. Organic cation transporters. Xenobiotica 2008, 38, 936–971. [Google Scholar] [CrossRef] [PubMed]
- Nigam, S.K. The SLC22 Transporter Family: A Paradigm for the Impact of Drug Transporters on Metabolic Pathways, Signaling, and Disease. Annu. Rev. Pharmacol. Toxicol. 2018, 58, 663–687. [Google Scholar] [CrossRef] [PubMed]
- Engelhart, D.C.; Granados, J.C.; Shi, D.; Saier, M.H., Jr.; Baker, M.E.; Abagyan, R.; Nigam, S.K. Systems Biology Analysis Reveals Eight SLC22 Transporter Subgroups, Including OATs, OCTs, and OCTNs. Int. J. Mol. Sci. 2020, 21, 1791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pelis, R.M.; Wright, S.H. SLC22, SLC44, and SLC47 transporters--organic anion and cation transporters: Molecular and cellular properties. Curr. Top. Membr. 2014, 73, 233–261. [Google Scholar]
- Koepsell, H.; Lips, K.; Volk, C. Polyspecific organic cation transporters: Structure, function, physiological roles, and biopharmaceutical implications. Pharm. Res. 2007, 24, 1227–1251. [Google Scholar] [CrossRef]
- Furubayashi, T.; Inoue, D.; Nishiyama, N.; Tanaka, A.; Yutani, R.; Kimura, S.; Katsumi, H.; Yamamoto, A.; Sakane, T. Comparison of Various Cell Lines and Three-Dimensional Mucociliary Tissue Model Systems to Estimate Drug Permeability Using an In Vitro Transport Study to Predict Nasal Drug Absorption in Rats. Pharmaceutics 2020, 12, 79. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.K.; Wolff, N.A.; Thevenod, F. Organic cation transporters: Physiology, toxicology and special focus on ethidium as a novel substrate. Curr. Drug Metab. 2009, 10, 617–631. [Google Scholar] [CrossRef]
- Nies, A.T.; Koepsell, H.; Damme, K.; Schwab, M. Organic cation transporters (OCTs, MATEs), in vitro and in vivo evidence for the importance in drug therapy. In Drug Transporters. Handbook of Experimental Pharmacology; Fromm, M., Kim, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; Volume 201, pp. 105–167. [Google Scholar]
- Koepsell, H. Organic Cation Transporters in Health and Disease. Pharmacol. Rev. 2020, 72, 253–319. [Google Scholar] [CrossRef]
- Drozdzik, M.; Busch, D.; Lapczuk, J.; Muller, J.; Ostrowski, M.; Kurzawski, M.; Oswald, S. Protein Abundance of Clinically Relevant Drug Transporters in the Human Liver and Intestine: A Comparative Analysis in Paired Tissue Specimens. Clin. Pharmacol. Ther. 2019, 105, 1204–1212. [Google Scholar] [CrossRef]
- Bosquillon, C. Drug transporters in the lung—Do they play a role in the biopharmaceutics of inhaled drugs? J. Pharm. Sci. 2010, 99, 2240–2255. [Google Scholar] [CrossRef]
- Gumbleton, M.; Al-Jayyoussi, G.; Crandon-Lewis, A.; Francombe, D.; Kreitmeyr, K.; Morris, C.J.; Smith, M.W. Spatial expression and functionality of drug transporters in the intact lung: Objectives for further research. Adv. Drug Deliv. Rev. 2011, 63, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Salomon, J.J.; Gausterer, J.C.; Yahara, T.; Hosoya, K.; Huwer, H.; Hittinger, M.; Schneider-Daum, N.; Lehr, C.M.; Ehrhardt, C. Organic cation transporter function in different in vitro models of human lung epithelium. Eur. J. Pharm. Sci. 2015, 80, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Salomon, J.J.; Endter, S.; Tachon, G.; Falson, F.; Buckley, S.T.; Ehrhardt, C. Transport of the fluorescent organic cation 4-(4-(dimethylamino)styryl)-N-methylpyridinium iodide (ASP+) in human respiratory epithelial cells. Eur. J. Pharm. Biopharm. 2012, 81, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Salomon, J.J.; Muchitsch, V.E.; Gausterer, J.C.; Schwagerus, E.; Huwer, H.; Daum, N.; Lehr, C.M.; Ehrhardt, C. The cell line NCl-H441 is a useful in vitro model for transport studies of human distal lung epithelial barrier. Mol. Pharm. 2014, 11, 995–1006. [Google Scholar] [CrossRef]
- Ingoglia, F.; Visigalli, R.; Rotoli, B.M.; Barilli, A.; Riccardi, B.; Puccini, P.; Dall’Asta, V. Functional characterization of the organic cation transporters (OCTs) in human airway pulmonary epithelial cells. Biochim. Biophys. Acta 2015, 1848, 1563–1572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forbes, B.; Ehrhardt, C. Human respiratory epithelial cell culture for drug delivery applications. Eur. J. Pharm. Biopharm. 2005, 60, 193–205. [Google Scholar] [CrossRef]
- Bi, Y.A.; Costales, C.; Mathialagan, S.; West, M.; Eatemadpour, S.; Lazzaro, S.; Tylaska, L.; Scialis, R.; Zhang, H.; Umland, J.; et al. Quantitative Contribution of Six Major Transporters to the Hepatic Uptake of Drugs: “SLC-Phenotyping” Using Primary Human Hepatocytes. J. Pharmacol. Exp. Ther. 2019, 370, 72–83. [Google Scholar] [CrossRef]
- Louisa, M.; Suyatna, F.D.; Wanandi, S.I.; Asih, P.B.; Syafruddin, D. Differential expression of several drug transporter genes in HepG2 and Huh-7 cell lines. Adv. Biomed. Res. 2016, 5, 104. [Google Scholar] [CrossRef]
- Gasser, P.J.; Lowry, C.A.; Orchinik, M. Corticosterone-sensitive monoamine transport in the rat dorsomedial hypothalamus: Potential role for organic cation transporter 3 in stress-induced modulation of monoaminergic neurotransmission. J. Neurosci. 2006, 26, 8758–8766. [Google Scholar] [CrossRef]
- Gazzola, G.C.; Dall’Asta, V.; Franchi-Gazzola, R.; White, M.F. The cluster-tray method for rapid measurement of solute fluxes in adherent cultured cells. Anal. Biochem. 1981, 115, 368–374. [Google Scholar] [CrossRef] [Green Version]
- Ingoglia, F.; Visigalli, R.; Rotoli, B.M.; Barilli, A.; Riccardi, B.; Puccini, P.; Dall’Asta, V. Functional activity of L-carnitine transporters in human airway epithelial cells. Biochim. Biophys. Acta 2016, 1858, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Rotoli, B.M.; Barilli, A.; Ingoglia, F.; Visigalli, R.; Bianchi, M.G.; Ferrari, F.; Martinelli, D.; Dionisi-Vici, C.; Dall’Asta, V. Analysis of LPI-causing mutations on y+LAT1 function and localization. Orphanet J. Rare Dis. 2019, 14, 63. [Google Scholar] [CrossRef] [PubMed]
- Rotoli, B.M.; Barilli, A.; Visigalli, R.; Ferrari, F.; Dall’Asta, V. y+LAT1 and y+LAT2 contribution to arginine uptake in different human cell models: Implications in the pathophysiology of Lysinuric Protein Intolerance. J. Cell. Mol. Med. 2020, 24, 921–929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ingoglia, F.; Visigalli, R.; Rotoli, B.M.; Barilli, A.; Riccardi, B.; Puccini, P.; Milioli, M.; Di Lascia, M.; Bernuzzi, G.; Dall’Asta, V. Human macrophage differentiation induces OCTN2-mediated L-carnitine transport through stimulation of mTOR-STAT3 axis. J. Leukoc. Biol. 2017, 101, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Braunlich, J.; Wirtz, H. Oral Versus Nasal High-Flow Bronchodilator Inhalation in Chronic Obstructive Pulmonary Disease. J. Aerosol. Med. Pulm. Drug Deliv. 2018, 31, 248–254. [Google Scholar]
- Cazzola, M.; Rogliani, P. Comparative effectiveness of indacaterol/glycopyrronium in the treatment of chronic obstructive pulmonary disease. J. Comp. Eff. Res. 2017, 6, 627–636. [Google Scholar] [CrossRef]
- Hamelmann, E. Managing Severe Asthma: A Role for the Long-Acting Muscarinic Antagonist Tiotropium. Biomed. Res. Int. 2018, 2018, 7473690. [Google Scholar] [CrossRef] [Green Version]
- Panduga, V.; Stocks, M.J.; Bosquillon, C. Ipratropium is ‘luminally recycled’ by an inter-play between apical uptake and efflux transporters in Calu-3 bronchial epithelial cell layers. Int. J. Pharm. 2017, 532, 328–336. [Google Scholar] [CrossRef]
- Teague, W.G. Tiotropium: An Effective Bronchodilator in Severe Asthma Independent of Type 2 Inflammation. J. Allergy Clin. Immunol. Pract. 2019, 7, 2296–2297. [Google Scholar] [CrossRef]
- Wang, F.; Zheng, H.; Zhang, Y.; Zhu, H.; Shi, J.; Luo, Y.; Zhang, X.; Mao, H.; Herth, F.J.F.; Luo, F. Nebulized Ipratropium bromide protects against tracheal and bronchial secretion during bronchoscopy: A randomized controlled trial. Medicine 2019, 98, e17942. [Google Scholar] [CrossRef]
- Rotoli, B.M.; Visigalli, R.; Barilli, A.; Ferrari, F.; Bianchi, M.G.; Di Lascia, M.; Riccardi, B.; Puccini, P.; Dall’Asta, V. Functional analysis of OCTN2 and ATB0,+ in normal human airway epithelial cells. PLoS ONE 2020, 15, e0228568. [Google Scholar]
- Lips, K.S.; Volk, C.; Schmitt, B.M.; Pfeil, U.; Arndt, P.; Miska, D.; Ermert, L.; Kummer, W.; Koepsell, H. Polyspecific cation transporters mediate luminal release of acetylcholine from bronchial epithelium. Am. J. Respir. Cell Mol. Biol. 2005, 33, 79–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rotoli, B.M.; Barilli, A.; Visigalli, R.; Ferrari, F.; Frati, C.; Lagrasta, C.A.; Lascia, M.D.; Riccardi, B.; Puccini, P.; Dall’Asta, V. Characterization of ABC Transporters in EpiAirway, a Cellular Model of Normal Human Bronchial Epithelium. Int. J. Mol. Sci. 2020, 21, 3190. [Google Scholar] [CrossRef] [PubMed]
- Galietta, L.J.; Musante, L.; Romio, L.; Caruso, U.; Fantasia, A.; Gazzolo, A.; Romano, L.; Sacco, O.; Rossi, G.A.; Varesio, L.; et al. An electrogenic amino acid transporter in the apical membrane of cultured human bronchial epithelial cells. Am. J. Physiol. 1998, 275, L917–L923. [Google Scholar] [CrossRef] [PubMed]
- Sloan, J.L.; Grubb, B.R.; Mager, S. Expression of the amino acid transporter ATB 0+ in lung: Possible role in luminal protein removal. Am. J. Physiol. Lung Cell Mol. Physiol. 2003, 284, L39–L49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rotoli, B.M.; Bussolati, O.; Sala, R.; Gazzola, G.C.; Dall’Asta, V. The transport of cationic amino acids in human airway cells: Expression of system y+L activity and transepithelial delivery of NOS inhibitors. FASEB J. 2005, 19, 810–812. [Google Scholar] [CrossRef]
- Hucke, A.; Ciarimboli, G. The Role of Transporters in the Toxicity of Chemotherapeutic Drugs: Focus on Transporters for Organic Cations. J. Clin. Pharmacol. 2016, 56 (Suppl. S7), S157–S172. [Google Scholar] [CrossRef] [Green Version]
- Chien, H.C.; Zur, A.A.; Maurer, T.S.; Yee, S.W.; Tolsma, J.; Jasper, P.; Scott, D.O.; Giacomini, K.M. Rapid Method to Determine Intracellular Drug Concentrations in Cellular Uptake Assays: Application to Metformin in Organic Cation Transporter 1-Transfected Human Embryonic Kidney 293 Cells. Drug Metab. Dispos. 2016, 44, 356–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukherjee, M.; Pritchard, D.I.; Bosquillon, C. Evaluation of air-interfaced Calu-3 cell layers for investigation of inhaled drug interactions with organic cation transporters in vitro. Int. J. Pharm. 2012, 426, 7–14. [Google Scholar] [CrossRef] [PubMed]






| Gene/Protein Name NCBI Reference Sequence Identifier | Forward Primer | Reverse Primer |
|---|---|---|
| SLC22A1 (NM_003057.3) | TGTCACCGAAAAGCTGAGCC | TCCGTGAACCACAGGTACATC |
| SLC22A2T2 (NM_003058.4) | CATCGTCACCGCGTTTAACCTG | AGCCGATACTCATAGAGCCAAT |
| SLC22A3 (NM_003058.4) | AGGTATGGCAGGATCGTCATT | GCAGGAAGCGGAAGATCACA |
| SLC22A5/OCTN2 (NM_001308122.1) | TCCACCATTGTGACCGAG | ACCCACGAAGAACAAGGAGAT |
| SLC6A14 (NM_007231.5) | CTGCTTGGTTTTGTTTCTTCTTGGTC | GCAATTAAAATGCCCCATCCAGCAC |
| SLC47A1 (NM_018242.3) | TCAACCAGGGAATTGTACTGC | CAGAGCCTATCACCCCAAGA |
| RPL15 (NM_001253379.2) | GCAGCCATCAGGTAAGCCAAG | AGCGGACCCTCAGAAGAAAGC |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barilli, A.; Visigalli, R.; Ferrari, F.; Di Lascia, M.; Riccardi, B.; Puccini, P.; Dall’Asta, V.; Rotoli, B.M. Organic Cation Transporters (OCTs) in EpiAirway™, a Cellular Model of Normal Human Bronchial Epithelium. Biomedicines 2020, 8, 127. https://doi.org/10.3390/biomedicines8050127
Barilli A, Visigalli R, Ferrari F, Di Lascia M, Riccardi B, Puccini P, Dall’Asta V, Rotoli BM. Organic Cation Transporters (OCTs) in EpiAirway™, a Cellular Model of Normal Human Bronchial Epithelium. Biomedicines. 2020; 8(5):127. https://doi.org/10.3390/biomedicines8050127
Chicago/Turabian StyleBarilli, Amelia, Rossana Visigalli, Francesca Ferrari, Maria Di Lascia, Benedetta Riccardi, Paola Puccini, Valeria Dall’Asta, and Bianca Maria Rotoli. 2020. "Organic Cation Transporters (OCTs) in EpiAirway™, a Cellular Model of Normal Human Bronchial Epithelium" Biomedicines 8, no. 5: 127. https://doi.org/10.3390/biomedicines8050127
APA StyleBarilli, A., Visigalli, R., Ferrari, F., Di Lascia, M., Riccardi, B., Puccini, P., Dall’Asta, V., & Rotoli, B. M. (2020). Organic Cation Transporters (OCTs) in EpiAirway™, a Cellular Model of Normal Human Bronchial Epithelium. Biomedicines, 8(5), 127. https://doi.org/10.3390/biomedicines8050127