Prediagnostic Blood Selenium Status and Mortality among Patients with Colorectal Cancer in Western European Populations
Abstract
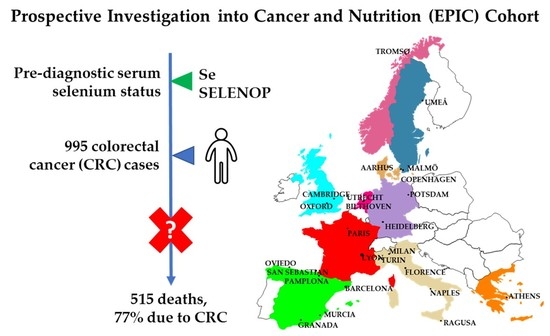
:1. Introduction
2. Materials and Methods
2.1. Study Population and Data Collection
2.2. Cancer Incidence Follow-Up
2.3. Vital Status Follow-Up
2.4. Case Ascertainment and Selection
2.5. Selenium and Selenoprotein P Measurements
2.6. Covariates
2.7. Statistical Analyses
3. Results
3.1. Characteristics of Study Participants
3.2. Selenium and Mortality among CRC Patients
3.3. Selenoprotein P and Mortality among CRC Patients
3.4. Stratified Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romaguera, D.; Ward, H.; Wark, P.A.; Vergnaud, A.-C.; Peeters, P.H.; van Gils, C.H.; Ferrari, P.; Fedirko, V.; Jenab, M.; Boutron-Ruault, M.-C.; et al. Pre-diagnostic concordance with the WCRF/AICR guidelines and survival in European colorectal cancer patients: A cohort study. BMC Med. 2015, 13, 107. [Google Scholar] [CrossRef]
- Haggar, F.A.; Boushey, R.P. Colorectal Cancer Epidemiology: Incidence, Mortality, Survival, and Risk Factors. Clin. Colon Rectal Surg. 2009, 22, 191–197. [Google Scholar] [CrossRef] [Green Version]
- Kornitzer, M.; Valente, F.; De Bacquer, D.; Neve, J.; De Backer, G. Serum selenium and cancer mortality: A nested case-control study within an age- and sex-stratified sample of the Belgian adult population. Eur. J. Clin. Nutr. 2004, 58, 98–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatfield, D.L.; Tsuji, P.A.; Carlson, B.A.; Gladyshev, V.N. Selenium and selenocysteine: Roles in cancer, health, and development. Trends Biochem. Sci. 2014, 39, 112–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurokawa, S.; Berry, M.J. Selenium. Role of the Essential Metalloid in Health. Metal. Ions Life Sci. 2013, 13, 499–534. [Google Scholar] [CrossRef] [Green Version]
- Zeng, H.; Combs, G.F., Jr. Selenium as an anticancer nutrient: Roles in cell proliferation and tumor cell invasion. J. Nutr. Biochem. 2008, 19, 1–7. [Google Scholar] [CrossRef]
- Schomburg, L.; Hughes, D.J. The missing link? The potential role of selenium in the development of liver cancer and significance for the general population. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 707–709. [Google Scholar] [CrossRef]
- Burk, R.F.; Hill, K.E. Regulation of Selenium Metabolism and Transport. Annu. Rev. Nutr. 2015, 35, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Richardson, D.R. More roles for selenoprotein P: Local selenium storage and recycling protein in the brain. Biochem. J. 2005, 386, E5. [Google Scholar] [CrossRef] [Green Version]
- Meplan, C. Selenium and chronic diseases: A nutritional genomics perspective. Nutrients 2015, 7, 3621–3651. [Google Scholar] [CrossRef]
- Hughes, D.J.; Fedirko, V.; Jenab, M.; Schomburg, L.; Meplan, C.; Freisling, H.; Bueno-de-Mesquita, H.B.; Hybsier, S.; Becker, N.P.; Czuban, M.; et al. Selenium status is associated with colorectal cancer risk in the European prospective investigation of cancer and nutrition cohort. Int. J. Cancer 2015, 136, 1149–1161. [Google Scholar] [CrossRef]
- Peters, K.M.; Carlson, B.A.; Gladyshev, V.N.; Tsuji, P.A. Selenoproteins in colon cancer. Free. Radic. Biol. Med. 2018, 127, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Speckmann, B.; Steinbrenner, H. Selenium and selenoproteins in inflammatory bowel diseases and experimental colitis. Inflamm. Bowel Dis. 2014, 20, 1110–1119. [Google Scholar] [CrossRef] [PubMed]
- Meplan, C.; Hesketh, J. The influence of selenium and selenoprotein gene variants on colorectal cancer risk. Mutagenesis 2012, 27, 177–186. [Google Scholar] [CrossRef] [Green Version]
- Kipp, A.; Banning, A.; van Schothorst, E.M.; Meplan, C.; Schomburg, L.; Evelo, C.; Coort, S.; Gaj, S.; Keijer, J.; Hesketh, J.; et al. Four selenoproteins, protein biosynthesis, and Wnt signalling are particularly sensitive to limited selenium intake in mouse colon. Mol. Nutr. Food Res. 2009, 53, 1561–1572. [Google Scholar] [CrossRef] [PubMed]
- Meplan, C.; Johnson, I.T.; Polley, A.C.; Cockell, S.; Bradburn, D.M.; Commane, D.M.; Arasaradnam, R.P.; Mulholland, F.; Zupanic, A.; Mathers, J.C.; et al. Transcriptomics and proteomics show that selenium affects inflammation, cytoskeleton, and cancer pathways in human rectal biopsies. FASEB J. 2016, 30, 2812–2825. [Google Scholar] [CrossRef] [Green Version]
- McKenzie, R.C.; Arthur, J.R.; Beckett, G.J. Selenium and the regulation of cell signaling, growth, and survival: Molecular and mechanistic aspects. Antioxid. Redox Signal. 2002, 4, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, A.; Basu, A.; Biswas, J.; Sen, T.; Bhattacharya, S. Chemoprotective and chemosensitizing properties of selenium nanoparticle (Nano-Se) during adjuvant therapy with cyclophosphamide in tumor-bearing mice. Mol. Cell. Biochem. 2017, 424, 13–33. [Google Scholar] [CrossRef]
- Chen, Y.C.; Prabhu, K.S.; Mastro, A.M. Is selenium a potential treatment for cancer metastasis? Nutrients 2013, 5, 1149–1168. [Google Scholar] [CrossRef] [Green Version]
- Kandas, N.O.; Randolph, C.; Bosland, M.C. Differential effects of selenium on benign and malignant prostate epithelial cells: Stimulation of LNCaP cell growth by noncytotoxic, low selenite concentrations. Nutr. Cancer 2009, 61, 251–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasaikina, M.V.; Turanov, A.A.; Avanesov, A.; Schweizer, U.; Seeher, S.; Bronson, R.T.; Novoselov, S.N.; Carlson, B.A.; Hatfield, D.L.; Gladyshev, V.N. Contrasting roles of dietary selenium and selenoproteins in chemically induced hepatocarcinogenesis. Carcinogenesis 2013, 34, 1089–1095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rose, A.H.; Bertino, P.; Hoffmann, F.W.; Gaudino, G.; Carbone, M.; Hoffmann, P.R. Increasing dietary selenium elevates reducing capacity and ERK activation associated with accelerated progression of select mesothelioma tumors. Am. J. Pathol. 2014, 184, 1041–1049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, Y.P.; Tang, J.M.; Tang, Y.; Gao, H.Y. Histological and ultrastructural changes induced by selenium in early experimental gastric carcinogenesis. World J. Gastroenterol. 2005, 11, 4457–4460. [Google Scholar] [CrossRef]
- Hughes, D.J.; Kunicka, T.; Schomburg, L.; Liska, V.; Swan, N.; Soucek, P. Expression of Selenoprotein Genes and Association with Selenium Status in Colorectal Adenoma and Colorectal Cancer. Nutrients 2018, 10, 1812. [Google Scholar] [CrossRef] [Green Version]
- Johnson, C.C.; Fordyce, F.M.; Rayman, M.P. Symposium on ‘Geographical and geological influences on nutrition’: Factors controlling the distribution of selenium in the environment and their impact on health and nutrition. Proc. Nutr. Soc. 2010, 69, 119–132. [Google Scholar] [CrossRef] [Green Version]
- Combs, G.F., Jr. Selenium in global food systems. Br. J. Nutr. 2001, 85, 517–547. [Google Scholar] [CrossRef] [Green Version]
- Lippman, S.M.; Klein, E.A.; Goodman, P.J.; Lucia, M.S.; Thompson, I.M.; Ford, L.G.; Parnes, H.L.; Minasian, L.M.; Gaziano, J.M.; Hartline, J.A.; et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: The Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2009, 301, 39–51. [Google Scholar] [CrossRef] [Green Version]
- Duffield-Lillico, A.J.; Reid, M.E.; Turnbull, B.W.; Combs, G.F., Jr.; Slate, E.H.; Fischbach, L.A.; Marshall, J.R.; Clark, L.C. Baseline characteristics and the effect of selenium supplementation on cancer incidence in a randomized clinical trial: A summary report of the Nutritional Prevention of Cancer Trial. Cancer Epidemiol. Biomark. Prev. 2002, 11, 630–639. [Google Scholar]
- Kantor, E.D.; Rehm, C.D.; Du, M.; White, E.; Giovannucci, E.L. Trends in Dietary Supplement Use Among US Adults From 1999–2012. JAMA 2016, 316, 1464–1474. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Riboli, E.; Hunt, K.J.; Slimani, N.; Ferrari, P.; Norat, T.; Fahey, M.; Charrondiere, U.R.; Hemon, B.; Casagrande, C.; Vignat, J.; et al. European Prospective Investigation into Cancer and Nutrition (EPIC): Study populations and data collection. Public Health Nutr. 2002, 5, 1113–1124. [Google Scholar] [CrossRef]
- Bingham, S.; Riboli, E. Diet and cancer—The European Prospective Investigation into Cancer and Nutrition. Nat. Rev. Cancer 2004, 4, 206–215. [Google Scholar] [CrossRef]
- Aleksandrova, K.; Pischon, T.; Jenab, M.; Bueno-de-Mesquita, H.B.; Fedirko, V.; Norat, T.; Romaguera, D.; Knüppel, S.; Boutron-Ruault, M.-C.; Dossus, L.; et al. Combined impact of healthy lifestyle factors on colorectal cancer: A large European cohort study. BMC Med. 2014, 12, 168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Couto, E.; Boffetta, P.; Lagiou, P.; Ferrari, P.; Buckland, G.; Overvad, K.; Dahm, C.C.; Tjonneland, A.; Olsen, A.; Clavel-Chapelon, F.; et al. Mediterranean dietary pattern and cancer risk in the EPIC cohort. Br. J. Cancer 2011, 104, 1493–1499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fedirko, V.; Riboli, E.; Tjonneland, A.; Ferrari, P.; Olsen, A.; Bueno-de-Mesquita, H.B.; van Duijnhoven, F.J.; Norat, T.; Jansen, E.H.; Dahm, C.C.; et al. Prediagnostic 25-hydroxyvitamin D, VDR and CASR polymorphisms, and survival in patients with colorectal cancer in western European ppulations. Cancer Epidemiol. Biomark. Prev. 2012, 21, 582–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosthoj, S.; Andersen, P.K.; Abildstrom, S.Z. SAS macros for estimation of the cumulative incidence functions based on a Cox regression model for competing risks survival data. Comput. Methods Programs Biomed. 2004, 74, 69–75. [Google Scholar] [CrossRef]
- Durrleman, S.; Simon, R. Flexible regression models with cubic splines. Stat. Med. 1989, 8, 551–561. [Google Scholar] [CrossRef]
- Govindarajulu, U.S.; Spiegelman, D.; Thurston, S.W.; Ganguli, B.; Eisen, E.A. Comparing smoothing techniques in Cox models for exposure-response relationships. Stat. Med. 2007, 26, 3735–3752. [Google Scholar] [CrossRef]
- Shah, Y.M.; Al-Dhaheri, M.; Dong, Y.; Ip, C.; Jones, F.E.; Rowan, B.G. Selenium disrupts estrogen receptor (alpha) signaling and potentiates tamoxifen antagonism in endometrial cancer cells and tamoxifen-resistant breast cancer cells. Mol. Cancer Ther. 2005, 4, 1239–1249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Li, F.; Younes, M.; Liu, H.; Chen, C.; Yao, Q. Reduced selenium-binding protein 1 in breast cancer correlates with poor survival and resistance to the anti-proliferative effects of selenium. PLoS ONE 2013, 8, e63702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vinceti, M.; Filippini, T.; Del Giovane, C.; Dennert, G.; Zwahlen, M.; Brinkman, M.; Zeegers, M.P.; Horneber, M.; D’Amico, R.; Crespi, C.M. Selenium for preventing cancer. Cochrane Database Syst. Rev. 2018, 1, Cd005195. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P.; Winther, K.H.; Pastor-Barriuso, R.; Cold, F.; Thvilum, M.; Stranges, S.; Guallar, E.; Cold, S. Effect of long-term selenium supplementation on mortality: Results from a multiple-dose, randomised controlled trial. Free. Radic. Biol. Med. 2018, 127, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Harris, H.R.; Bergkvist, L.; Wolk, A. Selenium intake and breast cancer mortality in a cohort of Swedish women. Breast Cancer Res. Treat. 2012, 134, 1269–1277. [Google Scholar] [CrossRef]
- Lubinski, J.; Marciniak, W.; Muszynska, M.; Huzarski, T.; Gronwald, J.; Cybulski, C.; Jakubowska, A.; Debniak, T.; Falco, M.; Kladny, J.; et al. Serum selenium levels predict survival after breast cancer. Breast Cancer Res. Treat. 2018, 167, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Meyer, H.A.; Endermann, T.; Stephan, C.; Stoedter, M.; Behrends, T.; Wolff, I.; Jung, K.; Schomburg, L. Selenoprotein P Status Correlates to Cancer-Specific Mortality in Renal Cancer Patients. PLoS ONE 2012, 7, e46644. [Google Scholar] [CrossRef] [Green Version]
- Pietrzak, S.; Wojcik, J.; Scott, R.J.; Kashyap, A.; Grodzki, T.; Baszuk, P.; Bielewicz, M.; Marciniak, W.; Wojcik, N.; Debniak, T.; et al. Influence of the selenium level on overall survival in lung cancer. J. Trace Elem. Med. Biol. Organ Soc. Miner. Trace Elem. (GMS) 2019, 56, 46–51. [Google Scholar] [CrossRef]
- Lubinski, J.; Marciniak, W.; Muszynska, M.; Jaworowska, E.; Sulikowski, M.; Jakubowska, A.; Kaczmarek, K.; Sukiennicki, G.; Falco, M.; Baszuk, P.; et al. Serum selenium levels and the risk of progression of laryngeal cancer. PLoS ONE 2018, 13, e0184873. [Google Scholar] [CrossRef] [Green Version]
- Yan, L.; Yee, J.A.; McGuire, M.H.; Graef, G.L. Effect of dietary supplementation of selenite on pulmonary metastasis of melanoma cells in mice. Nutr. Cancer 1997, 28, 165–169. [Google Scholar] [CrossRef]
- Jiang, C.; Ganther, H.; Lu, J. Monomethyl selenium—Specific inhibition of MMP-2 and VEGF expression: Implications for angiogenic switch regulation. Mol. Carcinog 2000, 29, 236–250. [Google Scholar] [CrossRef]
- Unni, E.; Kittrell, F.S.; Singh, U.; Sinha, R. Osteopontin is a potential target gene in mouse mammary cancer chemoprevention by Se-methylselenocysteine. Breast Cancer Res. 2004, 6, R586. [Google Scholar] [CrossRef] [Green Version]
- Yoon, S.O.; Kim, M.M.; Chung, A.S. Inhibitory effect of selenite on invasion of HT1080 tumor cells. J. Biol. Chem. 2001, 276, 20085–20092. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Zhou, R.; Hui, K.; Yang, Y.; Zhang, Q.; Ci, Y.; Shi, L.; Xu, C.; Huang, F.; Hu, Y. Selenite inhibits glutamine metabolism and induces apoptosis by regulating GLS1 protein degradation via APC/C-CDH1 pathway in colorectal cancer cells. Oncotarget 2017, 8, 18832–18847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meplan, C.; Hesketh, J. Selenium and cancer: A story that should not be forgotten-insights from genomics. Cancer Treat. Res. 2014, 159, 145–166. [Google Scholar] [CrossRef] [PubMed]
- Short, S.P.; Williams, C.S. Selenoproteins in Tumorigenesis and Cancer Progression. Adv. Cancer Res. 2017, 136, 49–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hurst, R.; Armah, C.N.; Dainty, J.R.; Hart, D.J.; Teucher, B.; Goldson, A.J.; Broadley, M.R.; Motley, A.K.; Fairweather-Tait, S.J. Establishing optimal selenium status: Results of a randomized, double-blind, placebo-controlled trial. Am. J. Clin. Nutr. 2010, 91, 923–931. [Google Scholar] [CrossRef] [Green Version]
- Xia, Y.; Hill, K.E.; Li, P.; Xu, J.; Zhou, D.; Motley, A.K.; Wang, L.; Byrne, D.W.; Burk, R.F. Optimization of selenoprotein P and other plasma selenium biomarkers for the assessment of the selenium nutritional requirement: A placebo-controlled, double-blind study of selenomethionine supplementation in selenium-deficient Chinese subjects. Am. J. Clin. Nutr. 2010, 92, 525–531. [Google Scholar] [CrossRef] [Green Version]
- Combs, G.F., Jr.; Jackson, M.I.; Watts, J.C.; Johnson, L.K.; Zeng, H.; Idso, J.; Schomburg, L.; Hoeg, A.; Hoefig, C.S.; Chiang, E.C.; et al. Differential responses to selenomethionine supplementation by sex and genotype in healthy adults. Br. J. Nutr. 2012, 107, 1514–1525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashton, K.; Hooper, L.; Harvey, L.J.; Hurst, R.; Casgrain, A.; Fairweather-Tait, S.J. Methods of assessment of selenium status in humans: A systematic review. Am. J. Clin. Nutr. 2009, 89, 2025s–2039s. [Google Scholar] [CrossRef] [Green Version]
- Longnecker, M.P.; Stram, D.O.; Taylor, P.R.; Levander, O.A.; Howe, M.; Veillon, C.; McAdam, P.A.; Patterson, K.Y.; Holden, J.M.; Morris, J.S.; et al. Use of selenium concentration in whole blood, serum, toenails, or urine as a surrogate measure of selenium intake. Epidemiology 1996, 7, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Allen, N.E.; Travis, R.C.; Appsleby, P.N.; Albanes, D.; Barnett, M.J.; Black, A.; Bueno-de-Mesquita, H.B.; Deschasaux, M.; Galan, P.; Goodman, G.E.; et al. Selenium and Prostate Cancer: Analysis of Individual Participant Data From Fifteen Prospective Studies. J. Natl. Cancer Inst. 2016, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Selenium, µg/L | ||||||
|---|---|---|---|---|---|---|
| Characteristic a | Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | |
| ≤67.5 | 67.6–77.4 | 77.5–88.0 | 88.1–99.9 | ≥100.0 | ||
| (N = 197) | (N = 201) | (N = 198) | (N = 199) | (N = 198) | ||
| Selenium (µg/L), mean (SD) | 56.7 (9.0) | 72.6 (2.9) | 82.6 (2.8) | 94.2 (3.4) | 115.6 (18.4) | |
| Selenoprotein P (mg/L), mean (SD) | 3.5 (0.8) | 4.1 (0.8) | 4.3 (0.7) | 4.6 (0.7) | 5.1 (0.9) | |
| Age at diagnosis (years), mean (SD) | 62.6 (7.8) | 61.7 (7.1) | 62.5 (7.1) | 62.0 (7.6) | 62.7 (7.0) | |
| BMI (kg/m2), mean (SD) | 26.4 (4.4) | 26.5 (4.3) | 26.8 (4.4) | 26.8 (4.6) | 26.9 (4.2) | |
| Women, N (%) | 117 (59.4) | 114 (56.7) | 100 (50.5) | 94 (47.2) | 91 (46.0) | |
| Location of primary tumor, N (%) | ||||||
| Colon | 139 (70.6) | 134 (66.7) | 123 (62.1) | 103 (51.8) | 127 (64.1) | |
| Rectum | 58 (29.4) | 67 (33.3) | 75 (37.9) | 96 (48.2) | 71 (35.9) | |
| Stage of tumor, N (%) | ||||||
| I | 28 (14.2) | 53 (26.4) | 38 (19.2) | 39 (19.6) | 35 (17.7) | |
| II | 52 (26.4) | 37 (18.4) | 43 (21.7) | 45 (22.6) | 43 (21.7) | |
| III | 62 (31.5) | 52 (25.9) | 67 (33.8) | 63 (31.7) | 77 (38.9) | |
| IV | 24 (12.2) | 31 (15.4) | 24 (12.1) | 25 (12.6) | 19 (9.6) | |
| Tumor grade, N (%) | ||||||
| Well differentiated | 11 (5.6) | 8 (4.0) | 15 (7.6) | 9 (4.5) | 7 (3.5) | |
| Moderately differentiated | 69 (35.0) | 73 (36.3) | 57 (28.8) | 51 (25.6) | 34 (17.2) | |
| Poorly differentiated | 18 (9.1) | 12 (6.0) | 13 (6.6) | 17 (8.5) | 10 (5.1) | |
| Unknown | 99 (50.3) | 108 (53.7) | 113 (57.1) | 122 (61.3) | 147 (74.3) | |
| Smoking status, N (%) | ||||||
| Never smoker | 80 (40.6) | 83 (41.3) | 79 (39.9) | 80 (40.2) | 72 (36.4) | |
| Former smoker | 60 (30.5) | 56 (27.9) | 69 (34.9) | 66 (33.2) | 72 (36.4) | |
| Current smoker | 56 (28.4) | 60 (29.9) | 49 (24.8) | 52 (26.1) | 54 (27.3) | |
| Physical activity, N (%) | ||||||
| Inactive | 27 (13.7) | 35 (17.4) | 34 (17.2) | 29 (14.6) | 34 (17.2) | |
| Moderately inactive | 59 (30.0) | 52 (25.9) | 58 (29.3) | 61 (30.7) | 64 (32.3) | |
| Moderately active | 92 (46.7) | 87 (43.3) | 84 (42.4) | 89 (44.7) | 83 (41.9) | |
| Active | 17 (8.6) | 24 (11.9) | 22 (11.1) | 20 (10.1) | 17 (8.6) | |
| Self-reported diabetes, N (%) | ||||||
| No | 148 (75.1) | 156 (77.6) | 167 (84.3) | 151 (75.9) | 146 (73.7) | |
| Yes | 13 (6.6) | 6 (3.0) | 6 (3.0) | 9 (4.5) | 15 (7.6) | |
| Alcohol (grams/day), mean (SD) | 17.2 (24.5) | 15.8 (19.7) | 20.3 (23.3) | 17.9 (21.1) | 19.5 (21.9) | |
| Overall mortality, N (%) | 103 (52.3) | 104 (51.7) | 104 (52.5) | 102 (51.3) | 101 (51.0) | |
| CRC-specific mortality, N (%) | 83 (42.1) | 82 (40.8) | 79 (39.9) | 76 (38.2) | 75 (37.9) | |
| Selenium | Selenoprotein P | |||||
|---|---|---|---|---|---|---|
| Deaths/Total | µg/L | HR (95% CI) | Deaths/Total | mg/L | HR (95% CI) | |
| Overall mortality | ||||||
| Age-, Sex-, Stage-adjusted a | ||||||
| Quintile 1 | 103/197 | ≤67.5 | 1.00 (ref) | 109/197 | ≤3.53 | 1.00 (ref) |
| Quintile 2 | 104/201 | 67.6–77.4 | 1.07 (0.80–1.42) | 104/199 | 3.54–4.06 | 0.96 (0.72–1.28) |
| Quintile 3 | 104/198 | 77.5–88.0 | 0.91 (0.68–1.22) | 95/199 | 4.07–4.50 | 0.77 (0.57–1.02) |
| Quintile 4 | 102/199 | 88.1–99.9 | 0.91 (0.68–1.22) | 91/197 | 4.51–5.06 | 0.75 (0.56–1.00) |
| Quintile 5 | 101/198 | ≥100.0 | 0.76 (0.56–1.02) | 114/198 | ≥5.07 | 0.87 (0.65–1.15) |
| Ptrend b | 0.04 | 0.27 | ||||
| per one SD c | 514/993 | 0.91 (0.83–1.00) | 513/990 | 0.95 (0.86–1.04) | ||
| Multivariable-adjusted d | ||||||
| Quintile 1 | 103/197 | ≤67.5 | 1.00 (ref) | 109/197 | ≤3.53 | 1.00 (ref) |
| Quintile 2 | 104/201 | 67.6–77.4 | 1.05 (0.79–1.41) | 104/199 | 3.54–4.06 | 1.00 (0.75–1.33) |
| Quintile 3 | 104/198 | 77.5–88.0 | 0.95 (0.71–1.27) | 95/199 | 4.07–4.50 | 0.80 (0.59–1.07) |
| Quintile 4 | 102/199 | 88.1–99.9 | 0.93 (0.69–1.25) | 91/197 | 4.51–5.06 | 0.74 (0.55–1.00) |
| Quintile 5 | 101/198 | ≥100.0 | 0.77 (0.57–1.03) | 114/198 | ≥5.07 | 0.83 (0.62–1.11) |
| Ptrend b | 0.04 | 0.17 | ||||
| per one SD c | 514/993 | 0.91 (0.83–1.00) | 513/990 | 0.94 (0.85–1.03) | ||
| CRC-specific mortality e | ||||||
| Age-, Sex-, Stage-adjusted a | ||||||
| Quintile 1 | 83/192 | ≤67.5 | 1.00 (ref) | 87/193 | ≤3.53 | 1.00 (ref) |
| Quintile 2 | 82/198 | 67.6–77.4 | 1.06 (0.76–1.47) | 78/191 | 3.54–4.06 | 0.99 (0.71–1.37) |
| Quintile 3 | 79/192 | 77.5–88.0 | 0.93 (0.67–1.30) | 70/194 | 4.07–4.50 | 0.73 (0.52–1.03) |
| Quintile 4 | 76/194 | 88.1–99.9 | 0.88 (0.63–1.23) | 69/196 | 4.51–5.06 | 0.70 (0.50–0.99) |
| Quintile 5 | 75/193 | ≥100.0 | 0.73 (0.52–1.03) | 91/192 | ≥5.07 | 0.92 (0.66–1.27) |
| Ptrend b | 0.07 | 0.48 | ||||
| per one SD c | 395/969 | 0.91 (0.81–1.01) | 395/966 | 0.96 (0.86–1.07) | ||
| Multivariable-adjusted d | ||||||
| Quintile 1 | 83/192 | ≤67.5 | 1.00 (ref) | 87/193 | ≤3.53 | 1.00 (ref) |
| Quintile 2 | 82/198 | 67.6–77.4 | 1.05 (0.76–1.46) | 78/191 | 3.54–4.06 | 1.04 (0.74–1.44) |
| Quintile 3 | 79/192 | 77.5–88.0 | 0.95 (0.68–1.32) | 70/194 | 4.07–4.50 | 0.76 (0.54–1.07) |
| Quintile 4 | 76/194 | 88.1–99.9 | 0.88 (0.63–1.24) | 69/196 | 4.51–5.06 | 0.71 (0.50–1.00) |
| Quintile 5 | 75/193 | ≥100.0 | 0.73 (0.52–1.02) | 91/192 | ≥5.07 | 0.89 (0.64–1.24) |
| Ptrend b | 0.06 | 0.39 | ||||
| per one SD c | 395/969 | 0.90 (0.81–1.00) | 395/966 | 0.95 (0.86–1.06) | ||
| Overall Mortality | CRC-Specific Mortality a | ||||||
|---|---|---|---|---|---|---|---|
| Risk Factor | Deaths/Total | HR (95% CI) b | Ptrend d or interaction c | Deaths/Total | HR (95% CI) b | Ptrend d or interaction c | |
| Selenium | |||||||
| All participants | 514/993 | 0.91 (0.83–1.00) | 0.04 d | 395/969 | 0.90 (0.81–1.00) | 0.06 d | |
| Sensitivity analyses | |||||||
| Complete CRC stage data e | 450/857 | 0.91 (0.82–1.00) | 0.05 d | 355/839 | 0.92 (0.82–1.03) | 0.14 d | |
| Imputed CRC stage data | 514/993 | 0.92 (0.84–1.01) | 0.07 d | 395/969 | 0.91 (0.82–1.02) | 0.09 d | |
| Follow-up (years) | |||||||
| ≥2 | 257/732 | 0.94 (0.83–1.07) | 0.37 d | 165/711 | 0.94 (0.80–1.10) | 0.42 d | |
| ≥4 | 158/631 | 1.02(0.87–1.19) | 0.83 d | 77/611 | 1.03 (0.84–1.28) | 0.76 d | |
| Time between blood collection and diagnosis (years) | |||||||
| <2.6 | 176/330 | 0.93 (0.78–1.12) | 0.03 | 138/325 | 0.89 (0.73–1.10) | 0.01 | |
| [2.6–4.8) | 175/332 | 0.86 (0.73–1.01) | 133/321 | 0.84 (0.70–1.02) | |||
| ≥4.8 | 163/331 | 1.00 (0.83–1.21) | 124/323 | 1.00 (0.80–1.25) | |||
| Stratified Analyses | |||||||
| Sex | |||||||
| Women | 252/516 | 0.92 (0.80–1.06) | 0.52 | 202/505 | 0.93 (0.79–1.08) | 0.48 | |
| Men | 262/477 | 0.87 (0.77–1.00) | 193/464 | 0.86 (0.73–1.01) | |||
| Age at diagnosis (years) | |||||||
| <62.4 | 215/497 | 0.94 (0.80–1.11) | 0.51 | 184/493 | 0.95 (0.80–1.13) | 0.38 | |
| ≥62.4 | 299/496 | 0.89 (0.79–1.00) | 211/476 | 0.86 (0.75–0.99) | |||
| Cancer site | |||||||
| Colon | 333/626 | 0.89(0.80–1.00) | 0.56 | 255/611 | 0.89 (0.78–1.01) | 0.87 | |
| Rectum | 181/367 | 0.92 (0.77–1.09) | 140/358 | 0.90 (0.74–1.09) | |||
| Stage e | |||||||
| I–II | 128/413 | 1.03 (0.84–1.25) | 0.11 | 79/399 | 1.08 (0.85–1.37) | 0.10 | |
| III–IV | 322/444 | 0.90 (0.80–1.01) | 276/440 | 0.89 (0.78–1.01) | |||
| Region f | |||||||
| Northern | 194/330 | 0.79 (0.68–0.91) | 0.03 | 158/325 | 0.75 (0.64–0.89) | 0.01 | |
| Central | 227/436 | 0.97 (0.83–1.13) | 163/418 | 0.94 (0.79–1.13) | |||
| Southern | 93/227 | 1.03 (0.81–1.30) | 74/226 | 1.21 (0.92–1.58) | |||
| Selenoprotein P | |||||||
| All participants | 513/990 | 0.94 (0.85, 1.03) | 0.17 d | 395/966 | 0.95 (0.86–1.06) | 0.39 d | |
| Sensitivity analyses | |||||||
| Complete CRC stage data e | 450/856 | 0.94 (0.84, 1.04) | 0.22 d | 355/838 | 0.95 (0.86–1.06) | 0.39 d | |
| Imputed CRC stage data | 513/990 | 0.94 (0.86, 1.04) | 0.24 d | 395/966 | 0.97 (0.87–1.08) | 0.58 d | |
| Follow-up (years) | |||||||
| ≥2 | 257/730 | 0.93 (0.82, 1.07) | 0.31 d | 165/709 | 0.97 (0.82–1.15) | 0.74 d | |
| ≥4 | 158/629 | 0.99 (0.83, 1.17) | 0.88 d | 77/609 | 1.10 (0.86–1.40) | 0.45 d | |
| Time between blood collection and diagnosis (years) | |||||||
| <2.6 | 176/329 | 0.88 (0.74–1.05) | 0.15 | 138/324 | 0.88 (0.72–1.07) | 0.45 | |
| [2.6–4.8) | 175/331 | 0.87(0.74–1.04) | 133/320 | 0.90 (0.74–1.10) | |||
| ≥4.8 | 162/330 | 1.06(0.88–1.28) | 124/322 | 1.16 (0.93–1.44) | |||
| Stratified Analyses | |||||||
| Sex | |||||||
| Women | 252/514 | 0.91(0.79–1.06) | 0.74 | 202/503 | 0.97 (0.82–1.15) | 0.76 | |
| Men | 261/476 | 0.92(0.81–1.05) | 193/463 | 0.92 (0.79–1.08) | |||
| Age at diagnosis (years) | |||||||
| <62.4 | 216/497 | 0.91(0.78–1.07) | 0.66 | 185/493 | 0.91 (0.76–1.08) | 0.83 | |
| ≥62.4 | 297/493 | 0.93(0.82–1.05) | 210/473 | 0.97 (0.84–1.12) | |||
| Cancer site | |||||||
| Colon | 332/626 | 0.94(0.84–1.06) | 0.80 | 255/611 | 0.97 (0.85–1.10) | 0.84 | |
| Rectum | 181/364 | 0.86(0.71–1.04) | 140/355 | 0.94 (0.76–1.17) | |||
| Stage e | |||||||
| I–II | 127/410 | 0.95(0.78–1.15) | 0.90 | 78/396 | 1.09 (0.85–1.38) | 0.37 | |
| III–IV | 323/446 | 0.95(0.84–1.08) | 277/442 | 0.96 (0.84–1.11) | |||
| Region f | |||||||
| Northern | 194/330 | 0.88(0.77–1.01) | 0.49 | 158/325 | 0.90 (0.77–1.05) | 0.31 | |
| Central | 226/432 | 0.96(0.82–1.13) | 163/414 | 0.94 (0.78–1.14) | |||
| Southern | 93/228 | 1.03(0.79–1.34) | 74/227 | 1.20 (0.88–1.62) | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baker, J.R.; Umesh, S.; Jenab, M.; Schomburg, L.; Tjønneland, A.; Olsen, A.; Boutron-Ruault, M.-C.; Rothwell, J.A.; Severi, G.; Katzke, V.; et al. Prediagnostic Blood Selenium Status and Mortality among Patients with Colorectal Cancer in Western European Populations. Biomedicines 2021, 9, 1521. https://doi.org/10.3390/biomedicines9111521
Baker JR, Umesh S, Jenab M, Schomburg L, Tjønneland A, Olsen A, Boutron-Ruault M-C, Rothwell JA, Severi G, Katzke V, et al. Prediagnostic Blood Selenium Status and Mortality among Patients with Colorectal Cancer in Western European Populations. Biomedicines. 2021; 9(11):1521. https://doi.org/10.3390/biomedicines9111521
Chicago/Turabian StyleBaker, Jacqueline Roshelli, Sushma Umesh, Mazda Jenab, Lutz Schomburg, Anne Tjønneland, Anja Olsen, Marie-Christine Boutron-Ruault, Joseph A. Rothwell, Gianluca Severi, Verena Katzke, and et al. 2021. "Prediagnostic Blood Selenium Status and Mortality among Patients with Colorectal Cancer in Western European Populations" Biomedicines 9, no. 11: 1521. https://doi.org/10.3390/biomedicines9111521
APA StyleBaker, J. R., Umesh, S., Jenab, M., Schomburg, L., Tjønneland, A., Olsen, A., Boutron-Ruault, M. -C., Rothwell, J. A., Severi, G., Katzke, V., Johnson, T., Schulze, M. B., Masala, G., Agnoli, C., Simeon, V., Tumino, R., Bueno-de-Mesquita, H. B., Gram, I. T., Skeie, G., ... Fedirko, V. (2021). Prediagnostic Blood Selenium Status and Mortality among Patients with Colorectal Cancer in Western European Populations. Biomedicines, 9(11), 1521. https://doi.org/10.3390/biomedicines9111521