A Complementary Sensory Tool for Children with Autism Spectrum Disorders
Abstract
:1. Introduction
1.1. Sensory Integration Process
1.2. Sensory Processing Disorders
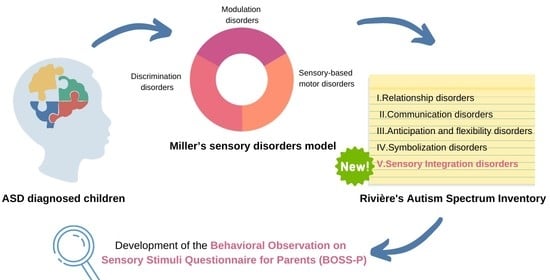
1.3. Autism Spectrum Disorders and SPD Relationships
1.4. Autism Spectrum Disorders and SPD Assessment
1.5. Aim
2. Methods
2.1. Participants
2.2. Procedure
2.3. Instrument
2.4. Statistics
3. Results
3.1. Exploratory Factor Analysis
3.2. Confirmatory Factor Analysis
3.3. Reliability
3.4. Questionnaire’s Capability to Classify between ASD and Typical Development
4. Discussion
4.1. About the BOSS-P Questionnaire
4.2. The BOSS-P and Other Instruments
4.3. Limitations and Future Lines
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ayres, A.J. Sensory Integration and Learning Disorders; Western Psychological Services: Los Angeles, CA, USA, 1972; ISBN 978-0-87424-303-1. [Google Scholar]
- Humphries, T.; Wright, M.; McDougall, B.; Vertes, J. The Efficacy of Sensory Integration Therapy for Children with Learning Disability. Phys. Occup. Ther. Pediatr. 1990, 10, 1–17. [Google Scholar] [CrossRef]
- Williams, M.S.; Shellenberger, S. The Alert Program for Self-Regulation; TherapyWorks Inc.: Albuquerque, NM, USA, 1995; ISBN 978-0-9643041-1-6. [Google Scholar]
- Dunn, W. Supporting Children to Participate Successfully in Everyday Life by Using Sensory Processing Knowledge. Infants Young Child. 2007, 20, 84–101. [Google Scholar] [CrossRef] [Green Version]
- Kilroy, E.; Aziz-Zadeh, L.; Cermak, S.A. Ayres Theories of Autism and Sensory Integration Revisited: What Contemporary Neuroscience Has to Say. Brain Sci. 2019, 9, 68. [Google Scholar] [CrossRef] [Green Version]
- Miller, L.J.; Anzalone, M.E.; Lane, S.J.; Cermak, S.A.; Osten, E.T. Concept Evolution in Sensory Integration: A Proposed Nosology for Diagnosis. Am. J. Occup. Ther. 2007, 61, 135–140. [Google Scholar] [CrossRef] [Green Version]
- Sher, B. Everyday Games for Sensory Processing Disorder: 100 Playful Activities to Empower Children with Sensory Differences; Althea Press: Berkeley, CA, USA, 2016; ISBN 978-1-62315-700-5. [Google Scholar]
- Lane, S.J.; Bundy, A.C. Kids Can Be Kids: A Childhood Occupations Approach; Lane, S., Bundy, A.C., Eds.; F.A. Davis Co.: Philadelphia, PA, USA, 2012; ISBN 978-0-8036-1228-0. [Google Scholar]
- Lane, S.J.; Mailloux, Z.; Schoen, S.; Bundy, A.; May-Benson, T.A.; Parham, L.D.; Roley, S.S.; Schaaf, R.C. Neural Foundations of Ayres Sensory Integration®. Brain Sci. 2019, 9, 153. [Google Scholar] [CrossRef] [Green Version]
- Cermak, S.A. Reflections on 25 Years of Dyspraxia Research. In Ayres Dyspraxia Monograph; Pediatric Therapy Network: Torrance, CA, USA, 2011. [Google Scholar]
- Ackerley, R.; Kavounoudias, A. The role of tactile afference in shaping motor behaviour and implications for prosthetic innovation. Neuropsychologia 2015, 79, 192–205. [Google Scholar] [CrossRef] [Green Version]
- Beaudry Bellefeuille, I.; Sánchez Padrón, O. Tengo Duendes en Las Piernas; Nobel: Oviedo, Spain, 2011; ISBN 978-84-8459-654-7. [Google Scholar]
- Pfeiffer, C.; Serino, A.; Blanke, O. The vestibular system: A spatial reference for bodily self-consciousness. Front. Integr. Neurosci. 2014, 8, 31. [Google Scholar] [CrossRef] [Green Version]
- Mahler, K.J.; Craig, A.D. Interoception: The Eighth Sensory System: Practical Solutions for Improving Self-Regulation, Self-Awareness and Social Understanding of Individuals with Autism Spectrum and Related Disorders; AAPC Publishing: Shawnee Mission, KS, USA, 2016; ISBN 978-1-942197-14-0. [Google Scholar]
- Farb, N.A.S.; Daubenmier, J.; Price, C.J.; Gard, T.; E Kerr, C.; Dunn, B.D.; Klein, A.C.; Paulus, M.P.; Mehling, W.E. Interoception, contemplative practice, and health. Front. Psychol. 2015, 6, 763. [Google Scholar] [CrossRef] [Green Version]
- Ayres, A.J. Sensory Integration and Praxis Tests; Western Psychological Services: Los Angeles, CA, USA, 1989. [Google Scholar]
- Gourley, L.; Wind, C.; Henninger, E.M.; Chinitz, S. Sensory Processing Difficulties, Behavioral Problems, and Parental Stress in a Clinical Population of Young Children. J. Child Fam. Stud. 2013, 22, 912–921. [Google Scholar] [CrossRef] [Green Version]
- Miller, L.J.; Nielsen, D.M.; Schoen, S.; Brett-Green, B.A. Perspectives on sensory processing disorder: A call for translational research. Front. Integr. Neurosci. 2009, 3, 3. [Google Scholar] [CrossRef] [Green Version]
- Galiana-Simal, A.; Vela-Romero, M.; Romero-Vela, V.M.; Oliver-Tercero, N.; García-Olmo, V.; Benito-Castellanos, P.J.; Muñoz-Martinez, V.; Beato-Fernandez, L. Sensory processing disorder: Key points of a frequent alteration in neurodevelopmental disorders. Cogent Med. 2020, 7. [Google Scholar] [CrossRef]
- Butera, C.; Ring, P.; Sideris, J.; Jayashankar, A.; Kilroy, E.; Harrison, L.; Cermak, S.; Aziz-Zadeh, L. Impact of Sensory Processing on School Performance Outcomes in High Functioning Individuals with Autism Spectrum Disorder. Mind Brain Educ. 2020, 14, 243–254. [Google Scholar] [CrossRef]
- Baranek, G.T.; David, F.J.; Poe, M.D.; Stone, W.L.; Watson, L.R. Sensory Experiences Questionnaire: Discriminating sensory features in young children with autism, developmental delays, and typical development. J. Child Psychol. Psychiatry 2006, 47, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Dunn, W. The Sensations of Everyday Life: Empirical, Theoretical, and Pragmatic Considerations. Am. J. Occup. Ther. 2001, 55, 608–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ismael, N.; Lawson, L.M.; Hartwell, J. Relationship Between Sensory Processing and Participation in Daily Occupations for Children with Autism Spectrum Disorder: A Systematic Review of Studies That Used Dunn’s Sensory Processing Framework. Am. J. Occup. Ther. 2018, 72. [Google Scholar] [CrossRef]
- Schaaf, R.C.; Mailloux, Z. Clinician’s Guide for Implementing Ayres Sensory Integration: Promoting Participation for Children with Autism; AOTA Press: Bethesda, MD, USA, 2015; ISBN 978-1-56900-365-7. [Google Scholar]
- Mulligan, S.; A Schoen, S.; Miller, L.J.; Valdez, A.; Magalhaes, D. The Sensory Processing 3-Dimensions Scale: Initial Studies of Reliability and Item Analyses. Open J. Occup. Ther. 2019, 7, 4. [Google Scholar] [CrossRef] [Green Version]
- APA. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013; p. 5. [Google Scholar]
- Bölte, S.; Girdler, S.; Marschik, P.B. The contribution of environmental exposure to the etiology of autism spectrum disorder. Cell. Mol. Life Sci. 2019, 76, 1275–1297. [Google Scholar] [CrossRef] [Green Version]
- Steinman, G.; Mankuta, D. Molecular biology of autism’s etiology—An alternative mechanism. Med. Hypotheses 2019, 130, 109–272. [Google Scholar] [CrossRef]
- Linke, A.C.; Keehn, R.J.J.; Pueschel, E.B.; Fishman, I.; Müller, R.-A. Children with ASD show links between aberrant sound processing, social symptoms, and atypical auditory interhemispheric and thalamocortical functional connectivity. Dev. Cogn. Neurosci. 2018, 29, 117–126. [Google Scholar] [CrossRef]
- Chang, Y.-S.; Owen, J.P.; Desai, S.S.; Hill, S.S.; Arnett, A.B.; Harris, J.; Marco, E.J.; Mukherjee, P. Autism and Sensory Processing Disorders: Shared White Matter Disruption in Sensory Pathways but Divergent Connectivity in Social-Emotional Pathways. PLoS ONE 2014, 9, e103038. [Google Scholar] [CrossRef]
- Schaaf, R.C.; Lane, A.E. Toward a Best-Practice Protocol for Assessment of Sensory Features in ASD. J. Autism Dev. Disord. 2015, 45, 1380–1395. [Google Scholar] [CrossRef] [PubMed]
- Posar, A.; Visconti, P. Sensory abnormalities in children with autism spectrum disorder. J. Pediatr. 2018, 94, 342–350. [Google Scholar] [CrossRef]
- Marco, E.J.; Hinkley, L.B.N.; Hill, S.S.; Nagarajan, S.S. Sensory Processing in Autism: A Review of Neurophysiologic Findings. Pediatr. Res. 2011, 69, 48R–54R. [Google Scholar] [CrossRef] [PubMed]
- Suarez, M.A. Sensory Processing in Children with Autism Spectrum Disorders and Impact on Functioning. Pediatr. Clin. North Am. 2012, 59, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Glod, M.; Riby, D.M.; Rodgers, J. Sensory processing profiles and autistic symptoms as predictive factors in autism spectrum disorder and Williams syndrome. J. Intellect. Disabil. Res. 2020, 64, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Schoen, S.; Lane, S.J.; Mailloux, Z.; May-Benson, T.; Parham, L.D.; Roley, S.S.; Schaaf, R.C. A systematic review of ayres sensory integration intervention for children with autism. Autism Res. 2019, 12, 6–19. [Google Scholar] [CrossRef] [PubMed]
- Bodison, S.C.; Parham, L.D. Specific Sensory Techniques and Sensory Environmental Modifications for Children and Youth with Sensory Integration Difficulties: A Systematic Review. Am. J. Occup. Ther. 2017, 72, 72011–90040. [Google Scholar] [CrossRef]
- Ben-Sasson, A.; Hen, L.; Fluss, R.; Cermak, S.A.; Engel-Yeger, B.; Gal, E. A Meta-Analysis of Sensory Modulation Symptoms in Individuals with Autism Spectrum Disorders. J. Autism Dev. Disord. 2009, 39, 1–11. [Google Scholar] [CrossRef]
- Di Renzo, M.; Di Castelbianco, F.B.; Vanadia, E.; Petrillo, M.; Racinaro, L.; Rea, M. Sensory Processing and Repetitive Behaviors in Clinical Assessment of Preschool Children with Autism Spectrum Disorder. J. Child Adolesc. Behav. 2017, 5, 5. [Google Scholar] [CrossRef] [Green Version]
- Dakopolos, A.J.; Jahromi, L.B. Differences in sensory responses among children with autism spectrum disorder and typical development: Links to joint attention and social competence. Infant Child Dev. 2018, 28, e2117. [Google Scholar] [CrossRef] [Green Version]
- Thye, M.D.; Bednarz, H.M.; Herringshaw, A.J.; Sartin, E.B.; Kana, R.K. The impact of atypical sensory processing on social impairments in autism spectrum disorder. Dev. Cogn. Neurosci. 2018, 29, 151–167. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.; Chukoskie, L.; Zinni, M.; Townsend, J.; Trauner, D. Dyspraxia, motor function and visual–motor integration in autism. Behav. Brain Res. 2014, 269, 95–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, M.; Srinivasan, S.M.; Bhat, A.N. Comparing motor performance, praxis, coordination, and interpersonal synchrony between children with and without Autism Spectrum Disorder (ASD). Res. Dev. Disabil. 2018, 72, 79–95. [Google Scholar] [CrossRef] [PubMed]
- Günal, A.; Bumin, G.; Huri, M. The Effects of Motor and Cognitive Impairments on Daily Living Activities and Quality of Life in Children with Autism. J. Occup. Ther. Sch. Early Interv. 2019, 12, 444–454. [Google Scholar] [CrossRef]
- Wong, C.; Odom, S.L.; Hume, K.A.; Cox, A.W.; Fettig, A.; Kucharczyk, S.; Brock, M.E.; Plavnick, J.B.; Fleury, V.P.; Schultz, T.R. Evidence-Based Practices for Children, Youth, and Young Adults with Autism Spectrum Disorder: A Comprehensive Review. J. Autism Dev. Disord. 2015, 45, 1951–1966. [Google Scholar] [CrossRef]
- Jorquera-Cabrera, S.; Romero-Ayuso, D.; Rodriguez-Gil, G.; Triviño-Juárez, J.-M. Assessment of Sensory Processing Characteristics in Children between 3 and 11 Years Old: A Systematic Review. Front. Pediatr. 2017, 5, 57. [Google Scholar] [CrossRef] [Green Version]
- Eeles, A.L.; Spittle, A.J.; Anderson, P.J.; Brown, N.; Lee, K.J.; Boyd, R.; Doyle, L.W. Assessments of sensory processing in infants: A systematic review. Dev. Med. Child Neurol. 2012, 55, 314–326. [Google Scholar] [CrossRef] [Green Version]
- Burns, C.O.; Dixon, D.R.; Novack, M.; Granpeesheh, D. A Systematic Review of Assessments for Sensory Processing Abnormalities in Autism Spectrum Disorder. Rev. J. Autism Dev. Disord. 2017, 4, 209–224. [Google Scholar] [CrossRef]
- Dubois, D.; Lymer, E.; Gibson, B.E.; Desarkar, P.; Nalder, E.J. Assessing Sensory Processing Dysfunction in Adults and Adolescents with Autism Spectrum Disorder: A Scoping Review. Brain Sci. 2017, 7, 108. [Google Scholar] [CrossRef]
- Yeung, L.H.J.; Thomacos, N. Assessments of sensory processing in infants and children with autism spectrum disorder between 0–12 years old: A scoping review. Res. Autism Spectr. Disord. 2020, 72, 101517. [Google Scholar] [CrossRef]
- Dunn, W. Sensory Profile User’s Manual; Pearson Psychcop: San Antonio, TX, USA, 1999. [Google Scholar]
- Dunn, W. Sensory Profile 2 Manual; Pearson Psychcop: San Antonio, TX, USA, 2014. [Google Scholar]
- Parham, L.D.; Ecker, C.; Miller Kuhaneck, H.; Henry, D.A.; Glennon, T.J. Sensory Processing Measure (SPM): Manual; Western Psychological Services: Los Angeles, CA, USA, 2007. [Google Scholar]
- Mulligan, S.; Schoen, S.; Miller, L. Scientific Research Panel 304C Reliability and Item Analyses of the Sensory Processing 3-Dimensions Scale. Am. J. Occup. Ther. 2018, 72, 7211500055. [Google Scholar] [CrossRef]
- Mailloux, Z.; Parham, L.D.; Roley, S.S.; Ruzzano, L.; Schaaf, R.C. Introduction to the Evaluation in Ayres Sensory Integration® (EASI). Am. J. Occup. Ther. 2017, 72, 72011–95030. [Google Scholar] [CrossRef]
- Petrocchi, S.; Levante, A.; Lecciso, F. Systematic Review of Level 1 and Level 2 Screening Tools for Autism Spectrum Disorders in Toddlers. Brain Sci. 2020, 10, 180. [Google Scholar] [CrossRef] [Green Version]
- Towle, P.O.; Patrick, P.A. Autism Spectrum Disorder Screening Instruments for Very Young Children: A Systematic Review. Autism Res. Treat. 2016, 2016, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Payakachat, N.; Tilford, J.M.; Kovacs, E.; Kuhlthau, K. Autism spectrum disorders: A review of measures for clinical, health services and cost–effectiveness applications. Expert Rev. Pharm. Outcomes Res. 2012, 12, 485–503. [Google Scholar] [CrossRef] [Green Version]
- Randall, M.; Egberts, K.J.; Samtani, A.; Scholten, R.J.; Hooft, L.; Livingstone, N.; Sterling-Levis, K.; Woolfenden, S.; Williams, K. Diagnostic tests for autism spectrum disorder (ASD) in preschool children. Cochrane Database Syst. Rev. 2018, 7, CD009044. [Google Scholar] [CrossRef] [Green Version]
- Rivière, Á. Tratamiento y Definición del Espectro Autista. In Tratamiento del Autismo. Nuevas Perspectivas; Instituto de Migraciones y Servicios Sociales: Madrid, Spain, 1998; ISBN 84-88986-70-X. [Google Scholar]
- Rivière, A. IDEA: Inventario de Espectro Autista; Fundec: Buenos Aires, Argentina, 2002. [Google Scholar]
- García-Gómez, A. A proposal of three additional dimensions to the Rivière’s Autism Spectrum Inventory. Psicol. Educ. in press. [CrossRef]
- Williams, Z.J.; Failla, M.D.; Gotham, K.O.; Woynaroski, T.G.; Cascio, C. Psychometric Evaluation of the Short Sensory Profile in Youth with Autism Spectrum Disorder. J. Autism Dev. Disord. 2018, 48, 4231–4249. [Google Scholar] [CrossRef]
- Dunn, W. Perfil Sensorial 2 Breve; Adaptación Española; Pearson Psychcop: Madrid, Spain, 2016; ISBN 978-84-9035-547-3. [Google Scholar]
- Ferrando, P.J.; Lorenzo-Seva, U. Program FACTOR at 10: Origins, development and future directions. Psicothema 2017, 29, 236–240. [Google Scholar]
- Lorenzo-Seva, U.; Ferrando, P.J. Factor: A computer program to fit the exploratory factor analysis model. Behav. Res. Methods 2006, 38, 88–91. [Google Scholar] [CrossRef] [Green Version]
- Manual of the Program. Available online: http://psico.fcep.urv.es/utilitats/factor/documentation/Manual-of-the-Factor-Program-v92.pdf (accessed on 20 August 2020).
- Timmerman, M.E.; Lorenzo-Seva, U. Dimensionality assessment of ordered polytomous items with parallel analysis. Psychol. Methods 2011, 16, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Arbuckle, J. Amos 24.0 User’s Guide; IBM SPSS: Chicago, IL, USA, 2015. [Google Scholar]
- Flora, D.B.; LaBrish, C.; Chalmers, R.P. Old and New Ideas for Data Screening and Assumption Testing for Exploratory and Confirmatory Factor Analysis. Front. Psychol. 2012, 3, 3. [Google Scholar] [CrossRef] [Green Version]
- Lloret-Segura, S.; Ferreres-Traver, A.; Hernández-Baeza, A.; Tomás-Marco, I. Exploratory Item Factor Analysis: A practical guide revised and updated. An. Psicol. 2014, 30, 1151–1169. [Google Scholar] [CrossRef]
- Ferrando, P.J.; Anguiano-Carrasco, C. Factor analysis as a research technique in psychology. Pap. Psicólogo 2010, 31, 18–33. [Google Scholar]
- Byrne, B.M. A Primer of LISREL. In Texto original: Basic Applications and Programming for Confirmatory Factor Analytic Models; Springer: New York, NY, USA, 1989; ISBN 978-1-4613-8887-6. [Google Scholar]
- Hu, L.-T.; Bentler, P.M. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struct. Equ. Model. Multidiscip. J. 1999, 6, 1–55. [Google Scholar]
- Hair, J.F. Multivariate Data Analysis: A Global Perspective; Pearson Education: London, UK, 2010; ISBN 978-0-13-515309-3. [Google Scholar]
- Browne, M.W.; Cudeck, R. Alternative Ways of Assessing Model Fit. Sociol. Methods Res. 1992, 21, 230–258. [Google Scholar] [CrossRef]
- Gadermann, A.M.; Guhn, M.; Zumbo, B.D. Ordinal Alpha. Encycl. Qual. Life Well Being Res. 2014, 4513–4515. [Google Scholar] [CrossRef]
- Oliden, P.E.; Zumbo, B.D. Reliability coefficients for ordinal response scales. Psicothema 2008, 20, 896–901. [Google Scholar]
- Pallant, J. SPSS Survival Manual: A Step by Step Guide to Data Analysis Using IBM SPSS, 5th ed.; McGraw Hill: Berkshire, UK, 2013; ISBN 978-0-335-26258-8. [Google Scholar]
- IBM Corp IBM SPSS Statistics for Windows, Version 24.0. Available online: https://he02.tci-thaijo.org/index.php/ramajournal/statistical_software_references_format (accessed on 22 August 2020).
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences. Lawrence Earlbaum Associates Hillsdale, NJ, USA. Available online: http://www.utstat.toronto.edu/~brunner/oldclass/378f16/readings/CohenPower.pdf (accessed on 15 October 2020).
- Jöreskog, K.G.; Sörbom, D. LISREL 8: Structural Equation Modeling with the SIMPLIS Command Language; Scientific Software International: Lincolnwood, IL, USA, 1993; ISBN 978-0-89498-033-6. [Google Scholar]
- Salgado, J.F. Transforming the Area under the Normal Curve (AUC) into Cohen’s d, Pearson’s r pb, Odds-Ratio, and Natural Log Odds-Ratio: Two Conversion Tables. Eur. J. Psychol. Appl. Leg. Context 2018, 10, 35–47. [Google Scholar] [CrossRef] [Green Version]
- Roley, S.S.; Blanche, E.I.; Schaaf, R.C. Understanding the Nature of Sensory Integration with Diverse Populations; PRO-ED: Indianapolis, IN, USA, 2007; ISBN 978-1-4164-0332-6. [Google Scholar]
- Schoen, S.; Miller, L.J.; Sullivan, J.C. Measurement in Sensory Modulation: The Sensory Processing Scale Assessment. Am. J. Occup. Ther. 2014, 68, 522–530. [Google Scholar] [CrossRef] [Green Version]
- Ministerio de Sanidad, Servicios Sociales e Igualdad. Salud Mental y Calidad de Vida en la Población Infantil; Serie Informes Monográficos no2; Ministerio de Sanidad, Servicios Sociales e Igualdad: Madrid, Spain, 2014.
- Van Gameren-Oosterom, H.B.; Van Dommelen, P.; Schönbeck, Y.; Oudesluys-Murphy, A.M.; Van Wouwe, J.P.; Buitendijk, S.E. Prevalence of Overweight in Dutch Children with Down Syndrome. Pediatrics 2012, 130, e1520–e1526. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Items | F1 | F2 | F3 |
|---|---|---|---|
| 1. Shows disproportionate reactions if touched. | 0.491 | ||
| 2. Shows panic reactions to loud noises. | 0.624 | ||
| 3. Shows rejection of water when showering or washing. | 0.340 | ||
| 4. He is bothered by noisy and crowded places. | 0.829 | ||
| 5. When something goes wrong, it takes a long time to calm down. | 0.566 | ||
| 6. Shows discomfort with activities that involve spinning. | 0.507 | ||
| 7. Cannot concentrate or perform tasks when background noise. | 0.627 | ||
| 8. He gets agitated in the presence of very powerful light sources. | 0.760 | ||
| 9. Frequently touches or puts body parts or objects in his mouth. | 0.394 | ||
| 10. He is bothered with strong smells. | 0.702 | ||
| 11. Some clothes bother him; he feels itchy about some fabrics. | 0.730 | ||
| 12. He dislikes personal hygiene or grooming activities. | 0.452 | ||
| 13. Quick movements are unpleasant for him. | 0.643 | ||
| 14. Attends to his name or when he is called. | 0.492 | ||
| 15. Communicates feelings aimed at satisfying basic needs. | 0.620 | ||
| 16. Realizes when he is tired or exhausted. | 0.639 | ||
| 17. Shows comfort when hugged by parents or close relatives. | 0.837 | ||
| 18. Shows satisfaction when basic needs are met | 0.959 | ||
| 19. When he is disconsolate, he gets calmed by his parents. | 0.720 | ||
| 20. Expresses enjoyment or feels comfortable in certain situations. | 0.897 | ||
| 21. Can perceive danger in situations that could harm. | 0.475 | ||
| 22. Can identify basic emotions in himself and others. | 0.442 | ||
| 23. Can orientate himself in the environment. | 0.418 | ||
| 24. Notices that his heart is racing when he is tired or excited. | 0.522 | ||
| 25. Recognizes the elements that make him nervous. | 0.578 | ||
| 26. Has difficulty in recognizing people’s faces. | 0.374 | ||
| 27. Has difficulty identifying parts of his own body. | 0.655 | ||
| 28. Presents inability to reproduce speech movements. | 0.737 | ||
| 29. Can ride a bicycle, rollerblades or a skateboard. | 0.623 | ||
| 30. Can perform simple motor imitations. | 0.724 | ||
| 31. Can fasten buttons or make loops to get dressed. | 0.927 | ||
| 32. Can stack small blocks or string beads on a string. | 0.569 | ||
| 33. Can use cutlery with both hands. | 0.634 | ||
| 34. Can make copies from simple drawings. | 0.930 | ||
| 35. Shows clumsiness in typing or using the computer keyboard. | 0.814 | ||
| 36. Shows insecurity going downstairs/hills, holds on to railings. | 0.485 | ||
| 37. Can adjust his strength when grasping objects. | 0.452 | ||
| 38. Can cut with scissors properly for his age. | 0.929 | ||
| 39. Can draw or colour within the proposed margins. | 0.924 | ||
| 40. Can follow motor imitations containing multiple steps. | 0.892 | ||
| 41. Can complete drawings with one half of it missing. | 0.930 |
| Indices | Cut-Off | Value |
|---|---|---|
| CMIN/DF | <2 | 1.995 |
| p (χ2) | >0.05 | 0.000 |
| TLI | >0.90 | 0.912 |
| CFI | >0.90 | 0.925 |
| RMSEA | <0.06 | 0.047 (0.043–0.051) |
| RMSR | <0.08 | 0.071 |
| BOSS-P | SP2 | ||||||
|---|---|---|---|---|---|---|---|
| F1 | F2 | F3 | Total | Sensory | Behavioral | Total | |
| F1 | 1 | ||||||
| F2 | −0.076 | 1 | |||||
| F3 | −0.134 | 0.297 | 1 | ||||
| Total | 0.438 * | 0.636 ** | 0.701 ** | 1 | |||
| Sensory | 0.448 * | 0.084 | 0.027 | 0.309 | 1 | ||
| Behaviour | 0.600 ** | 0.034 | 0.147 | 0.446 * | 0.613 ** | 1 | |
| total | 0.590 ** | 0.063 | 0.103 | 0.426 * | 0.879 ** | 0.915 ** | 1 |
| Autism Spectrum Disorder | Typical Development | |||||||
|---|---|---|---|---|---|---|---|---|
| F1 | F2 | F3 | Total | F1 | F2 | F3 | Total | |
| x | 33.8 | 31.9 | 40.3 | 106 | 24.8 | 20.5 | 23.1 | 68.4 |
| SD | 8.9 | 8.0 | 11.9 | 19 | 7.7 | 5.5 | 7.8 | 16.0 |
| BOSS-P | ||||
|---|---|---|---|---|
| F1 | F2 | F3 | ||
| Rivière’s inventory levels of severity | 1 (8 points) | >40 | >36 | >50 |
| 2 (6 points) | 34–40 | 30–36 | 40–50 | |
| 3 (4 points) | 27–34 | 27–30 | 31.5–40 | |
| 4 (2 points) | <27 | <27 | <31.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barrios-Fernández, S.; Gozalo, M.; Díaz-González, B.; García-Gómez, A. A Complementary Sensory Tool for Children with Autism Spectrum Disorders. Children 2020, 7, 244. https://doi.org/10.3390/children7110244
Barrios-Fernández S, Gozalo M, Díaz-González B, García-Gómez A. A Complementary Sensory Tool for Children with Autism Spectrum Disorders. Children. 2020; 7(11):244. https://doi.org/10.3390/children7110244
Chicago/Turabian StyleBarrios-Fernández, Sabina, Margarita Gozalo, Beatriz Díaz-González, and Andrés García-Gómez. 2020. "A Complementary Sensory Tool for Children with Autism Spectrum Disorders" Children 7, no. 11: 244. https://doi.org/10.3390/children7110244
APA StyleBarrios-Fernández, S., Gozalo, M., Díaz-González, B., & García-Gómez, A. (2020). A Complementary Sensory Tool for Children with Autism Spectrum Disorders. Children, 7(11), 244. https://doi.org/10.3390/children7110244