Patent Ductus Arteriosus Clinical Trials: Lessons Learned and Future Directions
Abstract
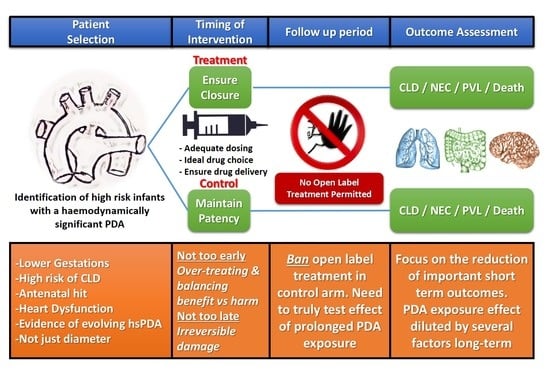
:1. Introduction
2. Challenges with PDA Trials to Date
2.1. Defining Haemodynamic Significance in PDA Trials
2.2. Creating True Intervention and Control Arms
2.3. Choosing the Right Outcomes
3. Conclusions
- (1)
- Ensuring that infants at the highest risk of PDA related morbidities with a low chance of spontaneous closure and are most likely to benefit from treatment are included in the trials. This requires a robust and comprehensive echocardiography assessment of PDA physiology incorporating aspects of myocardial performance with important clinical features integrated into the risk assessment. Reliance on single markers for inclusion to trials (such as PDA diameter alone) should no longer be implemented.
- (2)
- The timing of enrolment and intervention should balance avoiding intervention that may be too early, which could result in overtreatment and potentially dilute beneficial effects of shunt elimination in high risk infants, avoiding late treatment where prolonged shunt exposure may cause irreversible damage.
- (3)
- Treatment regiments in the intervention arm should focus on optimising PDA closure and echocardiography confirmed shunt elimination, while banning open label treatment in the placebo arm is vital to ensure ductal patency persists in this group of infants. This approach will facilitate a true comparison between early shunt elimination versus chronic exposure to left to right shunting.
- (4)
- Selecting outcome measures relevant to infants and parents with the focus on reducing important short term morbidities while reserving longer term neurodevelopmental impairment as a safety measure in those trials.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Knight, D.B. The treatment of patent ductus arteriosus in preterm infants. A review and overview of randomized trials. Semin. Neonatol. 2001, 6, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Evans, N.; Kluckow, M. Early ductal shunting and intraventricular haemorrhage in ventilated preterm infants. Arch. Dis. Child. Fetal Neonatal Ed. 1996, 75, F183–F186. [Google Scholar] [CrossRef] [PubMed]
- Harkin, P.; Marttila, R.; Pokka, T.; Saarela, T.; Hallman, M. Morbidities associated with patent ductus arteriosus in preterm infants. Nationwide cohort study. J. Matern. Fetal Neonatal Med. 2018, 31, 2576–2583. [Google Scholar] [CrossRef] [PubMed]
- Majed, B.; Bateman, D.A.; Uy, N.; Lin, F. Patent ductus arteriosus is associated with acute kidney injury in the preterm infant. Pediatr. Nephrol. 2019, 34, 1129–1139. [Google Scholar] [CrossRef] [PubMed]
- Bixler, G.M.; Powers, G.C.; Clark, R.H.; Walker, M.W.; Tolia, V.N. Changes in the Diagnosis and Management of Patent Ductus Arteriosus from 2006 to 2015 in United States Neonatal Intensive Care Units. J. Pediatrics 2017, 189, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Lokku, A.; Mirea, L.; Lee, S.K.; Shah, P.S. Trends and Outcomes of Patent Ductus Arteriosus Treatment in Very Preterm Infants in Canada. Am. J. Perinatol. 2017, 34, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Sung, S.I.; Chang, Y.S.; Chun, J.Y.; Yoon, S.A.; Yoo, H.S.; Ahn, S.Y.; Park, W.S. Mandatory Closure Versus Nonintervention for Patent Ductus Arteriosus in Very Preterm Infants. J. Pediatrics 2016, 177, 66–71.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zonnenberg, I.; de Waal, K. The definition of a haemodynamic significant duct in randomized controlled trials: A systematic literature review. Acta Paediatr. 2012, 101, 247–251. [Google Scholar] [CrossRef] [PubMed]
- McNamara, P.J.; Sehgal, A. Towards rational management of the patent ductus arteriosus: The need for disease staging. Arch. Dis. Child. Fetal Neonatal Ed. 2007, 92, F424–F427. [Google Scholar] [CrossRef] [PubMed]
- El-Khuffash, A.; James, A.T.; Corcoran, J.D.; Dicker, P.; Franklin, O.; Elsayed, Y.N.; Ting, J.Y.; Sehgal, A.; Malikiwi, A.; Harabor, A.; et al. A Patent Ductus Arteriosus Severity Score Predicts Chronic Lung Disease or Death before Discharge. J. Pediatrics 2015, 167, 1354–1361.e2. [Google Scholar] [CrossRef] [PubMed]
- van Laere, D.; van Overmeire, B.; Gupta, S.; El Khuffash, A.; Savoia, M.; McNamara, P.J.; Schwarz, C.E.; de Boode, W.P.; European Special Interest Group ‘Neonatologist Performed Echocardiography’ (NPE). Application of NPE in the assessment of a patent ductus arteriosus. Pediatr. Res. 2018, 84 (Suppl. 1), 46–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broadhouse, K.M.; Price, A.N.; Durighel, G.; Cox, D.J.; Finnemore, A.E.; Edwards, A.D.; Hajnal, J.V.; Groves, A.M. Assessment of PDA shunt and systemic blood flow in newborns using cardiac MRI. NMR Biomed. 2013, 26, 1135–1141. [Google Scholar] [CrossRef] [Green Version]
- Sehgal, A.; McNamara, P.J. Coronary artery perfusion and myocardial performance after patent ductus arteriosus ligation. J. Thorac. Cardiovasc. Surg. 2012, 143, 1271–1278. [Google Scholar] [CrossRef] [Green Version]
- Kluckow, M.; Lemmers, P. Hemodynamic assessment of the patent ductus arteriosus: Beyond ultrasound. Semin. Fetal Neonatal Med. 2018, 23, 239–244. [Google Scholar] [CrossRef]
- El-Khuffash, A.; Bussmann, N.; Breatnach, C.R.; Smith, A.; Tully, E.; Griffin, J.; McCallion, N.; Corcoran, J.D.; Fernandez, E.; Looi, C.; et al. A Pilot Randomized Controlled Trial of Early Targeted Patent Ductus Arteriosus Treatment Using a Risk Based Severity Score (The PDA RCT). J. Pediatrics 2020. [Google Scholar] [CrossRef]
- Evans, N. Preterm patent ductus arteriosus: A continuing conundrum for the neonatologist? Semin. Fetal Neonatal Med. 2015, 20, 272–277. [Google Scholar] [CrossRef]
- Marlow, N. Is survival and neurodevelopmental impairment at 2 years of age the gold standard outcome for neonatal studies? Arch. Dis. Child. Fetal Neonatal Ed. 2015, 100, F82–F84. [Google Scholar] [CrossRef] [Green Version]
- Webbe, J.W.H.; Duffy, J.M.N.; Afonso, E.; Al-Muzaffar, I.; Brunton, G.; Greenough, A.; Hall, N.J.; Knight, M.; Latour, J.M.; Lee-Davey, C.; et al. Core outcomes in neonatology: Development of a core outcome set for neonatal research. Arch. Dis. Child. Fetal Neonatal Ed. 2019, 105, 425–431. [Google Scholar] [CrossRef] [Green Version]
- Keller, R.L.; Feng, R.; DeMauro, S.B.; Ferkol, T.; Hardie, W.; Rogers, E.E.; Stevens, T.P.; Voynow, J.A.; Bellamy, S.L.; Shaw, P.A.; et al. Bronchopulmonary Dysplasia and Perinatal Characteristics Predict 1-Year Respiratory Outcomes in Newborns Born at Extremely Low Gestational Age: A Prospective Cohort Study. J. Pediatrics 2017, 187, 89–97.e3. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith, A.; EL-Khuffash, A. Patent Ductus Arteriosus Clinical Trials: Lessons Learned and Future Directions. Children 2021, 8, 47. https://doi.org/10.3390/children8010047
Smith A, EL-Khuffash A. Patent Ductus Arteriosus Clinical Trials: Lessons Learned and Future Directions. Children. 2021; 8(1):47. https://doi.org/10.3390/children8010047
Chicago/Turabian StyleSmith, Aisling, and Afif EL-Khuffash. 2021. "Patent Ductus Arteriosus Clinical Trials: Lessons Learned and Future Directions" Children 8, no. 1: 47. https://doi.org/10.3390/children8010047
APA StyleSmith, A., & EL-Khuffash, A. (2021). Patent Ductus Arteriosus Clinical Trials: Lessons Learned and Future Directions. Children, 8(1), 47. https://doi.org/10.3390/children8010047