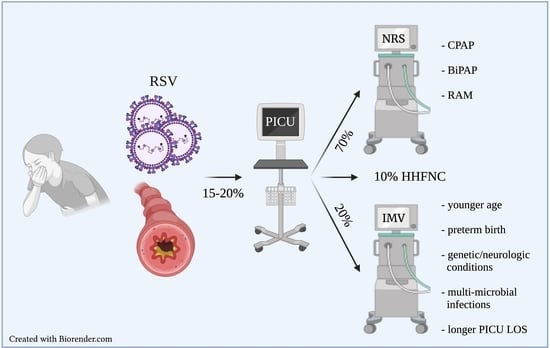
Risk Factors Associated with Mechanical Ventilation in Critical Bronchiolitis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Setting
2.2. Study Design
2.3. Eligibility
2.4. Variables
2.5. Definitions
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
Appendix A
| Diagnosis | ICD-9 | ICD-10 |
|---|---|---|
| Acute bronchiolitis due to respiratory syncytial virus | 466.11 | J21.0 |
| Acute bronchiolitis due to human metapneumovirus | 466.19 | J21.1 |
| Acute bronchiolitis due to other specified organisms | 466.19 | J21.8 |
| Acute bronchiolitis, unspecified | 466.19 | J21.9 |
References
- McLaurin, K.K.; Farr, A.M.; Wade, S.W.; Diakun, D.R.; Stewart, D.L. Respiratory syncytial virus hospitalization outcomes and costs of full-term and preterm infants. J. Perinatol. 2016, 36, 990–996. [Google Scholar] [CrossRef] [Green Version]
- Meissner, H.C. Viral Bronchiolitis in Children. N. Engl. J. Med. 2016, 374, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Silver, A.H.; Nazif, J.M. Bronchiolitis. Pediatr. Rev. 2019, 40, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Stockman, L.J.; Curns, A.T.; Anderson, L.J.; Fischer-Langley, G. Respiratory syncytial virus-associated hospitalizations among infants and young children in the United States, 1997–2006. Pediatr. Infect. Dis. J. 2012, 31, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Freire, G.; Kuppermann, N.; Zemek, R.; Plint, A.C.; Babl, F.E.; Dalziel, S.R.; Freedman, S.B.; Atenafu, E.G.; Stephens, D.; Steele, D.W.; et al. Predicting Escalated Care in Infants With Bronchiolitis. Pediatrics 2018, 142, e20174253. [Google Scholar] [CrossRef] [Green Version]
- Hall, C.B.; Weinberg, G.A.; Iwane, M.K.; Blumkin, A.K.; Edwards, K.M.; Staat, M.A.; Auinger, P.; Griffin, M.R.; Poehling, K.A.; Erdman, D.; et al. The burden of respiratory syncytial virus infection in young children. N. Engl. J. Med. 2009, 360, 588–598. [Google Scholar] [CrossRef] [Green Version]
- Garcia, C.G.; Bhore, R.; Soriano-Fallas, A.; Trost, M.; Chason, R.; Ramilo, O.; Mejias, A. Risk factors in children hospitalized with RSV bronchiolitis versus non-RSV bronchiolitis. Pediatrics 2010, 126, e1453–e1460. [Google Scholar] [CrossRef] [Green Version]
- Ralston, S.L.; Lieberthal, A.S.; Meissner, H.C.; Alverson, B.K.; Baley, J.E.; Gadomski, A.M.; Johnson, D.W.; Light, M.J.; Maraqa, N.F.; Mendonca, E.A.; et al. Clinical practice guideline: The diagnosis, management, and prevention of bronchiolitis. Pediatrics 2014, 134, e1474–e1502. [Google Scholar] [CrossRef] [Green Version]
- Gupta, P.; Beam, B.W.; Rettiganti, M. Temporal Trends of Respiratory Syncytial Virus-Associated Hospital and ICU Admissions Across the United States. Pediatr. Crit. Care Med. 2016, 17, e343–e351. [Google Scholar] [CrossRef]
- Rha, B.; Curns, A.T.; Lively, J.Y.; Campbell, A.P.; Englund, J.A.; Boom, J.A.; Azimi, P.H.; Weinberg, G.A.; Staat, M.A.; Selvarangan, R.; et al. Respiratory Syncytial Virus-Associated Hospitalizations Among Young Children: 2015–2016. Pediatrics 2020, 146, e20193611. [Google Scholar] [CrossRef]
- Hasegawa, K.; Pate, B.M.; Mansbach, J.M.; Macias, C.G.; Fisher, E.S.; Piedra, P.A.; Espinola, J.A.; Sullivan, A.F.; Camargo, C.A., Jr. Risk factors for requiring intensive care among children admitted to ward with bronchiolitis. Acad. Pediatr. 2015, 15, 77–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damore, D.; Mansbach, J.M.; Clark, S.; Ramundo, M.; Camargo, C.A., Jr. Prospective multicenter bronchiolitis study: Predicting intensive care unit admissions. Acad. Emerg. Med. 2008, 15, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Iyer, N.P.; Chatburn, R. Evaluation of a nasal cannula in noninvasive ventilation using a lung simulator. Respir. Care 2015, 60, 508–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, T.J.; Hovis, J.D.; Costantino, J.P.; Bierman, M.I.; Donahoe, M.P.; Rogers, R.M.; Kreit, J.W.; Sciurba, F.C.; Stiller, R.A.; Sanders, M.H. A randomized, prospective evaluation of noninvasive ventilation for acute respiratory failure. Am. J. Respir. Crit. Care Med. 2000, 161 Pt 1, 807–813. [Google Scholar] [CrossRef]
- Taha, A.; Larumbe-Zabala, E.; Abugroun, A.; Mohammedzein, A.; Naguib, M.T.; Patel, M. Outcomes of Noninvasive Positive Pressure Ventilation in Acute Respiratory Distress Syndrome and Their Predictors: A National Cohort. Crit. Care Res. Pract. 2019, 2019, 8106145. [Google Scholar] [CrossRef] [Green Version]
- Rochwerg, B.; Brochard, L.; Elliott, M.W.; Hess, D.; Hill, N.S.; Nava, S.; Navalesi, P.M.O.T.S.C.; Antonelli, M.; Brozek, J.; Conti, G.; et al. Official ERS/ATS clinical practice guidelines: Noninvasive ventilation for acute respiratory failure. Eur. Respir. J. 2017, 50, 1602426. [Google Scholar] [CrossRef]
- Yaman, A.; Kendirli, T.; Odek, C.; Ates, C.; Tasyapar, N.; Gunes, M.; Ince, E. Efficacy of noninvasive mechanical ventilation in prevention of intubation and reintubation in the pediatric intensive care unit. J. Crit. Care 2016, 32, 175–181. [Google Scholar] [CrossRef]
- Mayordomo-Colunga, J.; Medina, A.; Rey, C.; Diaz, J.J.; Concha, A.; Los Arcos, M.; Menendez, S. Predictive factors of non invasive ventilation failure in critically ill children: A prospective epidemiological study. Intensive Care Med. 2009, 35, 527–536. [Google Scholar] [CrossRef]
- Weichler, K.W.; Assal, O.E.; Forbes, M.; Pollauf, L. The Clinical impact of High Flow Nasal Cannula Utilization in the Emergency Department on the Endotracheal Intubation Rate for Infants and Young Children with Severe Bronchiolitis. Pediatrics 2019, 144, 416. [Google Scholar]
- Schlapbach, L.J.; Straney, L.; Gelbart, B.; Alexander, J.; Franklin, D.; Beca, J.; Whitty, J.A.; Ganu, S.; Wilkins, B.; Slater, A.; et al. Burden of disease and change in practice in critically ill infants with bronchiolitis. Eur. Respir. J. 2017, 49, 1601648. [Google Scholar] [CrossRef]
- Ganu, S.S.; Gautam, A.; Wilkins, B.; Egan, J. Increase in use of non-invasive ventilation for infants with severe bronchiolitis is associated with decline in intubation rates over a decade. Intensive Care Med. 2012, 38, 1177–1183. [Google Scholar] [CrossRef]
- Javouhey, E.; Barats, A.; Richard, N.; Stamm, D.; Floret, D. Non-invasive ventilation as primary ventilatory support for infants with severe bronchiolitis. Intensive Care Med. 2008, 34, 1608–1614. [Google Scholar] [CrossRef]
- Lazner, M.R.; Basu, A.P.; Klonin, H. Non-invasive ventilation for severe bronchiolitis: Analysis and evidence. Pediatr. Pulmonol. 2012, 47, 909–916. [Google Scholar] [CrossRef]
- Meduri, G.U.; Turner, R.E.; Abou-Shala, N.; Wunderink, R.; Tolley, E. Noninvasive positive pressure ventilation via face mask. First-line intervention in patients with acute hypercapnic and hypoxemic respiratory failure. Chest 1996, 109, 179–193. [Google Scholar] [CrossRef] [Green Version]
- Morris, J.V.; Ramnarayan, P.; Parslow, R.C.; Fleming, S.J. Outcomes for Children Receiving Noninvasive Ventilation as the First-Line Mode of Mechanical Ventilation at Intensive Care Admission: A Propensity Score-Matched Cohort Study. Crit. Care Med. 2017, 45, 1045–1053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernet, V.; Hug, M.I.; Frey, B. Predictive factors for the success of noninvasive mask ventilation in infants and children with acute respiratory failure. Pediatr. Crit. Care Med. 2005, 6, 660–664. [Google Scholar] [CrossRef] [PubMed]
- Straney, L.; Clements, A.; Parslow, R.C.; Pearson, G.; Shann, F.; Alexander, J.; Slater, A.; ANZICS Paediatric Study Group and the Paediatric Intensive Care Audit Network. Paediatric index of mortality 3: An updated model for predicting mortality in pediatric intensive care*. Pediatr. Crit. Care Med. 2013, 14, 673–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bressan, S.; Balzani, M.; Krauss, B.; Pettenazzo, A.; Zanconato, S.; Baraldi, E. High-flow nasal cannula oxygen for bronchiolitis in a pediatric ward: A pilot study. Eur. J. Pediatr. 2013, 172, 1649–1656. [Google Scholar] [CrossRef]
- Mayfield, S.; Bogossian, F.; O’Malley, L.; Schibler, A. High-flow nasal cannula oxygen therapy for infants with bronchiolitis: Pilot study. J. Paediatr. Child Health 2014, 50, 373–378. [Google Scholar] [CrossRef]
- Yehya, N.; Harhay, M.O.; Curley, M.A.Q.; Schoenfeld, D.A.; Reeder, R.W. Reappraisal of Ventilator-Free Days in Critical Care Research. Am. J. Respir. Crit. Care Med. 2019, 200, 828–836. [Google Scholar] [CrossRef]
- Stempel, H.E.; Martin, E.T.; Kuypers, J.; Englund, J.A.; Zerr, D.M. Multiple viral respiratory pathogens in children with bronchiolitis. Acta Paediatr. 2009, 98, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Shay, D.K.; Holman, R.C.; Newman, R.D.; Liu, L.L.; Stout, J.W.; Anderson, L.J. Bronchiolitis-associated hospitalizations among US children, 1980–1996. JAMA 1999, 282, 1440–1446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandrekar, J.N. Receiver operating characteristic curve in diagnostic test assessment. J. Thorac. Oncol. 2010, 5, 1315–1316. [Google Scholar] [CrossRef] [Green Version]
- Hasegawa, K.; Tsugawa, Y.; Brown, D.F.; Mansbach, J.M.; Camargo, C.A., Jr. Trends in bronchiolitis hospitalizations in the United States, 2000–2009. Pediatrics 2013, 132, 28–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mount, M.C.; Ji, X.; Kattan, M.W.; Slain, K.N.; Clayton, J.A.; Rotta, A.T.; Shein, S.L. Derivation and Validation of the Critical Bronchiolitis Score for the PICU. Pediatr. Crit. Care Med. 2021. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, I.; Rego, C.; Semedo, G.; Gomes, D.; Figueiras, A.; Roque, F.; Herdeiro, M.T. Systematic Review on the Impact of Guidelines Adherence on Antibiotic Prescription in Respiratory Infections. Antibiotics 2020, 9, 546. [Google Scholar] [CrossRef] [PubMed]
- Shein, S.L.; Kong, M.; McKee, B.; O’Riordan, M.; Toltzis, P.; Randolph, A.G. Antibiotic Prescription in Young Children With Respiratory Syncytial Virus-Associated Respiratory Failure and Associated Outcomes. Pediatr. Crit. Care Med. 2019, 20, 101–109. [Google Scholar] [CrossRef]
- Kopp, W.; Gedeit, R.G.; Asaro, L.A.; McLaughlin, G.E.; Wypij, D.; Curley, M.A.Q.; Randomized Evaluation of Sedation Titration for Respiratory Failure Study Investigators. The Impact of Preintubation Noninvasive Ventilation on Outcomes in Pediatric Acute Respiratory Distress Syndrome. Crit. Care Med. 2021, 49, 816–827. [Google Scholar] [CrossRef]


| NRS (440) | IMV (133) | p† | Early IMV (96) | Late IMV (37) | p† | |
|---|---|---|---|---|---|---|
| Age at PICU admission | ||||||
| Median (IQR), mo | 5 (2–11) | 3 (1.7–6.5) | 0.002 | 3 (1.5–6) | 3 (2–7.5) | 0.23 |
| n (%) | ||||||
| ≤1 month | 35 (8) | 19 (14) | 0.022 | 16 (17) | 3 (8) | 0.39 |
| 2–12 months | 315 (72) | 97 (73) | 69 (72) | 28 (76) | ||
| 13–24 months | 90 (20) | 17 (13) | 11 (11) | 6 (16) | ||
| Weight (kg), median (IQR) | 7.0 (5.1–9.3) | 5.3 (3.9–7.4) | <0.001 | 5.3 (3.6–7.2) | 5.3 (4.4–7.7) | 0.28 |
| Sex, n (%) | ||||||
| Female | 187 (43) | 62 (47) | 0.40 | 46 (48) | 16 (43) | 0.51 |
| Race, n (%) | ||||||
| Caucasian | 263 (60) | 77 (58) | 0.70 | 52 (54) | 25 (68) | 0.16 |
| Non-Caucasian | 177 (40) | 56 (42) | 44 (46) | 12 (32) | ||
| Ethnicity, n (%) | ||||||
| Hispanic | 87 (20) | 23 (17) | 0.52 | 14 (15) | 9 (24) | 0.18 |
| Non-Hispanic | 353 (80) | 110 (83) | 82 (85) | 28 (76) | ||
| Insurance, n (%) | ||||||
| Medicaid | 171 (39) | 56 (42) | 0.50 | 43 (45) | 13 (35) | 0.31 |
| Other | 269 (61) | 77 (58) | 53 (55) | 24 (65) |
| NRS (440) | IMV (133) | p† | Early IMV (96) | Late IMV (37) | p† | |
|---|---|---|---|---|---|---|
| Gestational age at delivery | ||||||
| Median (IQR), week | 38 (36–38) | 37 (34–38) | <0.001 | 36.5 (34–38) | 37 (34.5–38) | 0.52 |
| n (%) | ||||||
| Full-term at birth | 310 (70) | 68 (51) | <0.001 | 48 (50) | 18 (49) | 0.89 |
| Pre-term at birth | 130 (30) | 65 (49) | 48 (50) | 19 (51) | ||
| Late (33 to <37 weeks) | 82 (19) | 40 (30) | 0.56 | 28 (29) | 14 (38) | 0.43 |
| Very (28 to <32 weeks) | 38 (9) | 17 (13) | 13 (14) | 4 (11) | ||
| Extreme (<28 weeks) | 10 (2) | 8 (6) | 7 (7) | 1 (3) | ||
| Pre-existing genetic conditions, n (%) | 22 (5) | 16 (12) | 0.0042 | 8 (8) | 8 (22) | 0.035 |
| Pre-existing neurologic conditions, n (%) | 25 (6) | 26 (20) | <0.001 | 18 (19) | 8 (22) | 0.71 |
| NRS | IMV | p† | Early IMV | Late IMV | p† | |
|---|---|---|---|---|---|---|
| Intubation, n (%) | 0 | 133 | – | 96 (72) | 37 (28) | – |
| Duration (d), median (IQR) | n/a | 7 (5–9) | – | 6 (4–9) | 7 (6–13.5) | 0.043 |
| Ventilator-free days, median (IQR) | n/a | 21 (19–23) | – | 22 (19–24) | 21 (15–22) | 0.041 |
| PICU LOS (d), median (IQR) | 2 (1–3) | 8 (6–11) | <0.001 | 7 (4.75–10) | 10 (7–19.5) | <0.001 |
| PIM-III ROM, % (IQR) | 1.0 (0.9–1.2) | 1.1 (0.9–1.7) | 0.007 | 1.1 (0.9–1.8) | 1.0 (0.7–1.2) | 0.008 |
| Vasoactives usage, n (%) | 0 (0) | 16 (12) | <0.001 | 7 (7) | 9 (24) | 0.007 |
| Mortality, n (%) | 0 (0) | 2 (1.5) | 0.01 | 2 (2) | 0 (0) | 0.38 |
| Pathogen f (#), median (IQR) | 1 (1–1) | 2 (2–3) | <0.001 | 2 (1–3) | 3 (2–4) | 0.007 |
| Pathogen positive, n (%) | 398 (97) | 131 (98) | 0.54 | 95 (99) | 36 (97) | 0.48 |
| Single | 299 (75) | 29 (22) | <0.001 | 25 (26) | 4 (11) | 0.061 |
| Multiple | 99 (25) | 102 (78) | 70 (74) | 32 (89) | ||
| Virus only | 391 (98) | 36 (27) | <0.001 | 29 (31) | 7 (19) | 0.48 |
| Bacteria only | 0 (0) | 4 (3) | 3 (3) | 1 (3) | ||
| Virus + Bacteria | 7 (2) | 91 (69) | 63 (66) | 28 (78) | ||
| Viral count (>1) | 94 (24) | 29 (22) | 0.73 | 18 (19) | 11 (31) | 0.15 |
| NRS (440) | IMV (133) | p† | Early IMV (96) | Late IMV (37) | p† | |
|---|---|---|---|---|---|---|
| Total, n (%) | 126 (29) | 125 (94) | <0.001 | 90 (94) | 35 (95) | 0.85 |
| Duration (d), median (IQR) | 7 (3–10) | 7 (7–10) | <0.001 | 7 (7–10) | 10 (7–14) | 0.017 |
| Time to initiation (h after intubation), median (IQR) | n/a | 2 (0–18) | - | 2 (0–10) | 8 (0–32) | 0.20 |
| WBC (×1000), median (IQR) | n.c. | 9.4 (6.8–13) | - | 8.9 (6.8–12) | 11 (7.7–15) | 0.15 |
| % band, median (IQR) | n.c. | 0.5 (0–8) | - | 1 (0–8) | 0 (0–5.3) | 0.46 |
| Procalcitonin, median (IQR) | n.c. | 1 (0.3–16) | - | 1.6 (0.3–16) | 0.6 (0.4–17) | 0.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marlow, R.K.; Brouillette, S.; Williams, V.; Lenihan, A.; Nemec, N.; Lukowski, J.D.; Zheng, C.; Cullimore, M.L.; Mahapatra, S. Risk Factors Associated with Mechanical Ventilation in Critical Bronchiolitis. Children 2021, 8, 1035. https://doi.org/10.3390/children8111035
Marlow RK, Brouillette S, Williams V, Lenihan A, Nemec N, Lukowski JD, Zheng C, Cullimore ML, Mahapatra S. Risk Factors Associated with Mechanical Ventilation in Critical Bronchiolitis. Children. 2021; 8(11):1035. https://doi.org/10.3390/children8111035
Chicago/Turabian StyleMarlow, Rachel K., Sydney Brouillette, Vannessa Williams, Ariann Lenihan, Nichole Nemec, Joseph D. Lukowski, Cheng Zheng, Melissa L. Cullimore, and Sidharth Mahapatra. 2021. "Risk Factors Associated with Mechanical Ventilation in Critical Bronchiolitis" Children 8, no. 11: 1035. https://doi.org/10.3390/children8111035
APA StyleMarlow, R. K., Brouillette, S., Williams, V., Lenihan, A., Nemec, N., Lukowski, J. D., Zheng, C., Cullimore, M. L., & Mahapatra, S. (2021). Risk Factors Associated with Mechanical Ventilation in Critical Bronchiolitis. Children, 8(11), 1035. https://doi.org/10.3390/children8111035