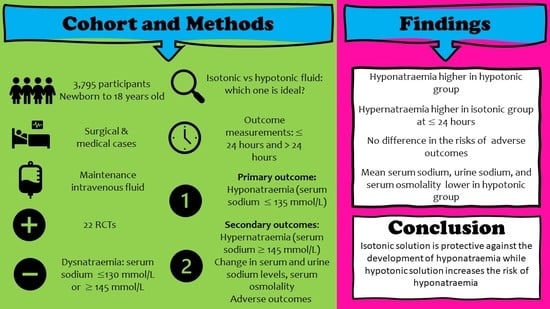
Efficacy and Safety of Isotonic and Hypotonic Intravenous Maintenance Fluids in Hospitalised Children: A Systematic Review and Meta-Analysis of Randomised Controlled Trials
Abstract
:1. Introduction
2. Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Study Selection
2.4. Data Extraction
2.5. Subgroup and Sensitivity Analyses
2.6. Quality Assessment and Publication Bias
2.7. Data Analysis
3. Results
3.1. Study Selection
3.2. Study Characteristics
3.3. Primary Outcomes
3.4. Secondary Outcomes
3.5. Quality Assessment and Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Holliday, M.A.; Segar, W.E. The maintenance need for water in parenteral fluid therapy. Pediatrics 1957, 19, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.N. Risk of acute hyponatremia in hospitalized children and youth receiving maintenance intravenous fluids. Paediatr. Child Health 2013, 18, 102–107. [Google Scholar] [CrossRef]
- Moritz, M.L.; Ayus, J.C. Prevention of hospital-acquired hyponatremia: A case for using isotonic saline. Pediatrics 2003, 111, 227–230. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence. Intravenous Fluid Therapy in Adults in Hospital; National Institute for Health and Care Excellence: London, UK, 2017. [Google Scholar]
- Feld, L.G.; Neuspiel, D.R.; Foster, B.A.; Leu, M.G.; Garber, M.D.; Austin, K.; Basu, R.K.; Conway, E.E., Jr.; Fehr, J.J.; Hawkins, C.; et al. Clinical Practice Guideline: Maintenance Intravenous Fluids in Children. Pediatrics 2018, 142, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Mathur, A.; Johnston, G.; Clark, L. Improving intravenous fluid prescribing. J. R. Coll. Physicians Edinb. 2020, 50, 181–187. [Google Scholar] [CrossRef]
- Hall, A.M.; Ayus, J.C.; Moritz, M.L. Things We Do For No Reason: The Default Use of Hypotonic Maintenance Intravenous Fluids in Pediatrics. J. Hosp. Med. 2018, 13, 637–640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McNab, S. Intravenous maintenance fluid therapy in children. J. Paediatr. Child Health 2016, 52, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Moritz, M.L.; Ayus, J.C. Maintenance intravenous fluids in acutely ill patients. N. Engl. J. Med. 2015, 373, 1350–1360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Severs, D.; Hoorn, E.J.; Rookmaaker, M.B. A critical appraisal of intravenous fluids: From the physiological basis to clinical evidence. Nephrol. Dial. Transplant. 2015, 30, 178–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, A.; Maltez, C. Intravenous fluid prescribing for medical inpatients: Are we getting it right? Clin. Med. 2019, 19, 57. [Google Scholar] [CrossRef]
- Lobo, D.; Dube, M.; Neal, K.; Simpson, J.; Rowlands, B.; Allison, S. Problems with solutions: Drowning in the brine of an inadequate knowledge base. Clin. Nutr. 2001, 20, 125–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramsay, G.; Baggaley, A.; Shaw, P.V.; Soltanmohammadi, E.; Ventham, N.; Shi, N.G.; Pearson, R.; Knight, S.; Forde, C.; Moore, N. Variability in the prescribing of intravenous fluids: A cross sectional multicentre analysis of clinical practice. Int. J. Surg. 2018, 51, 199–204. [Google Scholar] [CrossRef]
- Steele, A.; Gowrishankar, M.; Abrahamson, S.; Mazer, C.D.; Feldman, R.D.; Halperin, M.L. Postoperative hyponatremia despite near-isotonic saline infusion: A phenomenon of desalination. Ann. Intern. Med. 1997, 126, 20–25. [Google Scholar] [CrossRef]
- Alvis-Miranda, H.R.; Castellar-Leones, S.M.; Moscote-Salazar, L.R. Intravenous Fluid Therapy in Traumatic Brain Injury and Decompressive Craniectomy. Bull. Emerg. Trauma 2014, 2, 3–14. [Google Scholar]
- Tommasino, C.; Picozzi, V. Volume and electrolyte management. Best Pract. Res. Clin. Anaesthesiol. 2007, 21, 497–516. [Google Scholar] [CrossRef] [PubMed]
- Bockenhauer, D.; Aitkenhead, H. The kidney speaks: Interpreting urinary sodium and osmolality. Arch. Dis. Child. Educ. Pract. Ed. 2011, 96, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Kamel, K.S.; Ethier, J.H.; Richardson, R.M.; Bear, R.A.; Halperin, M.L. Urine electrolytes and osmolality: When and how to use them. Am. J. Nephrol. 1990, 10, 89–102. [Google Scholar] [CrossRef]
- Braun, M.M.; Barstow, C.; Pyzocha, N. Diagnosis and management of sodium disorders: Hyponatremia and hypernatremia. Am. Fam. Physician 2015, 91, 299–307. [Google Scholar]
- Yeates, K.E.; Singer, M.; Morton, A.R. Salt and water: A simple approach to hyponatremia. CMAJ 2004, 170, 365–369. [Google Scholar] [PubMed]
- Goh, K.P. Management of hyponatremia. Am. Fam. Physician 2004, 69, 2387–2394. [Google Scholar]
- National Institute for Health and Care Excellence. Intravenous Fluid Therapy in Children and Young People in Hospital; National Institute for Health and Care Excellence: London, UK, 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK563449/ (accessed on 11 June 2020).
- Kinlin, L.M.; Helmers, A.J.; Friedman, J.N.; Beck, C.E. Choice of maintenance intravenous fluids among paediatric residents in Canada. Paediatr. Child Health 2020, 25, 518–524. [Google Scholar] [CrossRef] [Green Version]
- Hall, A.M.; Ayus, J.C.; Moritz, M.L. How Salty Are Your Fluids? Pediatric Maintenance IV Fluid Prescribing Practices Among Hospitalists. Front. Pediatr. 2019, 7, 549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, P.; Hall, T.; Ali, T.; Lakhoo, K. Intravenous postoperative fluid prescriptions for children: A survey of practice. BMC Surg. 2008, 8, 10. [Google Scholar] [CrossRef] [Green Version]
- Freeman, M.A.; Ayus, J.C.; Moritz, M.L. Maintenance intravenous fluid prescribing practices among paediatric residents. Acta Paediatr. 2012, 101, 465–468. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.M.; Jung, Y.; Lee, S.E.; Lee, J.H.; Kim, K.H.; Koo, J.W.; Park, Y.S.; Cheong, H.I.; Ha, I.-S.; Choi, Y.; et al. Intravenous fluid prescription practices among pediatric residents in Korea. Korean J. Pediatr. 2013, 56, 282–285. [Google Scholar] [CrossRef]
- Way, C.; Dhamrait, R.; Wade, A.; Walker, I. Perioperative fluid therapy in children: A survey of current prescribing practice. Br. J. Anaesth. 2006, 97, 371–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shukla, S.; Basu, S.; Moritz, M.L. Use of hypotonic maintenance intravenous fluids and hospital-acquired hyponatremia remain common in children admitted to a general pediatric ward. Front. Pediatr. 2016, 4, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nordstrom, M.; Landman, G.; Pfaff, N.; Kaiser, S.V. Improving Isotonic Maintenance Intravenous Fluid Use at a Tertiary Children’s Hospital. Hosp. Pediatr. 2021, 11, 374–379. [Google Scholar] [CrossRef]
- Akinsola, B.; Cheng, J.; Iyer, S.B.; Jain, S. Improving Isotonic Maintenance Intravenous Fluid Use in the Emergency Department. Pediatrics 2021, 148, e2020022947. [Google Scholar] [CrossRef]
- Moritz, M.L.; Ayus, J.C. Isotonic fluids prevent hospital-acquired hyponatraemia. Nat. Rev. Nephrol. 2015, 11, 202–203. [Google Scholar] [CrossRef] [PubMed]
- Kannan, L.; Lodha, R. Appropriate fluid for intravenous maintenance therapy in hospitalized children--current status. Indian J. Pediatr. 2011, 78, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, 1–36. [Google Scholar]
- Varrier, M.; Ostermann, M. Fluid Composition and Clinical Effects. Crit. Care Clin. 2015, 31, 823–837. [Google Scholar] [CrossRef]
- Saniasiaya, J.; Islam, M.A.; Abdullah, B. Prevalence and characteristics of taste disorders in cases of COVID-19: A meta-analysis of 29,349 patients. Otolaryngol. Head Neck Surg. 2020, 165, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Alam, S.S.; Kundu, S.; Hossan, T.; Kamal, M.A.; Cavestro, C. Prevalence of Headache in Patients With Coronavirus Disease 2019 (COVID-19): A Systematic Review and Meta-Analysis of 14,275 Patients. Front. Neurol. 2020, 11, 562634. [Google Scholar] [CrossRef] [PubMed]
- Moola, S.; Munn, Z.; Tufanaru, C.; Aromataris, E.; Sears, K.; Sfetcu, R.; Currie, M.; Lisy, K.; Qureshi, R.; Mattis, P.; et al. Chapter 7: Systematic reviews of etiology and risk. In JBI Manual for Evidence Synthesis; Aromataris, E., Munn, Z., Eds.; JBI: Adelaide, Australia, 2020. [Google Scholar]
- Hajissa, K.; Marzan, M.; Idriss, M.I.; Islam, M.A. Prevalence of Drug-Resistant Tuberculosis in Sudan: A Systematic Review and Meta-Analysis. Antibiotics 2021, 10, 932. [Google Scholar] [CrossRef]
- Kannan, L.; Lodha, R.; Vivekanandhan, S.; Bagga, A.; Kabra, S.K.; Kabra, M. Intravenous fluid regimen and hyponatraemia among children: A randomized controlled trial. Pediatr. Nephrol. 2010, 25, 2303–2309. [Google Scholar] [CrossRef] [PubMed]
- Almeida, H.I.; Mascarenhas, M.I.; Loureiro, H.C.; Abadesso, C.S.; Nunes, P.S.; Moniz, M.S.; Machado, M.C. The effect of NaCl 0.9% and NaCl 0.45% on sodium, chloride, and acid-base balance in a PICU population. J. Pediatr. 2015, 91, 499–505. [Google Scholar] [CrossRef] [Green Version]
- Bagri, N.K.; Saurabh, V.K.; Basu, S.; Kumar, A. Isotonic versus Hypotonic Intravenous Maintenance Fluids in Children: A Randomized Controlled Trial. Indian J. Pediatr. 2019, 86, 1011–1016. [Google Scholar] [CrossRef]
- Balasubramaniam, K.; Kumar, P.; Saini, S.S.; Attri, S.V.; Dutta, S. Isotonic versus hypotonic fluid supplementation in term neonates with severe hyperbilirubinemia–A double-blind, randomized, controlled trial. Acta Paediatr. 2012, 101, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Brazel, P.W.; McPhee, I.B. Inappropriate secretion of antidiuretic hormone in postoperative scoliosis patients: The role of fluid management. Spine 1996, 21, 724–727. [Google Scholar] [CrossRef] [PubMed]
- Choong, K.; Arora, S.; Cheng, J.; Farrokhyar, F.; Reddy, D.; Thabane, L.; Walton, J.M. Hypotonic versus isotonic maintenance fluids after surgery for children: A randomized controlled trial. Pediatrics 2011, 128, 857–866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coulthard, M.G.; Long, D.A.; Ullman, A.J.; Ware, R.S. A randomised controlled trial of Hartmann’s solution versus half normal saline in postoperative paediatric spinal instrumentation and craniotomy patients. Arch. Dis. Child. 2012, 97, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Flores Robles, C.M.; Cuello García, C.A. A prospective trial comparing isotonic with hypotonic maintenance fluids for prevention of hospital-acquired hyponatraemia. Paediatr. Int. Child Health 2016, 36, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.N.; Beck, C.E.; DeGroot, J.; Geary, D.F.; Sklansky, D.J.; Freedman, S.B. Comparison of isotonic and hypotonic intravenous maintenance fluids: A randomized clinical trial. JAMA Pediatr. 2015, 169, 445–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baron, F.; Meregalli, C.N.; Rombola, V.; Bolasell, C.; Pigliapoco, V.; Bartoletti, S.; Debaisi, G. Hypotonic versus isotonic intravenous maintenance fluids in critically ill pediatric patients: A randomized clinical trial. Arch. Argent. Pediatr. 2013, 111, 281–287. [Google Scholar] [CrossRef]
- Kumar, M.; Mitra, K.; Jain, R. Isotonic versus hypotonic saline as maintenance intravenous fluid therapy in children under 5 years of age admitted to general paediatric wards: A randomised controlled trial. Paediatr. Int. Child Health 2019, 40, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Lehtiranta, S.; Honkila, M.; Kallio, M.; Paalanne, N.; Peltoniemi, O.; Pokka, T.; Renko, M.; Tapiainen, T. Risk of electrolyte disorders in acutely ill children receiving commercially available plasmalike isotonic fluids: A randomized clinical trial. JAMA Pediatr. 2021, 175, 28–35. [Google Scholar] [CrossRef] [PubMed]
- McNab, S.; Duke, T.; South, M.; Babl, F.E.; Lee, K.J.; Arnup, S.J.; Young, S.; Turner, H.; Davidson, A. 140 mmol/L of sodium versus 77 mmol/L of sodium in maintenance intravenous fluid therapy for children in hospital (PIMS): A randomised controlled double-blind trial. Lancet 2015, 385, 1190–1197. [Google Scholar] [CrossRef]
- Mierzewska-Schmidt, M. Intraoperative fluid management in children—A comparison of three fluid regimens. Anaesthesiol. Intensive Ther. 2015, 47, 125–130. [Google Scholar] [CrossRef] [Green Version]
- Montanana, P.A.; i Alapont, V.M.; Ocon, A.P.; Lopez, P.O.; Prats, J.L.L.; Parreño, J.D.T. The use of isotonic fluid as maintenance therapy prevents iatrogenic hyponatremia in pediatrics: A randomized, controlled open study. Pediatr. Crit. Care Med. 2008, 9, 589–597. [Google Scholar] [CrossRef] [Green Version]
- Omoifo, C.E.; Edomwonyi, N.P.; Idogun, S.E. Incidence of hyponatraemia following the use of three different intravenous fluids in paediatric surgery. Afr. J. Paediatr. Surg. 2018, 15, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Pemde, H.K.; Dutta, A.K.; Sodani, R.; Mishra, K. Isotonic intravenous maintenance fluid reduces hospital acquired hyponatremia in young children with central nervous system infections. Indian J. Pediatr. 2015, 82, 13–18. [Google Scholar] [CrossRef]
- Ramanathan, S.; Kumar, P.; Mishra, K.; Dutta, A.K. Isotonic versus Hypotonic Parenteral Maintenance Fluids in Very Severe Pneumonia. Indian J. Pediatr. 2016, 83, 27–32. [Google Scholar] [CrossRef]
- Raksha, S.; Dakshayani, B. Full Volume Isotonic (0.9%) vs. Two-Thirds Volume Hypotonic (0.18%) Intravenous Maintenance Fluids in Preventing Hyponatremia in Children Admitted to Pediatric Intensive Care Unit—A Randomized Controlled Study. J. Trop. Pediatr. 2017, 63, 454–460. [Google Scholar]
- Saba, T.G.; Fairbairn, J.; Houghton, F.; Laforte, D.; Foster, B.J. A randomized controlled trial of isotonic versus hypotonic maintenance intravenous fluids in hospitalized children. BMC Pediatr. 2011, 11, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shamim, A.; Afzal, K.; Ali, S.M. Safety and efficacy of isotonic (0.9%) vs. hypotonic (0.18%) saline as maintenance intravenous fluids in children: A randomized controlled trial. Indian Pediatr. 2014, 51, 969–974. [Google Scholar] [CrossRef] [Green Version]
- Torres, S.F.; Iolster, T.; Schnitzler, E.J.; Serrate, A.J.S.; Sticco, N.A.; Rivarola, M.R. Hypotonic and isotonic intravenous maintenance fluids in hospitalised paediatric patients: A randomised controlled trial. BMJ Paediatr. Open 2019, 3, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.; Durward, A. Pouring salt on troubled waters. Arch. Dis. Child. 2004, 89, 411–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holliday, M.A.; Ray, P.E.; Friedman, A.L. Fluid therapy for children: Facts, fashions and questions. Arch. Dis. Child. 2007, 92, 546–550. [Google Scholar] [CrossRef] [Green Version]
- Choong, K.; Kho, M.E.; Menon, K.; Bohn, D. Hypotonic versus isotonic saline in hospitalised children: A systematic review. Arch. Dis. Child. 2006, 91, 828–835. [Google Scholar] [CrossRef]
- Foster, B.A.; Tom, D.; Hill, V. Hypotonic versus isotonic fluids in hospitalized children: A systematic review and meta-analysis. J. Pediatr. 2014, 165, 163–169. [Google Scholar] [CrossRef]
- McNab, S.; Ware, R.S.; Neville, K.A.; Choong, K.; Coulthard, M.G.; Duke, T.; Davidson, A.; Dorofaeff, T. Isotonic versus hypotonic solutions for maintenance intravenous fluid administration in children. Cochrane Database Syst. Rev. 2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Padua, A.P.; Macaraya, J.R.G.; Dans, L.F.; Anacleto, F.E. Isotonic versus hypotonic saline solution for maintenance intravenous fluid therapy in children: A systematic review. Pediatr. Nephrol. 2015, 30, 1163–1172. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Jiang, W.; Wang, X.; Liu, W. The efficacy of isotonic and hypotonic intravenous maintenance fluid for pediatric patients: A meta-analysis of randomized controlled trials. Pediatr. Emerg. Care 2015, 31, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, E.; Xiao, Y. Isotonic versus hypotonic maintenance IV fluids in hospitalized children: A meta-analysis. Pediatrics 2014, 133, 105–113. [Google Scholar] [CrossRef] [Green Version]
- Rangaraju, M.; Slator, R.; Richard, B. Post-operative intravenous fluid administration for infant cleft surgery: An observational study. J. Plast. Reconstr. Aesthet. Surg. 2021, 74, 839–844. [Google Scholar] [CrossRef]
- Langer, T.; Limuti, R.; Tommasino, C.; Van Regenmortel, N.; Duval, E.L.; Caironi, P.; Malbrain, M.L.; Pesenti, A. Intravenous fluid therapy for hospitalized and critically ill children: Rationale, available drugs and possible side effects. Anaesthesiol. Intensive Ther. 2018, 50, 49–58. [Google Scholar] [CrossRef] [Green Version]
- Sahay, M.; Sahay, R. Hyponatremia: A practical approach. Indian J. Endocrinol. Metab. 2014, 18, 760–771. [Google Scholar] [CrossRef]
- Rai, A.; Whaley-Connell, A.; McFarlane, S.; Sowers, J.R. Hyponatremia, arginine vasopressin dysregulation, and vasopressin receptor antagonism. Am. J. Nephrol. 2006, 26, 579–589. [Google Scholar] [CrossRef]
- Cavari, Y.; Pitfield, A.F.; Kissoon, N. Intravenous maintenance fluids revisited. Pediatr. Emerg. Care 2013, 29, 1225–1231. [Google Scholar] [CrossRef] [PubMed]
- Arieff, A.I.; Ayus, J.C.; Fraser, C.L. Hyponatraemia and death or permanent brain damage in healthy children. Br. Med. J. 1992, 304, 1218–1222. [Google Scholar] [CrossRef] [Green Version]
- Benzon, H.A.; Bobrowski, A.; Suresh, S.; Wasson, N.R.; Cheon, E.C. Impact of preoperative hyponatraemia on paediatric perioperative mortality. Br. J. Anaesth. 2019, 123, 618–626. [Google Scholar] [CrossRef]
- Singhi, S.; Prasad, S.; Chugh, K. Hyponatremia in sick children: A marker of serious illness. Age 1994, 125, 126–130. [Google Scholar]
- Kutz, A.; Ebrahimi, F.; Aghlmandi, S.; Wagner, U.; Bromley, M.; Illigens, B.; Siepmann, T.; Schuetz, P.; Mueller, B.; Christ-Crain, M. Risk of adverse clinical outcomes in hyponatremic adult patients hospitalized for acute medical conditions: A population-based cohort study. J. Clin. Endocrinol. Metab. 2020, 105, 3428–3436. [Google Scholar] [CrossRef] [PubMed]
- Au, A.K.; Ray, P.E.; McBryde, K.D.; Newman, K.D.; Weinstein, S.L.; Bell, M.J. Incidence of postoperative hyponatremia and complications in critically-ill children treated with hypotonic and normotonic solutions. J. Pediatr. 2008, 152, 33–38. [Google Scholar] [CrossRef]
- Chua, H.-R.; Venkatesh, B.; Stachowski, E.; Schneider, A.G.; Perkins, K.; Ladanyi, S.; Kruger, P.; Bellomo, R. Plasma-Lyte 148 vs 0.9% saline for fluid resuscitation in diabetic ketoacidosis. J. Crit. Care 2012, 27, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Mahler, S.A.; Conrad, S.A.; Wang, H.; Arnold, T.C. Resuscitation with balanced electrolyte solution prevents hyperchloremic metabolic acidosis in patients with diabetic ketoacidosis. Am. J. Emerg. Med. 2011, 29, 670–674. [Google Scholar] [CrossRef]
- McFarlane, C.; Lee, A. A comparison of Plasmalyte 148 and 0.9% saline for intra-operative fluid replacement. Anaesthesia 1994, 49, 779–781. [Google Scholar] [CrossRef]
- Scheingraber, S.; Rehm, M.; Sehmisch, C.; Finsterer, U. Rapid saline infusion produces hyperchloremic acidosis in patients undergoing gynecologic surgery. Anesthesiology 1999, 90, 1265–1270. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.L.; Hildebrand, K.L.; McCormick, S.A.; Bedel, M.J. The effect of intravenous lactated Ringer’s solution versus 0.9% sodium chloride solution on serum osmolality in human volunteers. Anesth. Analg. 1999, 88, 999–1003. [Google Scholar] [CrossRef] [PubMed]
- Young, J.B.; Utter, G.H.; Schermer, C.R.; Galante, J.M.; Phan, H.H.; Yang, Y.; Anderson, B.A.; Scherer, L.A. Saline versus Plasma-Lyte A in initial resuscitation of trauma patients: A randomized trial. Ann. Surg. 2014, 259, 255–262. [Google Scholar] [CrossRef] [Green Version]
- Bulfon, A.F.; Alomani, H.L.; Anton, N.; Comrie, B.T.; Rochwerg, B.; Stef, S.A.; Thabane, L.; Vanniyasingam, T.; Choong, K. Intravenous fluid prescription practices in critically ill children: A shift in focus from natremia to chloremia? J. Pediatr. Intensive Care 2019, 8, 218–225. [Google Scholar] [CrossRef]
- Neville, K.A.; Sandeman, D.J.; Rubinstein, A.; Henry, G.M.; McGlynn, M.; Walker, J.L. Prevention of Hyponatremia during Maintenance Intravenous Fluid Administration: A Prospective Randomized Study of Fluid Type versus Fluid Rate. J. Pediatr. 2010, 156, 313–319. [Google Scholar] [CrossRef]
- Sulemanji, M.; Vakili, K. Neonatal renal physiology. Semin. Pediatr. Surg. 2013, 22, 195–198. [Google Scholar] [CrossRef]
- Blackburn, S.T. Renal function in the neonate. J. Perinat. Neonatal. Nurs. 1994, 8, 37–47. [Google Scholar] [CrossRef]
- Tuzun, F.; Akcura, Y.; Duman, N.; Ozkan, H. Comparison of isotonic and hypotonic intravenous fluids in term newborns: Is it time to quit hypotonic fluids. J. Matern. Fetal Neonatal Med. 2020, 1–6. [Google Scholar] [CrossRef]







| No. | Study ID [References] | Country | Follow-Up Duration | Condition | Isotonic | Hypotonic | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Age (Mean ± SD/Median (IQR)/Range) | Solution | N | Age (Mean ± SD/Median (IQR)/Range) | Solution | |||||
| 1 | Almeida 2014 [41] | Portugal | 24 h | Surgical and medical | 130 | 49.9 ± 62.5 (months) | NaCl 0.9%, with 154 mEq Na and Cl/L in 5% dextrose | 103 | 41.1 ± 64.4 (months) | NaCl 0.45%, with 75 mEq Na and Cl/L in 5% dextrose |
| 2 | Bagri 2019 [42] | India | 48 h | Medical | 74 | 36.0 (12.0–108.0) (months) | 0.9% saline in 5% dextrose with 20 mEq/L of potassium chloride | 74 | 60.0 (13.0–120.0) (months) | 0.45% saline in 5% dextrose with 20 mEq/L of potassium chloride |
| 3 | Balasubramaniam 2011 [43] | India | 24 h | Medical | 42 | 4.9 ± 2.0 (days) | 0.9% saline in 5% dextrose | 42 | 5.5 ± 1.9 (days) | 0.2% saline in 5% dextrose |
| 4 | Brazel 1996 [44] | Australia | NR | Surgical | 5 | 12.3–18.1 (years) | Hartman’s solution | 7 | 12.3–18.1 (years) | 0.3% NS in 3% dextrose 0.18% NS in 4% dextrose |
| 5 | Choong 2011 [45] | Canada | 48 h | Surgical | 128 | 9.2 ± 5.5 (years) | 0.9% saline in 5% dextrose | 130 | 9.0 ± 5.7 (years) | 0.45% saline in 5% dextrose |
| 6 | Coulthard 2012 [46] | Australia | 18 h | Surgical | 39 | 136.0 (52.0–167.0) (months) | Hartmann’s and 5% dextrose | 40 | 138.0 (72.0–169.0) (months) | 0.45% NaCl and 5% dextrose |
| 7 | Flores Robles 2015 [47] | Canada | 8 h | Surgical and medical | 52 | 58.8 ± 57.7 (months) | 0.9% saline in 5% dextrose | 49 | 63.5 ± 56.1 (months) | 0.3% saline in 3.3% dextrose |
| 50 | 54.6 ± 55.9 (months) | 0.45% saline in 5% dextrose | ||||||||
| 8 | Friedman 2015 [48] | Canada | 24 h | Medical | 47 | 3.9 (2.0–6.9) (years) | 0.9% saline in 5% dextrose | 45 | 5.8 (1.4–11.2) (years) | 0.45% saline in 5% dextrose |
| 9 | Jorro Baron 2013 [49] | Argentina | 24 h | Surgical and medical | 31 | 5.0 (3.0–9.0) (months) | 154 mmol/L sodium + 20 mmol/L potassium in 5% dextrose | 32 | 5.0 (3.0–10.0) (months) | 77 mmol/L sodium + 20 mmol/L potassium in 5% dextrose |
| 10 | Kannan 2010 [40] | India | 24 h | Medical | 58 | 36.0 (12.0–84.0) (months) | 0.9% saline in 5% dextrose at standard maintenance rate | 56 | 48.0 (12.7–72.0) (months) | 0.18% saline in 5% dextrose at the standard maintenance rate |
| 53 | 36.0 (10.0–66.0) (months) | 0.18% saline in 5% dextrose at 2/3 of the standard maintenance rate | ||||||||
| 11 | Kumar 2019 [50] | India | 24 h | Medical | 84 | 16.0 (7.0–30.0) (months) | 0.9% saline in 5% dextrose | 84 | 11.0 (5.0–28.5) (months) | 0.45% saline in 5% dextrose |
| 12 | Lehtiranta 2020 [51] | Finland | 7 days | Surgical and medical | 308 | 4.0 ± 3.1 (years) | 140 mmol/L of sodium and 5 mmol/L potassium in 5% dextrose | 306 | 4.1 ± 3.1 (years) | 80 mmol/L sodium and 20 mmol/L potassium in 5% dextrose |
| 13 | McNab 2014 [52] | Australia | 72 h | Surgical | 319 | 8.2 ± 5.4 (years) | 140 mmol/L of sodium | 322 | 8.9 ± 5.3 (years) | 77 mmol/L of sodium |
| 14 | Mierzewska-Schmidt 2015 [53] | Poland | NR | Surgical | 30 | 6.1 ± 2.1 (years) | Ringer’s acetate | 33 | 6.2 ± 2.1 (years) | 5% glucose in water solution |
| 27 | 6.5 ± 2.5 (years) | 3.33% glucose in 0.3% NaCl | ||||||||
| 15 | Montanana 2008 [54] | Spain | 24 h | Surgical and medical | 51 | 3.2 (1.3–10.0) (years) | 140 mEq/L sodium + 15 mEq/L potassium in 5% dextrose | 52 | 3.0 (0.9–7.0) (years) | 20 and 100 mEq/L sodium in 5% dextrose |
| 16 | Omoifo 2018 [55] | Nigeria | NR | Surgical | 20 | 5.9 ± 3.5 (years) | Normal saline | 25 | 6.5 ± 3.7 (years) | 4.3% dextrose in 0.18 saline |
| 17 | Pemde 2015 [56] | India | 24 h | Medical | 31 | 26.2 ± 19.6) (months) | 0.9% saline in 5% dextrose | 30 | 31.9 ± 20.7 (months) | 0.45% saline in 5% dextrose |
| 31 | 28.2 ± 21.2 (months) | 0.18% saline in 5% dextrose | ||||||||
| 18 | Ramanathan 2015 [57] | India | 24 h | Medical | 59 | 2.0–60.0 (months) | 0.9% saline in 5% dextrose and potassium chloride 20 meq/L | 60 | 2.0–60.0 (months) | 0.18% saline in 5% dextrose and potassium chloride 20 meq/L |
| 19 | Raksha 2017 [58] | India | 24 h | Medical | 120 | 1.0 month–18.0 years old | 0.9% saline in 5% dextrose with 20 mEq/L of potassium chloride at standard maintenance rate | 120 | 1.0 month–18.0 years old | 0.18% saline in 5% dextrose/isolyte-p at 2/3 standard maintenance rate |
| 20 | Saba 2011 [59] | Canada | 8 h | Surgical and medical | 16 | 8.2 (2.8–14.3) (years) | 0.9% saline in 5% dextrose | 21 | 8.9 (1.7–16.5) (years) | 0.45% saline in 5% dextrose |
| 21 | Shamim 2014 [60] | India | 48 h | Medical | 30 | 53.1 ± 39.5 (months) | 0.9% NaCl in 5% dextrose at the rate of 60% of standard maintenance volume | 30 | 54.4 ± 31.7 (months) | 0.18% NaCl in 5% dextrose at the rate of standard maintenance volume |
| 22 | Torres 2019 [61] | Argentina | 24 h | Surgical and medical | 145 | 18.0 (2.0–110.0) (months) | 0.9% saline in 5% dextrose | 154 | 21.0 (3.0–109.0) (months) | 0.45% saline in 5% dextrose |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasim, N.; Bakar, M.A.A.; Islam, M.A. Efficacy and Safety of Isotonic and Hypotonic Intravenous Maintenance Fluids in Hospitalised Children: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Children 2021, 8, 785. https://doi.org/10.3390/children8090785
Hasim N, Bakar MAA, Islam MA. Efficacy and Safety of Isotonic and Hypotonic Intravenous Maintenance Fluids in Hospitalised Children: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Children. 2021; 8(9):785. https://doi.org/10.3390/children8090785
Chicago/Turabian StyleHasim, Norfarahin, Mimi Azliha Abu Bakar, and Md Asiful Islam. 2021. "Efficacy and Safety of Isotonic and Hypotonic Intravenous Maintenance Fluids in Hospitalised Children: A Systematic Review and Meta-Analysis of Randomised Controlled Trials" Children 8, no. 9: 785. https://doi.org/10.3390/children8090785
APA StyleHasim, N., Bakar, M. A. A., & Islam, M. A. (2021). Efficacy and Safety of Isotonic and Hypotonic Intravenous Maintenance Fluids in Hospitalised Children: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Children, 8(9), 785. https://doi.org/10.3390/children8090785