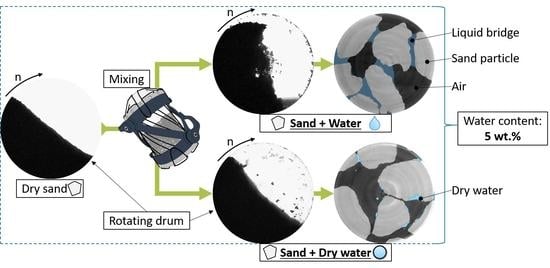
3.1. Bulk Properties of Single Substance Mixtures with Sand
A full understanding of the possibilities dry water (DW) creates for the coarse bulk material is only possible if the influence of the single components, water and R812S, are considered separately in mixtures with sand. Accordingly, a brief introduction to the resulting dynamic angle of repose (AoR) and cohesive index (CI) in dependence of the water content as well as R812S content is presented in
Figure 1. The mean value provides the AoR and fluctuations of the angle determine the CI. A CI of 0 implies that the bulk material flows ideally homogenously, whereas higher values suggest an irregular flow [
30]. Moreover, the portrayed results emerged from a mixing time of t
mix = 10 min as well as at a drum rotational speed of n
Drum = 10 min
−1.
Firstly, R812S marginally affects the flowability. The slight increase in AoR and CI may be associated with the added number or rather volume of fine silica particles. Thus, void volumes between aggregate particles are filled instead of solely coating the surface of aggregates, thereby obstructing the dynamic flow of mixtures. This assumption is substantiated by further inspecting the theoretical surface area coverage (SAC). The theoretical SACs of the aggregates with R812S at
wR812S ≈ 0.1%, 0.25% and 0.75% are approximately 26 m
2/m
2, 59 m
2/m
2 and 177 m
2/m
2, respectively. Moreover, the high values of SAC are a result of the coarse sand particles and their corresponding specific surface area, which do not represent the SAC of later mixtures containing DW. An increase in nano-particulate-induced surface roughness presumptively has a minor effect on the dynamic flow, as seen for the mixture of aggregate sand and R812S at
wR812S ≈ 0.1%. In contrast, Lüddecke showed that an increase in the surface roughness of finer host particles (≈40 µm) with nano-scale additives increases the flowability of metal powders due to the reduction in distance-dependent van der Waals forces between host particles [
14]. However, the relation of van der Waals forces to weight forces decreases with increasing particle size [
32]. For that reason, van der Waals forces are negligible in the size range of aggregates used within this study.
For mixtures containing water, on the other hand, severe effects on the dynamic flow properties of the coarse sand particles are evident. The dynamic AoR increases from an initial value of α
d,r = 38.7° without water to α
d,r = 56.2° at
wH2O = 0.5 wt.%. The degrading flowability is attributed to liquid capillary bridges, which form once water is added to the granular material [
33]. Furthermore, the theoretical degree of saturation for mixtures of aggregate sand and water at
wH2O = 0.5%, 1%, 3% and 5% are roughly 1.6%, 3.2%, 9.6% and 16.0%, respectively. After the initial rise, the dynamic AoR does not increase significantly with higher amounts of water within the mixture. This is possibly due to the measurement procedure, as the Granudrum calculates a mean flow angle in the centre of the flow and fluctuations create CI [
29]. At this point, it is important to state that mixtures containing
wH2O = 5% could not be evaluated.
Figure 2 underlines the aforementioned development of flowability with five consecutive images taken by the Granudrum at a rotating speed of
n = 10 min
−1.
For the starting material and
wH2O = 0.5%, substantial irregularities in the angle and form of the bulk material are not visible during the five consecutive images. All other water-based mixtures nevertheless exhibit an inhomogeneous flow regime at the given rotational speed, labelled additionally by an earlier and more distinct avalanching effect with higher water contents. Avalanching is presumptively a consequence of liquid bridges forming and, thus, clustering at higher liquid contents, as described by Herminghaus [
33]. For that reason, single particles are not able to flow independently, and a cohesive nature of the bulk material prevails.
Although the dynamic AoR may not reflect definite results from wH2O = 1% and higher, fluctuations of flow can perhaps assist with respect to mixture cohesiveness. Hence, the CI increases constantly with greater water contents, indicating the formation of extensive liquid bridges between single, non-absorbent particles and, thus, agglomerates or particle clusters emerge. As a result, an irregular flow of the bulk material emerges.
The physical properties of bulk granular materials cannot be described by one measurement method alone as the flow properties are subject to the stress state. Hence, multiple methods of analysis must be utilised to gain full knowledge of the flow behaviour of a bulk material and predict its behaviour for a given application [
34]. Further knowledge of the existing mixtures and influence of prevailing forces on the mixtures may be derived from the static AoR.
Figure 3 depicts the resulting AoR in a static environment for the single component mixtures with water and R812S. Moreover, the static AoR is displayed as a function of the contents for the respective substances and a mixing time t
mix of 10 min.
Within
Figure 3, R812S shows similar tendencies in the development of the static AoR when compared to the dynamic AoR in
Figure 1. The static AoR is slightly higher than that of the starting material (
wH2O = 0%), whereby a significant increase is not visible within the examined range. For mixtures including water, the static AoR is in agreement with the dynamic AoR to a certain extent. A sharp rise in the AoR is observed with the addition of water. The AoR begins at a value of α
s,r = 35.5° and increases to α
s,r = 46.2° for the starting material and the mixture with
wH2O = 0.5%, respectively. Thereafter, the slope decreases with an increasing water content until it seemingly approximates to a constant value of α
s,r ≈ 55°. These results coincide with the findings made by Samadani and Kudrolli [
35]. They found that the AoR rises abruptly, increases and, subsequently, saturates as the volume of a fluid is increased. In addition, Samadani and Kudrolli detected a greater surface roughness of bulk material whilst adding water, explaining it as a consequence of clustering or rather cohesion between particles [
35]. Clustering and cohesion can additionally be observed in
Figure 4. Herein, illustrative images taken during the analysis of the static AoR are displayed for dry sand (starting material) and all mixtures containing water.
The influence of water on the bulk material can additionally be analysed in a quasi-static environment, namely by conducting measurements with a ring shear tester at various degrees of consolidation.
Figure 5 demonstrates the termination locus resulting from the mean shear stress at steady-state flow for dry coarse sand as well as mixtures of coarse sand and water. Furthermore, mean shear stress values at steady-state flow were calculated as slip-stick effects were observed, especially for mixtures with coarse sand and water.
Noticeably, the shear stress of mixtures containing water does not deviate significantly from the values of the dry material at low consolidation stress. Mixtures containing water rather constantly demonstrate slightly lower shear stress at the lowest tested normal stress, which may be due to a lubricating effect between particles caused by liquid. These deviations are, however, minor in comparison to the effects at higher normal stress. The shear stress increases considerably at normal stresses of σ ≥ 1 kPa for mixtures with
wH2O ≥ 3%, whereas increased shear stress is postponed for the mixture with the lowest water content of
wH2O = 1%. Assuming water only resides on outer particle surfaces, higher normal stress consolidates the bulk solid and, consequently, forces the liquid downward between particles, thereby resulting in a higher water concentration within the given volume. Hence, tensile forces are presumably higher in the lateral direction and, thus, adhesive forces between particles increase. The shear stress increases accordingly under sufficient normal stress. For higher water contents, an increase in shear stress may occur at lower normal stress as a result of the degree of saturation within the bulk material, which, in turn, additionally increases when the bulk material is further consolidated. Cohesionless bulk materials are assumed to have a near linear progression that runs through the origin. However, similar nonlinearities were observed in previous studies once the Bond number, a dimensionless unit for cohesive strength, was increased [
36].
Further insight can be gained by transferring the above-portrayed data into an angle of internal friction at steady-state flow, which results from the horizontal cutting plane of a bulk material during ring shear measurements. The angle of internal friction at steady-state flow φ
sf is calculated from the arc tangent of the ratio of shear stress at steady-state, defined in
Figure 5 as τ
sf, to normal stress at pre-consolidation σ
pre [
31].
Figure 6a demonstrates the aforementioned angle of internal friction for different normal stresses of dry coarse sand and mixtures containing water and sand.
For dry coarse sand, φ
sf slightly decreases with increasing consolidation stress, which generally occurs due to the greater rise in frictional forces in comparison to adhesive forces at increased consolidation [
31]. For mixtures containing water, on the other hand, φ
sf is primarily close to that of dry sand until σ = 1 kPa and σ = 500 Pa for
wH2O = 1% and
wH2O ≥ 3%, respectively. φ
sf subsequently increases with increasing consolidation stress and decreases at roughly σ = 8 kPa. In addition, φ
sf is principally higher for mixtures containing higher amounts of water. Higher values for φ
sf are presumptively a result of increased adhesive forces acting between particles due to liquid capillary bridges. The following decrease in φ
sf may be due to heightened frictional forces between particles as consolidation proceeds and water is pressed into otherwise porous regions within the bulk material, as discussed before. As a result, contact points or rather contact surfaces between particles presumptively increase, which promotes friction.
Figure 6b additionally demonstrates the effects of single component mixture with R812S. The angle of internal friction of mixtures containing R812S does not significantly differ from that of coarse sand alone. Minor deviations occur for higher amounts of R812S in the form of lower angles, once again hinting towards a slightly higher influence of frictional forces. This may be a result of the R812S particles filling porous volumes between coarse sand particles.
The porous structure, as well as porosity of the bulk material, is another important factor for Selective Paste Intrusion (SPI) as it influences the penetration depth of the cement paste, the uniformity of penetration and the amount of cement paste required to fill void spaces. Thereby, the bulk density can be considered a process-related form of analysis. The results for the bulk density are displayed as a function of the water content by mass in
Figure 7. An increasing water content within the coarse bulk material evidently leads to lower bulk densities, which is inversely in accordance with the previously discussed results of the AoR. Thus, reduced flowability caused by accumulating liquid capillary bridges changes the porous structure by increasing the void spaces in the bulk material. Irregular porous structures, similar to the behaviour described in
Figure 2 and
Figure 4, were observed during experiments, which are validated by the combined given results.
3.2. Influence of Dry Water on Bulk Properties
The goal of utilising DW is primarily to incorporate water into the bulk material whilst counteracting the negative effects water has on bulk flowability and other properties, e.g., bulk density or inter-particle interactions. Naturally, the overall goal is to restore the aforementioned and, thus, reach the initial flowability of the starting material. From the previous investigation, it is also known that single component mixtures containing sand and R812S do not significantly affect bulk properties, thus, underlining the use of the starting material as a reference.
An example of typical curve progressions with respect to the dynamic AoR and related CI over the full spectrum of rotational speed are illustrated in
Figure 8. Apart from the starting material, the mixtures of coarse sand and DW contained only the additive variant DW
4:1. However, all water contents (
wH2O = 1, 3, and 5%) within the whole mixture, resulting in proportionate R812S-contents (
wR812S = 0.25, 0.75 and 1.25%), are included in
Figure 8. Mixing times are constant at t
mix = 10 min.
First and foremost, the mixture with
wH2O = 1% demonstrates a near equivalent dynamic AoR and CI to that of the starting material. This can be considered a significant improvement compared to water alone, where already
wH2O = 0.5% resulted in a sharp increase in the aforementioned values (cf.
Figure 1). Thereafter, in
Figure 8, the dynamic AoR and CI tend to increase with higher water contents. Noticeably, the dynamic AoR tends to start at higher values and, thus, initially decreases, followed by increasing angles at higher velocities.
wH2O = 5% does pose an exception in the tendency as the dynamic AoR does not decrease, but rather remains constant before increasing. The aforementioned findings with respect to initially decreasing values are clearer for the CI, whereby an initial cohesion must be overcome before approximately constant values are achieved between n
Drum = 10 min
−1 and n
Drum = 25 min
−1. Presumptively, the particles within the bulk are not in full motion at lower rotational speeds, carrying the whole bulk material to its maximum angle of stability before it slips or collapses within the cylinder. This form of inconsistent flow is followed by a continuous flow regime at higher velocities, which may be supported by a closer inspection of the CI at different rotational speeds. Hence, adhesive forces must initially be overcome in order to ensure dynamic flow, similar to the state of a fluidised bed [
37].
A closer inspection of the CI progression at two different drum rotational speeds is presented in
Figure 9. Within
Figure 9, mixing times are constant and all dry water additive variants are considered with different water contents within the entire mixtures.
Evidently, there is a slight decrease in the CI of the starting material (sand) from
n = 2 min
−1 to
n = 10 min
−1. These deviating values may be considered minor in comparison to the development of the CI for the mixtures containing DW. Firstly, the cohesive index at
n = 2 min
−1 increases with DW variants consisting of less R812S, possibly signifying the reduced prevention of liquid capillary bridges between particles. As a result, less R812S leads to an increase in cohesion at slow drum rotational speed. With the exception of the DW variant with the lowest R812S content (DW
19:1), the CI in dependence of the total water content remains near constant for the other three variants at
n = 10 min
−1. The values for
wH2O = 1% are exceptionally close to the values for pure sand (dry), which is a considerable improvement in comparison to results obtained with sand and water alone. For a mixture solely with water, the CI increases sixfold (cf.
Figure 1). Potentially, higher rotational speeds increase the porosity between particles, which, in turn, results in less friction between the particles, subsequently maintaining initial flow properties. This assumption is only plausible as long as dry water particles are able to sustain friction and, thus, liquid bridging is prohibited.
Similar commencing cohesion was observed by Forny et al. for dry water alone. They determined that slight agitation is required in order to overcome persisting cohesion and initiate flow [
25]. Corresponding effects were noticed whilst handling the complete mixtures within this study. However, according to Lumay et al., an intermittent flow within the rotating drum is not necessarily only the case for cohesive powders, but rather a general powder flow property related to low angular velocities as well as low Froude numbers [
29,
38].
Considerations must be made whether DW capsules are able to sustain stress during mixing for longer periods of time, especially with respect to later large-scale construction assignments. Hence, mixing times were varied as a method to increase the number of stress events within the created mixtures. The influence of DW variants and mixing times for different water contents
wH2O are depicted in
Figure 10. Furthermore, the DW variants are associated with the amount of R812S within the mixture, labelled as
wR812S. Values obtained by measuring the starting material (coarse dry sand) are represented henceforth by a red line, as these target values are the desired outcome.
For
wH2O = 1%, the dynamic AoR tends to increase with lower proportions of R812S within the DW. For shorter mixing times, the first significant reduction in bulk flowability, i.e., increasing AoR, is apparent for the mixture with DW
19:1, implying insufficient R812S to counteract cohesive effects caused by water. Moreover, longer mixing times seem to be counterproductive for bulk flowability at
wH2O = 1%, as the dynamic AoR increases over mixing time. Degradation of bulk flowability is exclusively postponed for the DW variant with the highest R812S content (DW
4:1), where the dynamic AoR increases after t
mix = 20 min. The aforementioned tendency is presumptively a consequence of the prevailing mechanical stress induced by considerably coarser sand particles during mixing combined with lower R812S contents. Several previous studies describe the sensitivity of dry water capsules towards mechanical stress, leading to the release of water [
23,
24,
25]. These findings suggest that a specific amount of R812S may be required to prevent liquid capillary bridges from forming. Another hypothesis may correspond with the particle size of DW capsules and their resistance to mechanical stress.
Figure 11 exhibits the characteristic particle sizes x
10, x
50 as well as x
90 for each produced DW variant.
It becomes evident in
Figure 11 that the ratio of water and R812S roughly correlates with the resulting particle size, which is possibly not only a consequence of the amount of R812S particles required to cover a given specific surface area of water droplets, but also the process conditions or parameters. For instance, deviations in the filling degree may influence the particle size distribution of the ensuing product. In
Figure 11, the x
10 values remain near constant for all DW variants. Furthermore, the median particle sizes (x
50) are approximately equal for the three DW variants with the highest proportions of R812S, whereas the variant DW
19:1 with the lowest R812S content has a significantly higher x
50. A moderately different tendency can be observed for the x
90 values, where the global progression shows an increase in particle size for DW variants with higher water contents. Altogether, DW
19:1 has the coarsest particles with the highest span within its particle size distribution.
Regarding the aforementioned hypothesis and
Figure 10, coarser DW particles are presumptively more vulnerable towards mechanical stress, whether pressure or friction, during mixing and thereby are more prone to releasing water into the bulk material. The occurrence of liquid bridges is, therefore, prolonged with higher proportions of R812S as well as finer particles at
wH2O = 1% in
Figure 10. In general, shorter mixing times are advantageous for
wH2O = 1%, as a higher number of stress events may result in eventual capsule disintegration.
Interestingly, for higher water contents of
wH2O ≥ 3%, on the other hand, longer mixing times lead to a lower dynamic AoR (cf.
Figure 10 centre and bottom). This is possibly due to the decreasing bulk density of the DW variants, resulting in higher volumes of additive within the mixture. As an example, the added volumes of DW
4:1 at
wH2O = 1% and
wH2O = 5% resulted in ratios of DW volume to sand volume (V
DW/V
Sand) of approximately 0.16 and 0.80, respectively. Consequently, longer mixing times are necessary to reach higher mixing qualities for a given water content. The DW variant DW
19:1 poses an exception of the aforementioned tendency. Shorter mixing times are still advantageous and the dynamic AoR of mixtures containing DW
19:1 increases thereafter to values similar to mixtures of sand and water alone (cf.
Figure 1). This outcome further suggests coalescence of water over the mixing time for DW variants containing insufficient amounts of R812S and coarser DW capsules. Sufficient stress events of DW capsules between coarse sand particles thereby result in DW fragmentation, allowing liquid bridges to form. However, the added volume of each DW variant with respect to their bulk density may have an additional effect. Higher numbers of R812S particles within DW variants (DW
4:1 > DW
6.66:1 > DW
9:1 > D
19:1) and their correlated volumes for a given water content within a mixture presumably cause attenuation of stress for the capsules. In addition, the total porosity of complete mixtures (sand and DW) is likely correlated with the aforementioned number of R812S particles and would, therefore, contribute to the dampening of stress events of DW capsules between aggregate sand particles.
Comparable developments are reflected with the CI as a function of the mixing time for all DW variants in
Figure 12. In general, the CI is significantly higher for the DW variant with the least amount of R812S and largest particle size (DW
19:1). The CI for the aforementioned variant also increases sharply at longer mixing times for all water contents, supporting previous assumptions with respect to capsule fragmentation. As opposed to the dynamic AoR, however, the CI does not converge to the values generated by mixtures with sand and water alone. In comparison, the highest value achieved with DW
19:1 at
wH2O = 3% is CI ≈ 38, whereas a mixture solely containing water lies at CI ≈ 85. Hence, minimal contents of R812S still contribute to a steadier flow of bulk granular materials containing water at shorter mixing times. Regarding the mixtures with the other DW variants, the CI increases slightly at longer mixing times with
wH2O = 1% but stays approximately constant for the other water contents. Moreover, the CI values are close to the reference starting material. In conclusion, the data emphasises the potential of DW as an additive with the function of storing water in coarse bulk materials and simultaneously maintaining initial flow properties. In comparison to the starting material (dry sand), results show that mixtures containing DW with water contents up to
wH2O = 3% have near equivalent dynamic flow properties. A minimum requirement of R812S as well as DW particle size, characterised by DW
9:1, is, however, necessary for stable mixtures. Long-term storage, nevertheless, may pose a problem due to constantly evaporating water whilst R812S remains in the mixture.
Further insight into the functionality of DW is demonstrated in
Figure 13, where micro-computed tomography (µCT) images of a mixture of coarse sand (grey) and water (blue) is depicted on the left as well as a mixture of coarse sand (grey) and DW (light blue) presented on the right. µCT images were taken of selected samples intending to explore events that prevail between DW and sand during mixing or rather the effect of mixing on the final product. The presented mixtures contain water contents of
wH2O = 5% for both solely water (left) as well as DW
6.66:1 (right).
Figure 13 demonstrates complete networks of liquid capillary bridges, which occur by adding the aforementioned water content without DW. For mixtures of aggregate sand and DW, DW mainly resides in the valleys of the particle surface and does not seem to create an even coating on sand particle surfaces. A clear distinction between DW and water alone was not achieved by µCT imaging. Possibly, layers of R812S are too thin to be detected or the difference in density between sand and hydrophobic silica is insufficient for a clear distinction of the respective materials. Liquid capillary bridges, however, are predominantly not visible, partially explaining the reason for maintaining flow properties with high water contents. Unfortunately, a clear distinction between water and R812S and, thus, whether DW capsules sustain mechanical stress, is not possible via µCT imaging. Multiple theories are plausible at this point as to whether capsules are fractured and how water and R812S are distributed on sand particle surfaces. The present µCT images do not portray dynamic events during the mixing process, but rather represent bulk behaviour in a static environment.
A closer inspection of DW capsule development during, or rather, after mixing, is possible via scanning electron cyromicroscopy (CyroSEM) imaging, which is displayed exemplarily with different magnifications in
Figure 14. These images do not demonstrate the exact outcome, as DW capsules did partially detach from sand particle surfaces due to sample pre-cooling with liquid nitrogen beforehand. Nevertheless, DW capsules in a variety of sizes are clearly visible within
Figure 14. In addition, the main proportion of DW capsules are found in the valleys of the particle surface, which supports findings from µCT imaging. Hence, DW particles are able to sustain mechanical stress during mixing until they are transported to protective cavities upon the surfaces of sand particles.
Further energy-dispersive X-ray spectroscopy (EDX) confirms the main components within the mixtures of this study.
Figure 15 visualises results from EDX measurements for both DW alone as well as DW on a coarse sand surface. Within
Figure 15a,b, the dominating element of solely DW is oxygen (blue) with traces of silicon (yellow) and carbon (green). This is a result of the abundance of water within the tested variant DW
9:1 as well as oxygen being an integral part of fumed silica (SiO
2). Note, hydrogen is not detectable in EDX measurements, for it does not have core electrons. Supplementary information to this topic can be found in [
39]. Mixtures of coarse sand and dry water, on the other hand, do have a higher proportion of silicon, as seen in
Figure 15c,d. Naturally, the higher amount of the element silicon is attributed to the coarse quartz particle. Moreover, the DW capsule is highly visible upon the quartz particle surface, once again denominated by an increased proportion of oxygen. The surface surrounding the DW capsule in
Figure 15d presents a fairly even distribution of silicon and oxygen, which coincides with estimated weight percentages of approximately 52 wt.% and 45 wt.%, respectively. Traces of carbon are found in
Figure 15b as well as c and presumably occur from hexamethyldisilazane (HMDS), which is used to treat Aerosil R812S. Further information with respect to the EDX measurements conducted here is available in the
Supplementary Materials.
Although the rotating drum method relates to flow properties at low consolidation stress and thereby particle bed additive manufacturing processes [
30], complementary information may be derived from flowability tests in a static or quasi-static surrounding. In relation to SPI, the static environment may also describe bulk behaviour in storage, shortly before the bulk material is transported to its designated surface. Consequently, results obtained regarding the static AoR as a function of the water content
wH2O for all DW variants are presented in
Figure 16.
The global tendency in
Figure 16 principally indicates that the static AoR increases with the water content (
wH2O) and, thus, the amount of DW is increased. The previous relation of static AoR to the DW variant is not evident for higher
wH2O. In contrast to the dynamic AoR, DW variant 19:1 does not pose an exception in regard to significantly higher AoR values. As a matter of fact, DW variant 19:1 marginally demonstrates the highest static AoR until
wH2O = 3%. Noticeable distinctions between samples were, however, observed, i.e., mixtures turning from quasi-dry to wet, which indicates the emergence of liquid capillary bridges as discussed and supported by the dynamic AoR. The aforementioned results are presumably a consequence of similar static friction that exists between particles. In addition, the prevailing mechanisms are the gravitational force and cohesion for a bulk material in a static environment. Once the bulk material at hand is set in motion, the cohesive forces become less dominant due to an increase in inter-particle porosity, or rather, void volume between particles. As mentioned beforehand, the decisive factor to overcome initial cohesion is, to an extent, the amount of hydrophobic silica in a mixture. Consequently, the effect of rolling friction in relation to adhesive forces caused by capillary liquid bridges increases once in motion. Noticeably, the mixture containing DW
4:1 and
wH2O = 1% has a near equivalent static AoR compared to the mixture of sand and R812S with the same
wR812S = 0.25% (cf.
Figure 3). In this case, water does not seem to have an effect on the AoR, presumably due to sufficient quantities of R812S particles and adequate DW particle size to protect capsules from fracturing. At water contents
wH2O ≥ 3%, the decisive factor characterising static bulk behaviour at higher water contents within mixtures, however, seems to be the water content and not the content of hydrophobic silica.
Results from the ring shear tester of mixtures containing one dry water variant, 9:1 (ratio H
2O:R812S), and different water contents are presented in
Figure 17. Herein, the angle of internal friction at steady-state flow φ
sf is depicted for different levels of normal stress or rather pre-consolidation stress.
Whilst observing
Figure 17, it becomes evident that the results of the mixtures containing dry water are not clearly distinguishable from the results obtained from dry coarse sand. However, mixtures containing
wH2O ≥ 3% show a slight decrease in their angles of internal friction, which coincides with results obtained for single component mixtures of coarse sand and R812S until σ = 1 kPa (cf.
Figure 6). Interestingly, φ
sf increases thereafter, signifying an increased effect of adhesive forces acting between coarse sand particles. An explanation for these occurrences at higher water contents presumably involves two combined effects. Firstly, less capsules are protected within the valleys of the pores with an increased amount of dry water added to the mixture. Secondly, sufficient normal stress during shear experiments may fracture unprotected capsules, thereby resulting in liquid bridging. As a consequence, adhesive forces become slightly more dominant in comparison to frictional forces between particles.