Improving the Efficiency and Antioxidant Activity of Essential Oil Extraction from Abies sachalinensis by Underwater Shockwave Pretreatment for the Construction of Low-Energy and Sustainable Essential Oil Extraction System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Chemicals
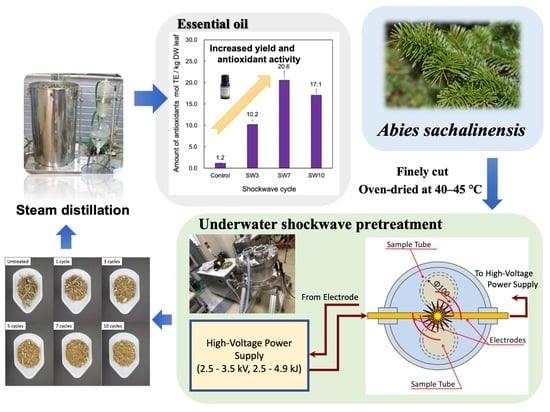
2.3. Underwater Shockwave Pretreatment of A. sachalinensis Leaves and Branches
2.4. Energy Evaluation of EO Extraction by Steam Distillation of Leaves and Branches of A. sachalinensis
2.5. Analysis of Volatile Components of EOs
2.6. DPPH Radical Scavenging Assay
2.7. Folin–Ciocalteu Assay of EOs
3. Results
3.1. Effect of Underwater Shockwave Pretreatment on Essential Oil Extraction
3.2. Effect of Underwater Shockwave Pretreatment on Volatile Contents
3.3. Evaluation of the Antioxidant Activity of EOs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, X.-W.; Li, S.; Shen, Y.-H.; Zhang, W.-D. Phytochemical and Biological Studies of Abies Species. Chem. Biodivers. 2008, 5, 56–81. [Google Scholar] [CrossRef] [PubMed]
- Wada, S.-I.; Hitomi, T.; Tanaka, R. Phenolic Compounds Isolated from the Bark of Abies sachalinensis. Helvetica Chim. Acta 2009, 92, 1610–1620. [Google Scholar] [CrossRef]
- Yatagai, M.; Sato, T. Terpenes of Leaf Oils from Conifers. Biochem. Syst. Ecol. 1986, 14, 469–478. [Google Scholar] [CrossRef]
- Satou, T.; Matsuura, M.; Murakami, S.; Hayashi, S.; Koike, K. Composition and Seasonal Variation of the Essential Oil from Abies Sachalinensis from Hokkaido, Japan. Nat. Prod. Commun. 2009, 4, 845–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawai, K.; Takahashi, C.; Miyamoto, T.; Numata, A.; Iwabuchi, H.; Yoshikura, M. Chemical differences between two populations of Abies sachalinensis. Phytochemistry 1993, 32, 331–334. [Google Scholar] [CrossRef]
- Takashima, Y.; Tamura, A.; Noseda, N.; Tanabe, J.; Makino, K.; Ishiguri, F.; Habu, N.; Iizuka, K.; Yokota, S. Diversities of decay resistance and n-hexane-extractive contents in seven half-sib families from plus trees in todomatsu (Abies sachalinensis). J. Wood Sci. 2014, 61, 192–198. [Google Scholar] [CrossRef]
- Satou, T.; Matsuura, M.; Takahashi, M.; Umezu, T.; Hayashi, S.; Sadamoto, K.; Koike, K. Anxiolytic-like effect of essential oil extracted from Abies sachalinensis. Flavour Fragr. J. 2011, 26, 416–420. [Google Scholar] [CrossRef]
- Wada, S.-I.; Hitomi, T.; Tokuda, H.; Tanaka, R. Anti-Tumor-Initiating Effects of Spiro-biflavonoids from Abies sachalinensis. Chem. Biodivers. 2010, 7, 2303–2308. [Google Scholar] [CrossRef] [PubMed]
- Ohira, T. Improvement of Air Quality by Essential Oils from Woody Plants. Mokuzai Gakkaishi 2015, 61, 226–231. [Google Scholar] [CrossRef] [Green Version]
- Brewer, M.S. Natural Antioxidants: Sources, Compounds, Mechanisms of Action, and Potential Applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 221–247. [Google Scholar] [CrossRef]
- Phutdhawong, W.; Kawaree, R.; Sanjaiya, S.; Sengpracha, W.; Buddhasukh, D. Microwave-Assisted Isolation of Essential oil of Cinnamomum iners Reinw. ex Bl.: Comparison with Conventional Hydrodistillation. Molecules 2007, 12, 868–877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavićević, V.P.; Marković, M.S.; Milojević, S.; Ristić, M.S.; Povrenović, D.S.; Veljković, V.B. Microwave-assisted hydrodistillation of juniper berry essential oil: Kinetic modeling and chemical composition. J. Chem. Technol. Biotechnol. 2015, 91, 883–891. [Google Scholar] [CrossRef]
- Sifton, H.B. Air-space tissue in plants. Bot. Rev. 1945, 11, 108–143. [Google Scholar] [CrossRef]
- Ohtani, K.; Ogawa, T. Expansion wave and cavitation bubble generation by underwater shock wave reflection from the interface. Mech. Eng. J. 2016, 3, 16–00298. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.-H.; Liu, X.-Y.; Zhang, H.-Q.; Wang, Y.; Xu, Y.-F.; Peng, B.-L.; Liu, Y. Modeling of kinetic characteristics of alkaline-surfactant-polymer-strengthened foams decay under ultrasonic standing wave. Pet. Sci. 2022, 19, 1825–1839. [Google Scholar] [CrossRef]
- Kuraya, E.; Miyafuji, Y.; Takemoto, A.; Itoh, S. The Effect of Underwater Shock Waves on Steam Distillation of Alpinia zerumbet leaves. Trans. Mater. Res. Soc. Jpn. 2014, 39, 447–449. [Google Scholar] [CrossRef] [Green Version]
- Maroušek, J.; Itoh, S.; Higa, O.; Kondo, Y.; Ueno, M.; Suwa, R.; Tominaga, J.; Kawamitsu, Y. Enzymatic hydrolysis enhanced by pressure shockwaves opening new possibilities in Jatropha Curcas L. processing. J. Chem. Technol. Biotechnol. 2013, 88, 1650–1653. [Google Scholar] [CrossRef]
- Maroušek, J.; Itoh, S.; Higa, O.; Kondo, Y.; Ueno, M.; Suwa, R.; Komiya, Y.; Tominaga, J.; Kawamitsu, Y. Pressure Shockwaves to Enhance Oil Extraction from Jatropha Curcas L. Biotechnol. Biotechnol. Equip. 2013, 27, 3654–3658. [Google Scholar] [CrossRef]
- Kuraya, E.; Nakada, S.; Touyama, A.; Itoh, S. Improving the antioxidant functionality of Citrus junos Tanaka (yuzu) fruit juice by underwater shockwave pretreatment. Food Chem. 2016, 216, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Kuraya, E.; Touyama, A.; Nakada, S.; Higa, O.; Itoh, S. Underwater Shockwave Pretreatment Process to Improve the Scent of Extracted Citrus junos Tanaka (Yuzu) Juice. Int. J. Food Sci. 2017, 2017, 2375181. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, A.; Higa, O.; Kuraya, E.; Higa, Y.; Shimojima, K.; Hokamoto, K.; Itoh, S. Using Underwater Shockwaves for the Development of Processing Devices for Plant Materials. Mater. Sci. Forum 2018, 910, 169–175. [Google Scholar] [CrossRef]
- Yasuda, A.; Kuraya, E.; Touyama, A.; Higa, O.; Hokamoto, K.; Itoh, S. Underwater shockwave pretreatment process for improving carotenoid content and yield of extracted carrot (Daucus carota L.) juice. J. Food Eng. 2017, 211, 15–21. [Google Scholar] [CrossRef]
- Périno-Issartier, S.; Ginies, C.; Cravotto, G.; Chemat, F. A comparison of essential oils obtained from lavandin via different extraction processes: Ultrasound, microwave, turbohydrodistillation, steam and hydrodistillation. J. Chromatogr. A 2013, 1305, 41–47. [Google Scholar] [CrossRef]
- Kawai, H.; Kuraya, E.; Touyama, A.; Higa, O.; Hokamoto, K.; Tokeshi, K.; Yasuda, A.; Naragaki, T.; Itoh, S. Improved yield and antioxidant activity of essential oil from Alpinia zerumbet (Zingiberaceae) leaves by underwater shockwave pretreatment. Food Bioprod. Process. 2020, 125, 134–140. [Google Scholar] [CrossRef]
- Kuraya, E.; Touyama, A.; Higa, O.; Tsujikawa, Y.; Tanaka, S.; Itoh, S. Improving the efficiency of essential-oil extraction from Abies sachalinensis with an underwater shockwave pretreatment. FACTA Univ. Ser. Physics Chem. Technol. 2018, 16, 83. [Google Scholar]
- Blois, M.S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Bampouli, A.; Kyriakopoulou, K.; Papaefstathiou, G.; Louli, V.; Krokida, M.; Magoulas, K. Comparison of different extraction methods of Pistacia lentiscus var. chia leaves: Yield, antioxidant activity and essential oil chemical composition. J. Appl. Res. Med. Aromat. Plants 2014, 1, 81–91. [Google Scholar] [CrossRef]
- Jezler, C.N.; Batista, R.S.; Alves, P.; Silva, D.D.C.; Costa, L.C.D.B. Histochemistry, content and chemical composition of essential oil in different organs of Alpinia zerumbet. Cienc. Rural 2013, 43, 1811–1816. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, T.; Takamura, H.; Matoba, T.; Terao, J. HPLC Method for Evaluation of the Free Radical-scavenging Activity of Foods by Using 1,1-Diphenyl-2-picrylhydrazyl. Biosci. Biotechnol. Biochem. 1998, 62, 1201–1204. [Google Scholar] [CrossRef] [PubMed]
- Özcan, M.M.; Arslan, D. Antioxidant effect of essential oils of rosemary, clove and cinnamon on hazelnut and poppy oils. Food Chem. 2011, 129, 171–174. [Google Scholar] [CrossRef]
- Tu, P.T.B.; Tawata, S. Anti-Oxidant, Anti-Aging, and Anti-Melanogenic Properties of the Essential Oils from Two Varieties of Alpinia zerumbet. Molecules 2015, 20, 16723–16740. [Google Scholar] [CrossRef]
- Ruberto, G.; Baratta, M.T. Antioxidant activity of selected essential oil components in two lipid model systems. Food Chem. 2000, 69, 167–174. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]




| Essential Oil Yield g/kg DW Leaf | Energy of SW Pretreatment kJ/kg DW Leaf | Energy of Pretreatment QEP MJ/kg EO *1 | Energy of Steam Distillation QESD MJ/kg EO | Total Energy QTE MJ/kg EO *3 | ||
|---|---|---|---|---|---|---|
| Fresh | Dried | |||||
| Control | 5.1 | 2.4 | - | - | 596.6/1267.9 | 596.6/1267.9 |
| SW1 *2 | - | 16.7 | 11.8 | 0.71 | 182.2 | 182.9 |
| SW3 | - | 19.7 | 40.3 | 2.05 | 154.4 | 156.5 |
| SW5 | 16.2 | 24.1 | 69.8 | 2.90 | 187.7/126.3 | 187.7/129.2 |
| SW7 | - | 28.7 | 97.4 | 3.39 | 106.0 | 109.4 |
| SW10 | - | 32.7 | 134.6 | 4.12 | 93.0 | 97.2 |
| Compounds | RI* DB-WAX | Relative Concentration (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Untreated | SW Pretreatment | |||||||
| Fresh | Dried | SW1 | SW3 | SW5 | SW7 | SW10 | ||
| Santene | 990 | 0.81 | 0.07 | 1.07 | 1.00 | 0.95 | 0.93 | 0.96 |
| Tricyclene | 1012 | 0.64 | 0.04 | 1.19 | 1.30 | 1.32 | 1.58 | 1.59 |
| α-Pinene | 1027 | 7.73 | 0.66 | 13.25 | 14.21 | 15.35 | 15.07 | 16.15 |
| α-Thujene | 1031 | 0.02 | - | 0.06 | 0.06 | 0.07 | 0.05 | 0.06 |
| α-Fenchene | 1063 | 0.01 | - | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 |
| Camphene | 1072 | 9.71 | 1.22 | 12.57 | 13.16 | 12.80 | 15.12 | 14.69 |
| β-Pinene | 1115 | 5.92 | 0.85 | 7.25 | 7.36 | 8.51 | 6.35 | 7.49 |
| Sabinene | 1128 | 0.01 | tr | 0.05 | 0.04 | 0.06 | 0.04 | 0.05 |
| 3-Carene | 1157 | 0.01 | 0.12 | 0.90 | 0.86 | 1.13 | 0.49 | 0.79 |
| Myrcene | 1171 | 2.36 | 0.62 | 1.78 | 1.87 | 1.93 | 2.00 | 1.90 |
| α-Phellandrene | 1173 | 0.08 | 0.08 | 0.23 | 0.23 | 0.23 | 0.20 | 0.22 |
| α-Terpinene | 1188 | 0.07 | 0.06 | 0.12 | 0.12 | 0.12 | 0.10 | 0.11 |
| Limonene | 1208 | 5.02 | 2.76 | 5.28 | 5.12 | 4.96 | 4.96 | 4.97 |
| β-Phellandrene | 1218 | 10.56 | 5.80 | 16.51 | 16.20 | 18.24 | 13.33 | 15.23 |
| γ-Terpinene | 1256 | 0.12 | 0.13 | 0.17 | 0.17 | 0.11 | 0.15 | 0.17 |
| p-Cymene | 1281 | 0.06 | 0.06 | 0.06 | 0.06 | 0.33 | 0.05 | 0.05 |
| Terpinolene | 1295 | 0.70 | 0.73 | 0.94 | 0.91 | 0.60 | 0.90 | 0.91 |
| Fenchyl acetate | 1484 | 1.19 | 2.37 | 1.42 | 1.39 | 0.87 | 1.41 | 1.36 |
| Camphor | 1537 | 1.94 | 1.60 | 0.53 | 0.43 | 0.41 | 0.46 | 0.38 |
| Linalool | 1559 | 0.03 | 3.31 | 0.84 | 0.77 | 0.58 | 0.76 | 0.64 |
| Linalyl acetate | 1568 | 0.35 | 1.31 | 0.47 | 0.46 | 0.33 | 0.46 | 0.38 |
| Bornyl acetate | 1599 | 27.65 | 38.28 | 22.87 | 22.12 | 13.49 | 23.86 | 22.09 |
| Isobornyl acetate | 1605 | 0.23 | 0.29 | 0.14 | 0.14 | 0.40 | 0.14 | 0.13 |
| Caryophyllene | 1617 | 0.40 | 0.64 | 1.06 | 1.10 | 0.69 | 1.27 | 1.18 |
| Terpinen-4-ol | 1621 | 0.14 | 0.48 | 0.11 | 0.11 | 0.08 | 0.09 | 0.08 |
| (E)-β-Farnesene | 1676 | - | 0.21 | 0.10 | 0.10 | 0.07 | 0.10 | 0.09 |
| Humulene | 1690 | 0.14 | 0.24 | 0.43 | 0.46 | 0.27 | 0.54 | 0.49 |
| α-Terpinyl acetate | 1715 | 1.53 | 1.23 | 0.42 | 0.42 | 0.34 | 0.37 | 0.33 |
| Borneol | 1722 | 19.45 | 30.44 | 7.19 | 7.22 | 3.93 | 6.46 | 5.02 |
| β-Himachalene | 1731 | - | 0.12 | 0.13 | 0.13 | 0.17 | 0.11 | 0.12 |
| δ-Cadinene | 1736 | - | 0.13 | 0.22 | 0.22 | 0.33 | 0.20 | 0.20 |
| β-Bisabolene | 1742 | - | 0.52 | 0.52 | 0.52 | 0.35 | 0.51 | 0.49 |
| α-Farnesene | 1760 | - | 0.11 | 0.08 | 0.08 | 0.06 | 0.11 | 0.10 |
| Nerolidol | 2053 | tr | 0.24 | 0.15 | 0.16 | 0.27 | 0.15 | 0.14 |
| Juniper camphor | 2264 | 1.06 | 1.47 | 0.52 | 0.51 | 0.33 | 0.50 | 0.49 |
| Total (%) | 97.93 | 96.21 | 98.66 | 99.01 | 89.69 | 98.87 | 99.08 | |
| Yield g/kg DW Leaf | |||||||
|---|---|---|---|---|---|---|---|
| Untreated | SW Pretreatment | ||||||
| Fresh | Dried | SW1 *1 | SW3 | SW5 | SW7 | SW10 | |
| β-Phellandrene | 0.54 | 0.14 | 2.76 | 3.20 | 4.40 | 3.83 | 4.98 |
| γ-Terpinene | 0.006 | 0.003 | 0.028 | 0.033 | 0.027 | 0.043 | 0.056 |
| Myrcene | 0.12 | 0.015 | 0.30 | 0.37 | 0.47 | 0.57 | 0.62 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawai, H.; Kuraya, E.; Touyama, A.; Higa, O.; Tokeshi, K.; Tsujikawa, Y.; Hokamoto, K.; Itoh, S. Improving the Efficiency and Antioxidant Activity of Essential Oil Extraction from Abies sachalinensis by Underwater Shockwave Pretreatment for the Construction of Low-Energy and Sustainable Essential Oil Extraction System. Processes 2022, 10, 2534. https://doi.org/10.3390/pr10122534
Kawai H, Kuraya E, Touyama A, Higa O, Tokeshi K, Tsujikawa Y, Hokamoto K, Itoh S. Improving the Efficiency and Antioxidant Activity of Essential Oil Extraction from Abies sachalinensis by Underwater Shockwave Pretreatment for the Construction of Low-Energy and Sustainable Essential Oil Extraction System. Processes. 2022; 10(12):2534. https://doi.org/10.3390/pr10122534
Chicago/Turabian StyleKawai, Hideaki, Eisuke Kuraya, Akiko Touyama, Osamu Higa, Kazuki Tokeshi, Yoshie Tsujikawa, Kazuyuki Hokamoto, and Shigeru Itoh. 2022. "Improving the Efficiency and Antioxidant Activity of Essential Oil Extraction from Abies sachalinensis by Underwater Shockwave Pretreatment for the Construction of Low-Energy and Sustainable Essential Oil Extraction System" Processes 10, no. 12: 2534. https://doi.org/10.3390/pr10122534
APA StyleKawai, H., Kuraya, E., Touyama, A., Higa, O., Tokeshi, K., Tsujikawa, Y., Hokamoto, K., & Itoh, S. (2022). Improving the Efficiency and Antioxidant Activity of Essential Oil Extraction from Abies sachalinensis by Underwater Shockwave Pretreatment for the Construction of Low-Energy and Sustainable Essential Oil Extraction System. Processes, 10(12), 2534. https://doi.org/10.3390/pr10122534