Optimization of the Biotreatment of GTL Process Water Using Pseudomonas aeruginosa Immobilized in PVA Hydrogel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. GLT PW Samples
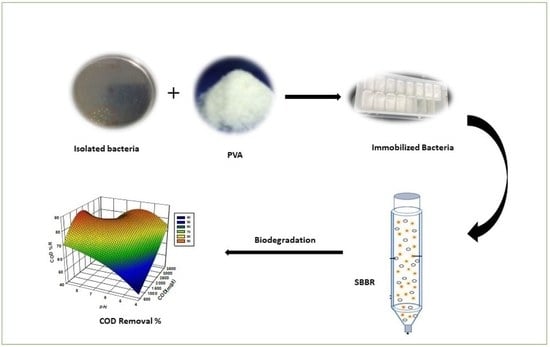
2.3. Isolation and Immobilization of Bacterial Culture
2.4. Biomass Acclimatization
2.5. Spouted Bed Bioreactor System (SBBR)
2.6. Batch Biological Treatment of GTL PW
2.7. Continuous Biological Treatment of GTL PW
2.8. Analytical Methods
2.9. Statistical Analysis and Optimization Using RSM
3. Results and Discussion
3.1. Statistical Analysis
3.2. Effect of Initial COD
3.3. Effect of pH
3.4. Effect of PVA Volume %
3.5. Model Validation
3.6. Continues Biological Treatment of GTL PW
3.6.1. Effect of Air Flow Rate
3.6.2. Effect of Liquid Flow Rate
3.6.3. Dynamic Behavior
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elbashir, N.O.; Eljack, F.T. A Method to Design an Advanced Gas-to-Liquid Technology Reactor for Fischer-Tropsch Synthesis. In Proceedings of the 2nd Annual Gas Processing Symposium, Doha, Qatar, 10–14 January 2010; pp. 369–377. [Google Scholar]
- Saravanan, N.P.; van Vuuren, M.J. Process wastewater treatment and management in gas-to-liquids industries. In Proceedings of the SPE Oil and Gas India Conference and Exhibition, Mumbai, India, 20–22 January 2010; Volume 1, pp. 176–184. [Google Scholar]
- Surkatti, R.; El-Naas, M.H.; van Loosdrecht, M.C.M.; Benamor, A.; Al-Naemi, F.; Onwusogh, U. Biotechnology for gas-to-liquid (GTL) wastewater treatment: A review. Water 2020, 12, 2126. [Google Scholar] [CrossRef]
- Ding, P.; Chu, L.; Wang, J. Biological treatment of actual petrochemical wastewater using anaerobic/anoxic/oxic process and the microbial diversity analysis. Appl. Microbiol. Biotechnol. 2016, 100, 10193–10202. [Google Scholar] [CrossRef] [PubMed]
- Satish, G.P.; Ashokrao, D.M.; Arun, S.K. Microbial degradation of pesticide: A review. Afr. J. Microbiol. Res. 2017, 11, 992–1012. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Ma, W.; Han, H.; Li, K.; Hao, X. Enhanced treatment of Fischer–Tropsch (F-T) wastewater by novel anaerobic biofilm system with scrap zero valent iron (SZVI) assisted. Biochem. Eng. J. 2017, 117, 66–76. [Google Scholar] [CrossRef]
- Laurinonyte, J.; Meulepas, R.J.W.; van den Brink, P.; Temmink, H. Membrane Bioreactor (MBR) as Alternative to a Conventional Activated Sludge System Followed by Ultrafiltration (CAS-UF) for the Treatment of Fischer-Tropsch Reaction Water from Gas-to-Liquids Industries. Water Air Soil Pollut. 2017, 228, 1–13. [Google Scholar] [CrossRef]
- Majone, M.; Aulenta, F.; Dionisi, D.; D’Addario, E.N.; Sbardellati, R.; Bolzonella, D.; Beccari, M. High-rate anaerobic treatment of Fischer–Tropsch wastewater in a packed-bed biofilm reactor. Water Res. 2010, 44, 2745–2752. [Google Scholar] [CrossRef]
- Moreroa, M.; Hildebrandt, D.; Matambo, T. Aerobic bioremediation of fischer-tropsch effluent—Short chain alcohols and volatile fatty acids. Lect. Notes Eng. Comput. 2018, 2238, 528–531. [Google Scholar]
- Enyi, G.C.; Nasr, G.G.; Burby, M. Economics of wastewater treatment in GTL plant using spray technique. Int. J. Energy Environ. 2013, 4, 2076–2909. [Google Scholar]
- Vashi, H.; Iorhemen, O.T.; Tay, J.H. Aerobic granulation: A recent development on the biological treatment of pulp and paper wastewater. Environ. Technol. Innov. 2018, 9, 265–274. [Google Scholar] [CrossRef]
- Anayo, O.F.; Scholastica, E.C.; Peter, O.C.; Nneji, U.G.; Obinna, A.; Mistura, L.O. The Beneficial Roles of Pseudomonas in Medicine, Industries, and Environment: A Review. In Pseudomonas aeruginosa: An Armory Within; IntechOpen: Lagos, Nigeria, 2019. [Google Scholar]
- Moradali, M.F.; Ghods, S.; Rehm, B.H.A. Pseudomonas aeruginosa Lifestyle: A Paradigm for Adaptation, Survival, and Persistence. Front. Cell. Infect. Microbiol. 2017, 7, 39. [Google Scholar] [CrossRef] [Green Version]
- Retmana, B.P.; Sitaresmi, S.; Yasman, Y.; Oetari, A. Biodegradation of 0.02% Naphthalene by Pseudomonas aeruginosa DRK 9.1 Isolated from Volcanic Mud in Renokenongo Village, Sidoarjo. AIP Conf. Proc. 2020, 2242, 50025. [Google Scholar]
- Yoshikawa, M.; Zhang, M.; Toyota, K. Biodegradation of Volatile Organic Compounds and Their Effects on Biodegradability under Co-Existing Conditions. Microbes Environ. 2017, 32, 188–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darsa, K.V.; Thatheyus, A.J. Biodegradation of Petroleum Compound Using Pseudomonas aeruginosa. OALib 2014, 1, 1–9. [Google Scholar] [CrossRef]
- He, S.; Ni, Y.; Lu, L.; Chai, Q.; Liu, H.; Yang, C. Enhanced biodegradation of n-hexane by Pseudomonas sp. strain NEE2. Sci. Rep. 2019, 9, 16615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, R.; Mavi, G.K.; Raghav, S. Bioremediation of Sludge using Pseudomonas aeruginosa. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 69–79. [Google Scholar] [CrossRef]
- Al Disi, Z.; Jaoua, S.; Al-Thani, D.; Al-Meer, S.; Zouari, N. Considering the Specific Impact of Harsh Conditions and Oil Weathering on Diversity, Adaptation, and Activity of Hydrocarbon-Degrading Bacteria in Strategies of Bioremediation of Harsh Oily-Polluted Soils. BioMed Res. Int. 2017, 2017, 8649350. [Google Scholar] [CrossRef] [Green Version]
- El-Naas, M.H.; Mourad, A.-H.I.; Surkatti, R. Evaluation of the characteristics of polyvinyl alcohol (PVA) as matrices for the immobilization of Pseudomonas putida. Int. Biodeterior. Biodegrad. 2013, 85, 413–420. [Google Scholar] [CrossRef]
- El-Naas, M.H.; Al-Zuhair, S.; Makhlouf, S. Continuous biodegradation of phenol in a spouted bed bioreactor (SBBR). Chem. Eng. J. 2010, 160, 565–570. [Google Scholar] [CrossRef]
- El-Naas, M.H.; Al-Zuhair, S.; Makhlouf, S. Batch degradation of phenol in a spouted bed bioreactor system. J. Ind. Eng. Chem. 2010, 16, 267–272. [Google Scholar] [CrossRef]
- El-Naas, M.H.; Al-Zuhair, S.; Alhaija, M.A. Removal of phenol from petroleum refinery wastewater through adsorption on date-pit activated carbon. Chem. Eng. J. 2010, 162, 997–1005. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, S.; Kumar, S. Biodegradation kinetics of phenol and catechol using Pseudomonas putida MTCC 1194. Biochem. Eng. J. 2005, 22, 151–159. [Google Scholar] [CrossRef]
- El-Naas, M.H.; Al-Muhtaseb, S.A.; Makhlouf, S. Biodegradation of phenol by Pseudomonas putida immobilized in polyvinyl alcohol (PVA) gel. J. Hazard. Mater. 2009, 164, 720–725. [Google Scholar] [CrossRef] [PubMed]
- Burns, T. How to Reduce COD in Water|Bioprocess H2O. 2021. Available online: https://www.bioprocessh2o.com/blog/cod (accessed on 11 September 2022).
- El-Naas, M.H.; Al-Zuhair, S.; Al-Lobaney, A.; Makhlouf, S. Assessment of electrocoagulation for the treatment of petroleum refinery wastewater. J. Environ. Manag. 2009, 91, 180–185. [Google Scholar] [CrossRef] [PubMed]
- You, C.; Okano, H.; Hui, S.; Zhang, Z.; Kim, M.; Gunderson, C.W.; Wang, Y.-P.; Lenz, P.; Yan, D.; Hwa, T. Coordination of bacterial proteome with metabolism by cyclic AMP signalling. Nature 2013, 500, 301–306. [Google Scholar] [CrossRef] [Green Version]
- Achrem, M.; Skuza, L.; Kalinka, A.; Szućko, I.; Filip, E.; Słominska-Walkowiak, R.; Rogalska, S.M. Role of Epigenetic Mechanisms in Plant Response to Low Temperature. Acta Biol. Crac. Bot. 2012, 54, 7–15. [Google Scholar] [CrossRef]
- El Telib, A.E.; El-Naas, M.H.; Acio, J.A. Biodegradation of BTEX: Optimization through Response Surface Methodology. Am. J. Eng. Appl. Sci. 2017, 10, 20–31. [Google Scholar] [CrossRef]
- Gopalakrishnan, B.; Muthukumarapandian, A.; Sujatha, S.; Raja, S.; Rajamohan, N.; Rajasimman, M. Statistical modeling and optimization of tannery wastewater treatment in a fluidized bed bioreactor with low density biomass support. Model. Earth Syst. Environ. 2021, 8, 1099–1107. [Google Scholar] [CrossRef]
- Azimi, N.; Hassani, A.H.; Darzi, G.N.; Borghei, S.M. Biodegradation of Wastewater Containing High Concentration of Sulfamethoxazole by Antibiotic Adopted Biofilm in Attached Growth Bioreactor. Pol. J. Environ. Stud. 2017, 26, 2463–2469. [Google Scholar] [CrossRef]
- Mohanty, S.S.; Jena, H.M. Biodegradation of Herbicide by the Immobilized Microbial Consortium SMC1 in Continuous Packed-Bed Biofilm Reactor. Front. Chem. Eng. 2022, 4, 44. [Google Scholar] [CrossRef]
- Kawan, J.A.; Suja’, F.; Pramanik, S.K.; Yusof, A.; Rahman, R.A.; Hasan, H.A. Effect of Hydraulic Retention Time on the Performance of a Compact Moving Bed Biofilm Reactor for Effluent Polishing of Treated Sewage. Water 2022, 14, 81. [Google Scholar] [CrossRef]
- Chen, Y.; Jay, J.; Cheng, J.J.; Creamer, K.S. Inhibition of anaerobic digestion process: A review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef] [PubMed]
- El-Naas, M.H.; Mohammad, A.F.; Suleiman, M.I.; Al Musharfy, M.; Al-Marzouqi, A.H. Evaluation of a novel gas-liquid contactor/reactor system for natural gas applications. J. Nat. Gas Sci. Eng. 2017, 39, 133–142. [Google Scholar] [CrossRef]
- Luis, K.; Francois, D.; Trevor, P. Method for Purifying Fischer-Tropsch Derived Water. 2003, pp. 1–25. Available online: https://patents.google.com/patent/US20050131086A1/en (accessed on 15 September 2022).







| Characteristic | GTL PW | Pretreated GTL PW |
|---|---|---|
| COD (mg/L) | 5000–7000 | 2000 to 4000 |
| TOC (mg/L) | 1500–1700 | 700–1400 |
| pH | 2.9 | 3.3 |
| Component | Concentration (mg/L) |
|---|---|
| MgSO4·7H2O | 300 |
| K2HPO4 | 250 |
| CaCl2·2H2O | 150 |
| (NH4)2CO3 | 120 |
| FeSO4·7H2O | 3.5 |
| ZnSO4·7H2O | 1.3 |
| MnCl2·4H2O | 0.13 |
| CuSO4·5H2O | 0.018 |
| CoCl2·6H2O | 0.015 |
| Na2MoO4·2H2O | 0.013 |
| Total | 824.98 |
| Factor | Units | −α | Lower Limit (−1) | 0 | Upper Limit (+1) | +α |
|---|---|---|---|---|---|---|
| Concentration | mg/L | 318.21 | 1000 | 2000 | 3000 | 3681.79 |
| pH | - | 3.98 | 5 | 6.5 | 8 | 9.02 |
| PVA v% | - | 16.59 | 20 | 25 | 30 | 33.41 |
| RunOrder | PtType | Blocks | COD | pH | PVA Vol. % |
|---|---|---|---|---|---|
| 1 | 0 | 1 | 2000 | 6.5 | 25 |
| 2 | 1 | 1 | 3000 | 8 | 20 |
| 3 | 1 | 1 | 1000 | 5 | 30 |
| 4 | −1 | 1 | 2000 | 3.98 | 25 |
| 5 | −1 | 1 | 2000 | 6.5 | 16.59 |
| 6 | 1 | 1 | 3000 | 5 | 20 |
| 7 | 1 | 1 | 3000 | 5 | 30 |
| 8 | 0 | 1 | 2000 | 6.5 | 25 |
| 9 | −1 | 1 | 2000 | 9.02 | 25 |
| 10 | −1 | 1 | 3681.79 | 6.5 | 25 |
| 11 | −1 | 1 | 318.20 | 6.5 | 25 |
| 12 | 1 | 1 | 1000 | 8 | 20 |
| 13 | 0 | 1 | 2000 | 6.5 | 25 |
| 14 | 0 | 1 | 2000 | 6.5 | 25 |
| 15 | 0 | 1 | 2000 | 6.5 | 25 |
| 16 | 1 | 1 | 1000 | 8 | 30 |
| 17 | −1 | 1 | 2000 | 6.5 | 33.40 |
| 18 | 1 | 1 | 1000 | 5 | 20 |
| 19 | 0 | 1 | 2000 | 6.5 | 25 |
| 20 | 1 | 1 | 3000 | 8 | 30 |
| Condition | Step Change | t1 (h) | t2 (h) | t63.2% (h) | td (h) | (h) |
|---|---|---|---|---|---|---|
| Liquid flow rate | 2.1–4.2 | 22 | 24 | 33.376 | 2 | 9.376 |
| Liquid flow rate | 4.2–2.1 | 48 | 48.5 | 56.848 | 0.5 | 6.848 |
| Characteristic | GTL PW | Pretreated GTL PW | Treated GTL PW (Batch Experiments) |
|---|---|---|---|
| COD (mg/L) | 5000–7000 | 2000 to 4000 | ~441 |
| TOC (mg/L) | 1500–1700 | 700–1400 | ~154 |
| pH | 2.9 | 3.3 | 7.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, S.A.; Surkatti, R.; Ba-Abbad, M.M.; El-Naas, M.H. Optimization of the Biotreatment of GTL Process Water Using Pseudomonas aeruginosa Immobilized in PVA Hydrogel. Processes 2022, 10, 2568. https://doi.org/10.3390/pr10122568
Ahmed SA, Surkatti R, Ba-Abbad MM, El-Naas MH. Optimization of the Biotreatment of GTL Process Water Using Pseudomonas aeruginosa Immobilized in PVA Hydrogel. Processes. 2022; 10(12):2568. https://doi.org/10.3390/pr10122568
Chicago/Turabian StyleAhmed, Somaya A., Riham Surkatti, Muneer M. Ba-Abbad, and Muftah H. El-Naas. 2022. "Optimization of the Biotreatment of GTL Process Water Using Pseudomonas aeruginosa Immobilized in PVA Hydrogel" Processes 10, no. 12: 2568. https://doi.org/10.3390/pr10122568
APA StyleAhmed, S. A., Surkatti, R., Ba-Abbad, M. M., & El-Naas, M. H. (2022). Optimization of the Biotreatment of GTL Process Water Using Pseudomonas aeruginosa Immobilized in PVA Hydrogel. Processes, 10(12), 2568. https://doi.org/10.3390/pr10122568