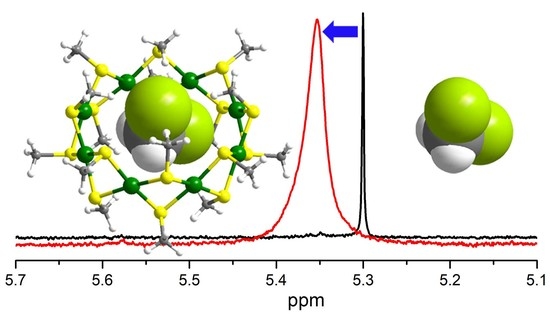
Cavity Size Effect on Host-Guest Property of Tiara-like Structural Mn(SR)2n Nanoclusters Probed by NMR Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis and Purification of Pdn(PET)2n
2.3. Characterization
2.4. Calculational Details
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Imaoka, T.; Akanuma, Y.; Haruta, N.; Tsuchiya, S.; Ishihara, K.; Okayasu, T.; Chun, W.-J.; Takahashi, M.; Yamamoto, K. Platinum clusters with precise numbers of atoms for preparative-scale catalysis. Nat. Commun. 2017, 8, 688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ananikov, V.P.; Orlov, N.V.; Zalesskiy, S.S.; Beletskaya, I.P.; Khrustalev, V.N.; Morokuma, K.; Musaev, D.G. Catalytic adaptive recognition of thiol (SH) and selenol (SeH) groups toward synthesis of functionalized vinyl monomers. J. Am. Chem. Soc. 2012, 134, 6637–6649. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Matsumoto, T.; Samoc, M.; Petrie, S.; Meng, S.; Corkery, T.C.; Stranger, R.; Zhang, J.; Humphrey, M.G.; Tatsumi, K. Dodecanuclear-ellipse and decanuclear-wheel nickel(II) thiolato clusters with efficient femtosecond nonlinear absorption. Angew. Chem. Int. Ed. 2010, 49, 4209–4212. [Google Scholar] [CrossRef] [PubMed]
- Tafen, D.N.; Kauffman, D.R.; Alfonso, D.R. Electrocatalytic oxygen evolution with pure and substituted M6(SR)12 (M = Pd, Fe, Rh) complexes. Comput. Mater. Sci. 2018, 150, 283–290. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Pan, Y.; Wang, Z.; Zhao, P. The fluorescence properties of tiara like structural thiolated palladium clusters. Dalton Trans. 2017, 46, 12964–12970. [Google Scholar] [CrossRef] [Green Version]
- Zhu, M.; Zhou, S.; Yao, C.; Liao, L.; Wu, Z. Reduction-resistant and reduction-catalytic double-crown nickel nanoclusters. Nanoscale 2014, 6, 14195–14199. [Google Scholar] [CrossRef]
- Zhuang, Z.; Yang, Q.; Chen, W. One-step rapid and facile synthesis of subnanometer-sized Pd6(C12H25S)11 clusters with ultra-high catalytic activity for 4-nitrophenol reduction. ACS Sustain. Chem. Eng. 2019, 7, 2916–2923. [Google Scholar] [CrossRef]
- Joya, K.S.; Sinatra, L.; AbdulHalim, L.G.; Joshi, C.P.; Hedhili, M.N.; Bakr, O.M.; Hussain, I. Atomically monodisperse nickel nanoclusters as highly active electrocatalysts for water oxidation. Nanoscale 2016, 8, 9695–9703. [Google Scholar] [CrossRef] [Green Version]
- Woodward, P.; Dahl, L.F.; Abel, E.W.; Crosse, B.C. A new type of cyclic transition metal complex, [Ni(SC2H5)2]6. J. Am. Chem. Soc. 1965, 87, 5251–5253. [Google Scholar] [CrossRef]
- Ivanov, S.A.; Kozee, M.A.; Merrill, W.A.; Agarwal, S.; Dahl, L.F. Cyclo-[Ni(μ2-SPh)2]9 and cyclo-[Ni(μ2-SPh)2]11: New oligomeric types of toroidal nickel(II) thiolates containing geometrically unprecedented 9- and 11-membered ring systems. J. Chem. Soc. Dalton Trans. 2002, 22, 4105–4115. [Google Scholar] [CrossRef]
- Zhang, C.; Takada, S.; Kölzer, M.; Matsumoto, T.; Tatsumi, K. Nickel(II) thiolate complexes with a flexible cyclo-{Ni10S20} framework. Angew. Chem. Int. Ed. 2006, 45, 3768–3772. [Google Scholar] [CrossRef] [PubMed]
- Shichibu, Y.; Yoshida, K.; Konishi, K. Hexanuclear platinum(II) thiolate macrocyclic host: Charge-transfer-driven inclusion of a AgI ion guest. Inorg. Chem. 2016, 55, 9147–9149. [Google Scholar] [CrossRef] [PubMed]
- Yamashina, Y.; Kataoka, Y.; Ura, Y. Tiara-like octanuclear palladium(II) and platinum(II) thiolates and their inclusion complexes with dihalo- or iodoalkanes. Inorg. Chem. 2014, 53, 3558–3567. [Google Scholar] [CrossRef] [PubMed]
- Yamashina, Y.; Kataoka, Y.; Ura, Y. Inclusion of an iodine molecule in a tiara-like octanuclear palladium thiolate complex. Eur. J. Inorg. Chem. 2014, 2014, 4073–4078. [Google Scholar] [CrossRef]
- Pan, Y.; Chen, J.; Gong, S.; Wang, Z. Co-synthesis of atomically precise nickel nanoclusters and the pseudo-optical gap of Ni4(SR)8. Dalton Trans. 2018, 47, 11097–11103. [Google Scholar] [CrossRef]
- Dance, I.G.; Scudder, M.L.; Secomb, R. c-Ni8(SCH2COOEt)16, a receptive octagonal toroid. Inorg. Chem. 1985, 24, 1201–1208. [Google Scholar] [CrossRef]
- Chen, J.; Liu, L.; Weng, L.; Lin, Y.; Liao, L.; Wang, C.; Yang, J.; Wu, Z. Synthesis and properties evolution of a family of tiara-like phenylethanethiolated palladium nanoclusters. Sci. Rep. 2015, 5, 16628. [Google Scholar] [CrossRef] [Green Version]
- Mezei, G.; Zaleski, C.M.; Pecoraro, V.L. Structural and functional evolution of metallacrowns. Chem. Rev. 2007, 107, 4933–5003. [Google Scholar] [CrossRef]
- Sobiech, T.A.; Zhong, Y.; Miller, D.P.; McGrath, J.K.; Scalzo, C.T.; Redington, M.C.; Zurek, E.; Gong, B. Ultra-tight host-guest binding with exceptionally strong positive cooperativity. Angew. Chem. Int. Ed. 2022, 61, e202213467. [Google Scholar] [CrossRef]
- Hu, S.-J.; Guo, X.-Q.; Zhou, L.-P.; Yan, D.-N.; Cheng, P.-M.; Cai, L.-X.; Li, X.-Z.; Sun, Q.-F. Guest-driven self-assembly and chiral induction of photofunctional lanthanide tetrahedral cages. J. Am. Chem. Soc. 2022, 144, 4244–4253. [Google Scholar] [CrossRef]
- Schäfer, F.; Mix, A.; Cati, N.; Lamm, J.-H.; Neumann, B.; Stammler, H.-G.; Mitzel, N.W. Host-guest chemistry of a bidentate silyl-triflate bis-Lewis acid—Complex complexation behaviour unravelled by diffusion NMR spectroscopy. Dalton Trans. 2022, 51, 7164–7173. [Google Scholar] [CrossRef] [PubMed]
- Boles, J.E.; Bennett, C.; Baker, J.; Hilton, K.L.F.; Kotak, H.A.; Clark, E.R.; Long, Y.; White, L.J.; Lai, H.Y.; Hind, C.K.; et al. Establishing the selective phospholipid membrane coordination, permeation and lysis properties for a series of ‘druggable’ supramolecular self-associating antimicrobial amphiphiles. Chem. Sci. 2022, 13, 9761–9773. [Google Scholar] [CrossRef]
- Mi, Y.; Ma, J.; Liang, W.; Xiao, C.; Wu, W.; Zhou, D.; Yao, J.; Sun, W.; Sun, J.; Gao, G.; et al. Guest-binding-induced interhetero hosts charge transfer crystallization: Selective coloration of commonly used organic solvents. J. Am. Chem. Soc. 2021, 143, 1553–1561. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.-S.; Ng, M.; Yeung, M.C.-L.; Yam, V.W.-W. Platinum(II)-based host–guest coordination-driven supramolecular Co-assembly assisted by Pt···Pt and π–π stacking interactions: A dual-selective luminescence sensor for cations and anions. J. Am. Chem. Soc. 2021, 143, 973–982. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision B.01; Gaussian, Inc.: Wallingford, UK, 2016. [Google Scholar]
- Wilson, P.J.; Bradley, T.J.; Tozer, D.J. Hybrid exchange-correlation functional determined from thermochemical data and ab initio potentials. J. Chem. Phys. 2001, 115, 9233–9242. [Google Scholar] [CrossRef] [Green Version]
- Flaig, D.; Maurer, M.; Hanni, M.; Braunger, K.; Kick, L.; Thubauville, M.; Ochsenfeld, C. Benchmarking hydrogen and carbon NMR chemical shifts at HF, DFT, and MP2 levels. J. Chem. Theory Comput. 2014, 10, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Jensen, F. Segmented contracted basis sets optimized for nuclear magnetic shielding. J. Chem. Theory Comput. 2015, 11, 132–138. [Google Scholar] [CrossRef]
- Andrae, D.; Haussermann, U.; Dolg, M.; Stoll, H.; Preuss, H. Energy-adjusted ab initio pseudopotentials for the second and third row transition elements. Theor. Chim. Acta 1990, 77, 123–141. [Google Scholar] [CrossRef]
- Chen, Z.; Wannere, C.S.; Corminboeuf, C.; Puchta, R.; Schleyer, P.V.R. Nucleus-independent chemical shifts (NICS) as an aromaticity criterion. Chem. Rev. 2005, 105, 3842. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Kagalwala, H.N.; Gottlieb, E.; Li, G.; Li, T.; Jin, R.; Bern-hard, S. Photocatalytic hydrogen generation system using a nickel-thiolate hexameric cluster. Inorg. Chem. 2013, 52, 9094–9101. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, H.E.; Kotlyar, V.; Nudelman, A. NMR chemical shifts of common laboratory solvents as trace impurities. J. Org. Chem. 1997, 62, 7512–7515. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Jin, M.; Ma, X.; Zhu, Q.; Huang, Y.; Wang, Y.; Hu, S.; Sheng, T.; Wu, X. In situ synthesis of nickel tiara-like clusters with two different thiolate bridges. Dalton Trans. 2012, 41, 8472–8476. [Google Scholar] [CrossRef]
- Higgins, J.D.; William Suggs, J. Preparation, structure and spectroscopic studies of the palladium mercaptides Pd8(S-nPr)16 and Pd6(S-nPr)12. Inorg. Chim. Acta 1988, 145, 247–252. [Google Scholar] [CrossRef]
- Muñoz-Castro, A. Bonding and magnetic response properties of several toroid structures. Insights of the role of Ni2S2 as a building block from relativistic density functional theory calculations. J. Phys. Chem. A 2011, 115, 10789–10794. [Google Scholar] [CrossRef] [PubMed]





| Series | H-G Molecules | Bonding Length (Å) | Bonding Angles (°) | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|
| M-M | M-S | S-α-C | M-S-M | S-M-Sa | S-M-Se | M-S-α-C | |||
| Pd8(SR)16 | Pd8(SCH2CH2CH3)16 | 3.23 | 2.32 | 1.84 | 88.05 | 97.77 | 82.27 | 107.06 | [35] |
| Pd8(SCH2CO2CH3)16 | 3.23 | 2.32 | 1.82 | 88.20 | 97.69 | 82.71 | 107.20 | [13] | |
| CH2Cl2@Pd8(SCH2CO2CH3)16 | 3.24 | 2.32 | 1.82 | 88.43 | 97.66 | 82.71 | 107.07 | [13] | |
| CH2Br2@Pd8(SCH2CO2CH3)16 | 3.24 | 2.32 | 1.81 | 88.44 | 97.77 | 82.62 | 107.24 | [13] | |
| (CH2Cl)2@Pd8(SCH2CO2CH3)16 | 3.24 | 2.32 | 1.82 | 88.29 | 97.74 | 82.66 | 107.00 | [13] | |
| CH3I@Pd8(SCH2CO2CH3)16 | 3.25 | 2.33 | 1.80 | 88.46 | 97.65 | 82.69 | 107.12 | [13] | |
| I2@Pd8(SCH2CO2CH3)16 | 3.26 | 2.33 | 1.82 | 88.60 | 97.79 | 82.41 | 106.25 | [14] | |
| Pt8(SR)16 | Pt8(SCH2CO2CH3)16 | 3.29 | 2.32 | 1.82 | 90.77 | 98.79 | 81.48 | 107.77 | [13] |
| CH2Cl2@Pt8(SCH2CO2CH3)16 | 3.29 | 2.33 | 1.82 | 90.37 | 98.04 | 82.11 | 108.22 | [13] | |
| CH2Br2@Pt8(SCH2CO2CH3)16 | 3.29 | 2.32 | 1.83 | 90.43 | 98.72 | 81.56 | 107.40 | [13] | |
| (CH2Cl)2@Pt8(SCH2CO2CH3)16 | 3.29 | 2.32 | 1.81 | 90.41 | 98.68 | 81.51 | 108.18 | [13] | |
| Pt6(SR)12 | Pt6[S-(CH2)11-CH3]12 | 3.17 | 2.32 | 1.83 | 86.26 | 98.41 | 81.35 | 108.94 | [12] |
| Ag@Pt6[S-(CH2)11-CH3]12 | 3.08 | 2.32 | 1.83 | 83.24 | 97.61 | 82.39 | 109.95 | [12] | |
| Ni10- | Ni10(StBu)10(SC2H5)10 | 3.15 | 2.20 | 1.83 | 91.18 | 96.91 | 83.12 | 109.67 | [34] |
| (StBu)10- | CH3C6H5@Ni10(StBu)10(etet)10 | 3.21 | 2.22 | — | 93.12 | 96.68 | 82.90 | — | [3] |
| (SR)10 | CH3C6H5@Ni10(StBu)10(pyet)10 | 3.16 | 2.21 | 1.85 | 91.31 | 97.42 | 83.04 | 109.92 | [3] |
| (0.5CH3C6H5)@Ni10(StBu)10(atet)10 | 3.17 | 2.21 | 1.85 | 92.06 | 96.96 | 83.07 | 110.02 | [3] | |
| Ni10(StBu)10(mtet)10 | 3.17 | 2.21 | 1.85 | 91.54 | 97.48 | 82.57 | 110.16 | [11] | |
| C6H6@Ni10(StBu)10(mtet)10 | 3.16 | 2.20 | 1.86 | 91.58 | 97.10 | 82.94 | 109.27 | [11] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, C.; Gong, S.; Chen, J.; Wang, Z. Cavity Size Effect on Host-Guest Property of Tiara-like Structural Mn(SR)2n Nanoclusters Probed by NMR Spectroscopy. Processes 2022, 10, 2683. https://doi.org/10.3390/pr10122683
Zhou C, Gong S, Chen J, Wang Z. Cavity Size Effect on Host-Guest Property of Tiara-like Structural Mn(SR)2n Nanoclusters Probed by NMR Spectroscopy. Processes. 2022; 10(12):2683. https://doi.org/10.3390/pr10122683
Chicago/Turabian StyleZhou, Changlin, Shida Gong, Jishi Chen, and Zonghua Wang. 2022. "Cavity Size Effect on Host-Guest Property of Tiara-like Structural Mn(SR)2n Nanoclusters Probed by NMR Spectroscopy" Processes 10, no. 12: 2683. https://doi.org/10.3390/pr10122683
APA StyleZhou, C., Gong, S., Chen, J., & Wang, Z. (2022). Cavity Size Effect on Host-Guest Property of Tiara-like Structural Mn(SR)2n Nanoclusters Probed by NMR Spectroscopy. Processes, 10(12), 2683. https://doi.org/10.3390/pr10122683