Modeling and Experimental Studies on Carbon Dioxide Absorption with Sodium Hydroxide Solution in a Rotating Zigzag Bed
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
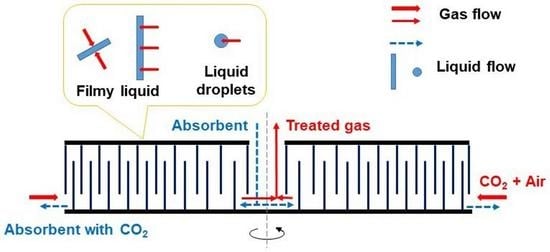
2.2. Experimental Apparatuses and Procedure
3. Theory
3.1. Reactions of CO2 in Sodium Hydroxide Solution
3.2. Gas and Liquid Flow in the RZB Rotor
3.3. Model Development
- (1)
- Velocity of droplets leaving the rotating baffle along the tangential direction in zone I was equal to the circumferential velocity of the rotating baffle [11].
- (2)
- Only the tangential velocity of gas phase in the RZB rotor was considered, and the radial and axial velocities of gas were ignored because the tangential velocity was the main component of the gas velocity [23].
- (3)
- It should be noted that the tangential gas velocity is smaller than the circumferential velocity of rotating baffles when the gas flow rate is low [23]. Because the gas flow rate was less than 1200 L/h in this study, the tangential gas velocity was much lower than the tangential liquid velocity in the space between the static baffle and rotating baffle. Thus, it was assumed that the effect of gas flow on the liquid form could be ignored in zone III, where the flying liquid remained in the form of an intact sheet [11]. Meanwhile, the liquid form in zones I and II was not affected by gas flow either.
- (4)
- (5)
- Liquid left the rotating baffle only through the lowest circle of perforations in the rotating baffles because the perforations were closely spaced [24]. Droplets formed by every perforation had the same amount, lifetime, velocity, and diameter.
- (6)
- Liquid film is a thin surface layer in the filmy liquid. The liquid film in the turbulent filmy liquid in zone II was renewed by impingement of fine droplets from zone I, and the liquid film in the filmy liquid in every zone of the RZB had the same lifetime [2].
- (7)
- The reaction between CO2 and NaOH was considered to be a pseudo-first-order reaction. The concentration of OH− in the liquid film was assumed to be constant during the CO2 absorption process, and thus kapp was regarded as a constant in the liquid element.
4. Results and Discussion
4.1. Model Validation
4.2. Effect of Liquid Flow Rate
4.3. Effect of Inlet Gas Flow Rate
4.4. Effect of Rotational Speed
4.5. Effect of Absorbent Temperature
4.6. Effect of Absorbent Concentration
4.7. Comparison between Spray Column and RZB
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| a | gas–liquid effective interfacial area, m2/m3 |
| aI, aII, aIII | gas–liquid effective interfacial area in zone I, II, and III, respectively, m2/m3 |
| aI,i | gas–liquid effective interfacial area in zone I of region i, m2/m3 |
| AI,i, AII,i | effective mass transfer area in zone I and II of region i, respectively, m2 |
| AIII,i’ | effective mass transfer area in zone III of region i’, m2 |
| A2 | turbulent coefficient |
| CA | difference between actual and equilibrium CO2 concentration in liquid phase, |
| kmol/m3, | CA = CCO2–CCO2* |
| CCO2 | concentration of CO2 in liquid phase, kmol/m3 |
| CCO2* | equilibrium concentration of CO2 in liquid bulk, kmol/m3 |
| C0 | concentration of CO2 at gas–liquid interface, kmol/m3 |
| CNaOH | concentration of NaOH solution, kmol/m3 |
| di | average droplet diameter in zone I of region i, m |
| DCO2 | diffusion coefficient of CO2 in NaOH solution, m2/s |
| DG | diffusion coefficient of CO2 in gas phase, m2/s |
| DW | diffusion coefficient of CO2 in water, m2/s |
| g | acceleration of gravity, m/s2 |
| G | inlet gas flow rate, L/h |
| G’ | inlet flow rate of inert gas (without reaction and dissolution), kmol/s |
| h | constant related to h+, h−, hg, m3/kmol |
| h+, h−, hg | constant of cation, anion, and gas, respectively, m3/kmol |
| hII | axial length of turbulent filmy liquid, m |
| H | Henry’s constant of NaOH solution, kPa m3/kmol |
| HW | Henry’s constant of water, kPa m3/kmol |
| I | ionic strength, kmol/m3 |
| k2 | second-order reaction rate constant, m3/kmol s |
| kapp | pseudo-first-order rate constant, 1/s |
| kG | gas-side mass-transfer coefficient, kmol/kPa m2 s |
| KGa | overall gas-phase volumetric mass-transfer coefficient, kmol/kPa m3 s |
| kL | liquid-side mass-transfer coefficient, m/s |
| kL−I, kL−II, kL−III | liquid-side mass-transfer coefficient in zone I, II, and III, respectively, m/s |
| kL−I,i, kL−II,i | liquid-side mass-transfer coefficient in zone I and II of region i, respectively, m/s |
| kL−III,i’ | liquid-side mass-transfer coefficient in zone III of region i’, m/s |
| kLa | liquid-side volumetric mass-transfer coefficient, 1/s |
| reaction rate constant in infinitely dilute NaOH solution, m3/kmol s | |
| ks | proportionality coefficient, kmol2 s/kPa2 m8 |
| L | liquid flow rate, L/h |
| na,i | number of perforations in rotating baffle ia |
| P | gas phase pressure, kPa |
| PCO2,0 | partial pressure of CO2 in gas phase at gas–liquid interface, kPa |
| QG | gas volumetric flow rate, m3/s |
| QL | liquid volumetric flow rate, m3/s |
| ra,i | radius of rotating baffle ia, m |
| rb,i | radius of static baffle ib, m |
| rh | hydraulic radius of annular region between rotating and static baffles, m |
| ri | inner radius of rotor, m |
| ro | outer radius of rotor, m |
| rm | radius of logarithmic mean, m |
| rCO2 | reaction rate of CO2 with NaOH solution, kmol/m3 s |
| R | radial coordinate of a droplet, m |
| RCO2-II,i | mass transfer rate of liquid film at the gas–liquid interface in zone II of region |
| i, | mol/m2 s |
| ReG | gas Reynolds number, |
| s | complex variable |
| Si | renewal frequency of liquid film in zone II of region i, 1/s |
| tI,i | lifetime of droplets in zone I of region i, s |
| tII,i | lifetime of liquid film in turbulent filmy liquid in zone II of region i, s |
| tIII,i | lifetime of liquid film in flying filmy liquid in zone III of region i’, s |
| T | absorbent temperature, K |
| Tgas | gas temperature at gas inlet of RZB, K |
| ua,i | tangential velocity of droplets leaving rotating baffle ia, m/s |
| ub,i | tangential velocity of flying filmy liquid leaving static baffle ib, m/s |
| ur,i | radial component of ua,i, m/s |
| ug,i | axial component of turbulent filmy liquid velocity on static baffle ib, m/s |
| vG | gas velocity in annular region between rotating and static baffles, m/s |
| vθ | average tangential component of gas velocity, m/s |
| vr | radial component of gas velocity, m/s |
| vz | axial component of gas velocity, m/s |
| vin | gas velocity at gas inlet of RZB, m/s |
| x | liquid film thickness of filmy liquid from gas–liquid interface, m |
| yCO2-in | molar fraction of CO2 in gas inlet of RZB, % |
| yCO2-out | molar fraction of CO2 in gas outlet of RZB, % |
| z | axial depth of rotor, m |
| α | angle between ua,i and tangent to static baffle ib, ° |
| ω | angular velocity, rad/s |
| εI,i | liquid holdup of droplets in zone Iof, region i |
| δ | average thickness of liquid film in zone II, m |
| ρG | density of gas phase, kg/m3 |
| ρL | density of NaOH solution, kg/m3 |
| μG | viscosity of gas phase, pa s |
| μL | viscosity of NaOH solution, pa s |
| μW | viscosity of water, pa s |
| σ | surface tension of NaOH solution, N/m |
References
- Li, Y.; Liu, P.; Wang, G.; Ji, J. Enhanced mass transfer and reduced pressure drop in a compound rotating zigzag bed. Sep. Purif. Technol. 2020, 250, 117188. [Google Scholar] [CrossRef]
- Wang, G.; Zhou, Z.; Li, Y.; Ji, J. Qualitative relationships between structure and performance of rotating zigzag bed in distillation. Chem. Eng. Process.-Process Intensif. 2019, 135, 141–147. [Google Scholar] [CrossRef]
- Wang, G.; Xu, Z.; Ji, J. Progress on higee distillation—Introduction to a new device and its industrial applications. Chem. Eng. Res. Des. 2011, 89, 1434–1442. [Google Scholar] [CrossRef]
- Wang, G.; Xu, Z.; Yu, Y.; Ji, J. Performance of a rotating zigzag bed—A new Higee. Chem. Eng. Process.-Process Intensif. 2008, 47, 2131–2139. [Google Scholar] [CrossRef]
- Wang, G.; Xu, O.; Xu, Z.; Ji, J. New Higee-rotating zigzag bed and its mass transfer performance. Ind. Eng. Chem. Res. 2008, 47, 8840–8846. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Wang, Y.; Chen, Y.; Ji, J.; Yu, Y.; Xu, Z. Distillation in a counterflow concentric-ring rotating bed. Ind. Eng. Chem. Res. 2014, 53, 4821–4837. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, T.; Liu, Z.; Wang, S.; Gao, Y.; Wu, M. Mass transfer in a rotating packed bed: A critical review. Chem. Eng. Process.-Process Intensif. 2019, 139, 78–94. [Google Scholar] [CrossRef]
- Karmakar, S.; Bhowal, A.; Das, P. A comparative study of liquid-liquid extraction in different rotating bed contactors. Chem. Eng. Process.-Process Intensif. 2018, 132, 187–193. [Google Scholar] [CrossRef]
- Liang, Z.; Wei, T.; Xie, J.; Li, H.; Liu, H. Direct conversion of terminal alkenes to aldehydes via ozonolysis reaction in rotating zigzag bed. J. Iran. Chem. Soc. 2020, 17, 2379–2384. [Google Scholar] [CrossRef]
- Li, Y.; Lu, Y.; Liu, X.; Wang, G.; Nie, Y.; Ji, J. Mass-transfer characteristics in a rotating zigzag bed as a Higee device. Sep. Purif. Technol. 2017, 186, 156–165. [Google Scholar] [CrossRef]
- Li, Y.; Yu, Y.; Xu, Z.; Li, X.; Liu, X.; Ji, J. Rotating zigzag bed as trayed Higee and its power consumption. Asia-Pac. J. Chem. Eng. 2013, 8, 494–506. [Google Scholar] [CrossRef]
- Liu, Z.; Esmaeili, A.; Zhang, H.; Xiao, H.; Yun, J.; Shao, L. Carbon dioxide absorption with aqueous amine solutions promoted by piperazine and 1-methylpiperazine in a rotating zigzag bed. Fuel 2021, 302, 121165. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, J.; Xiang, Y.; Zeng, X.; Chen, J. Absorption of carbon dioxide with ionic liquid in a rotating packed bed contactor: Mass transfer study. Ind. Eng. Chem. Res. 2011, 50, 6957–6964. [Google Scholar] [CrossRef]
- Yi, F.; Zou, H.; Chu, G.; Shao, L.; Chen, J. Modeling and experimental studies on absorption of CO2 by benfield solution in rotating packed bed. Chem. Eng. J. 2009, 145, 377–384. [Google Scholar] [CrossRef]
- Sun, B.; Wang, X.; Chen, J.; Chu, G.; Chen, J.; Shao, L. Simultaneous absorption of CO2 and NH3 into water in a rotating packed bed. Ind. Eng. Chem. Res. 2009, 48, 11175–11180. [Google Scholar] [CrossRef]
- Wang, D.; Liu, T.; Ma, L.; Wang, F.; Shao, L. Modeling and experimental studies on ozone absorption into phenolic solution in a rotating packed bed. Ind. Eng. Chem. Res. 2019, 58, 7052–7062. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, X.; Li, G.; Chu, G.; Sun, B.; Zou, H.; Arowo, M.; Shao, L. Mass transfer characteristics in a rotor-stator reactor. Chem. Eng. Technol. 2017, 40, 1078–1083. [Google Scholar] [CrossRef]
- Qian, Z.; Xu, L.; Cao, H.; Guo, K. Modeling study on absorption of CO2 by aqueous solutions of n-methyldiethanolamine in rotating packed bed. Ind. Eng. Chem. Res. 2009, 48, 9261–9267. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, J.; Liu, Z.; Lu, Y.; Chu, G.; Wang, W.; Chen, J. Efficient capture of carbon dioxide with novel mass-transfer intensification device using ionic liquids. AlChE J. 2013, 59, 2957–2965. [Google Scholar] [CrossRef]
- Luo, Y.; Chu, G.; Zou, H.; Wang, F.; Xiang, Y.; Shao, L.; Chen, J. Mass transfer studies in a rotating packed bed with novel rotors: Chemisorption of CO2. Ind. Eng. Chem. Res. 2012, 51, 9164–9172. [Google Scholar] [CrossRef]
- Tsai, C.; Chen, Y. Effective interfacial area and liquid-side mass transfer coefficients in a rotating bed equipped with baffles. Sep. Purif. Technol. 2015, 144, 139–145. [Google Scholar] [CrossRef]
- Li, Y.; Si, J.; Arowo, M.; Liu, Z.; Sun, B.; Song, Y.; Chu, G.; Shao, L. Experimental investigation of effective gas-liquid specific interfacial area in a rotor-stator reactor. Chem. Eng. Process.-Process Intensif. 2020, 148, 107801. [Google Scholar] [CrossRef]
- Wang, H.; Li, Y.; Ji, J. Experimental study on gas flow field in a rotating zigzag bed. Chin. J. Process Eng. 2010, 10, 56–59. (In Chinese) [Google Scholar]
- Li, Y.; Lu, Y.; Wang, G.; Nie, Y.; Ying, H.; Ji, J.; Liu, X. Liquid entrainment and flooding in a rotating zigzag bed. Ind. Eng. Chem. Res. 2015, 54, 2554–2563. [Google Scholar] [CrossRef]
- Lu, Y.; Li, Y.; Yu, Y.; Liu, X.; Ji, J. Study on liquid hold-up of rotating zigzag bed. Chin. J. Process Eng. 2014, 14, 568–572. (In Chinese) [Google Scholar]
- Cao, Z. Investigation into the Absorption in a Cyclone Absorber of Spraying Liquid from Side Wall. Ph.D. Thesis, South Yangtze University, Wuxi, China, 2008. (In Chinese). [Google Scholar]
- Guo, F.; Zheng, C.; Guo, K.; Feng, Y.; Gardner, N. Hydrodynamics and mass transfer in cross-flow rotating packed bed. Chem. Eng. Sci. 1997, 52, 3853–3859. [Google Scholar] [CrossRef]
- Liu, G.; Ma, L.; Xing, Z. Handbook of Chemical Property Chart; Chemical Industry Press: Beijing, China, 2002; pp. 47–163. (In Chinese) [Google Scholar]
- Dutcher, C.; Wexler, A.; Clegg, S.L. Surface tensions of inorganic multicomponent aqueous electrolyte solutions and melts. J. Phys. Chem. A 2010, 114, 12216–12230. [Google Scholar] [CrossRef] [PubMed]
- Sheng, M.; Xie, C.; Sun, B.; Luo, Y.; Zhang, L.; Chu, G.; Zou, H.; Chen, J. Effective mass transfer area measurement using a CO2–NaOH system: Impact of different sources of kinetics models and physical properties. Ind. Eng. Chem. Res. 2019, 58, 11082–11092. [Google Scholar] [CrossRef]
- Pohorecki, R.; Moniuk, W. Kinetics of reaction between carbon dioxide and hydroxyl ions in aqueous electrolyte solutions. Chem. Eng. Sci. 1988, 43, 1677–1684. [Google Scholar] [CrossRef]
- Rajan, S.; Kumar, M.; Ansari, M.J.; Rao, D.; Kaistha, N. Limiting gas liquid flows and mass transfer in a novel rotating packed bed (HiGee). Ind. Eng. Chem. Res. 2011, 50, 986–997. [Google Scholar] [CrossRef]
- Javed, K.; Mahmud, T.; Purba, E. The CO2 capture performance of a high-intensity vortex spray scrubber. Chem. Eng. J. 2010, 162, 448–456. [Google Scholar] [CrossRef]










| Item | Value |
|---|---|
| Inner diameter of the rotor (cm) | 5.7 |
| Outer diameter of the rotor (cm) | 18.3 |
| Inner diameter of the shell (cm) | 23.1 |
| Diameter of the static baffles (cm) | 5.7, 8.1, 9.9, 11.4, 12.7, 14.0, 15.2, 16.3, 17.3, 18.3 |
| Diameter of the rotating baffles (cm) | 7.2, 9.0, 10.7, 12.2, 13.5, 14.7, 15.8, 16.8, 17.8 |
| Volume of the rotor (cm3) | 665 |
| Axial depth of the rotor (cm) | 2.8 |
| Axial depth of the static baffles (cm) | 2.5 |
| Axial depth of the rotating baffles (cm) | 2.4 |
| Diameter of perforation in the rotating baffles (mm) | 2.1 |
| Parameter | Expression |
|---|---|
| H [30] | |
| HW [30] | |
| DCO2 [31] | |
| DW [31] | |
| DG [32] | |
| k2 [31] | |
| [31] | |
| di [27] | |
| vθ [23] | |
| ug,i [25] |
| Javed et al. [33] | This Work | |
|---|---|---|
| Reactor | Spray column | RZB |
| Mass transfer zone volume (cm3) | 9813 | 665 |
| Liquid flow rate (L/h) | 120–300 | 25–40 |
| Gas–liquid volume ratio | 32–42 | 25–40 |
| Rotational speed (rpm) | / | 400–1200 |
| Absorbent | 1.25 kmol/m3 NaOH | 0.10–0.20 kmol/m3 NaOH |
| Inlet CO2 concentration (%) | 2.5 | 4 |
| Absorbent temperature (K) | ca. 298 K | 298 K |
| KGa (105 × kmol/kPa m3 s) | 2.65–4.56 | 6.81–11.28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Esmaeili, A.; Zhang, H.; Wang, D.; Lu, Y.; Shao, L. Modeling and Experimental Studies on Carbon Dioxide Absorption with Sodium Hydroxide Solution in a Rotating Zigzag Bed. Processes 2022, 10, 614. https://doi.org/10.3390/pr10030614
Liu Z, Esmaeili A, Zhang H, Wang D, Lu Y, Shao L. Modeling and Experimental Studies on Carbon Dioxide Absorption with Sodium Hydroxide Solution in a Rotating Zigzag Bed. Processes. 2022; 10(3):614. https://doi.org/10.3390/pr10030614
Chicago/Turabian StyleLiu, Zhibang, Arash Esmaeili, Hanxiao Zhang, Dan Wang, Yuan Lu, and Lei Shao. 2022. "Modeling and Experimental Studies on Carbon Dioxide Absorption with Sodium Hydroxide Solution in a Rotating Zigzag Bed" Processes 10, no. 3: 614. https://doi.org/10.3390/pr10030614
APA StyleLiu, Z., Esmaeili, A., Zhang, H., Wang, D., Lu, Y., & Shao, L. (2022). Modeling and Experimental Studies on Carbon Dioxide Absorption with Sodium Hydroxide Solution in a Rotating Zigzag Bed. Processes, 10(3), 614. https://doi.org/10.3390/pr10030614