Sorbent Properties of Orange Peel-Based Biochar for Different Pollutants in Water
Abstract
:1. Introduction
2. Materials and Methods
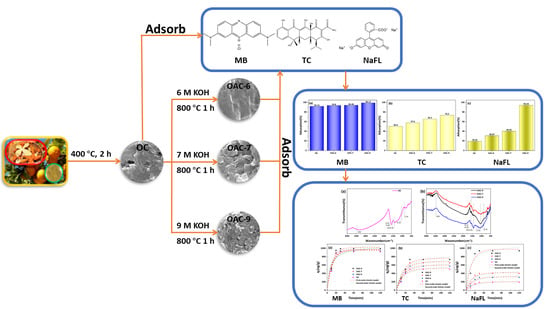
2.1. Material Preparation
2.2. Characterization of Adsorbents
2.3. Adsorption Capacity Analysis
2.4. Adsorption Kinetic Model
3. Results and Discussion
3.1. Characterization of Absorbents
3.2. Adsorption Performance of the Adsorbents
3.2.1. Adsorption Equilibrium
3.2.2. Adsorption Rate
3.2.3. Adsorption Kinetics
3.2.4. Regeneration of Adsorbents
3.2.5. Adsorption Mechanism and Comparison of Adsorption Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Madeira, J.G.F.; Oliveira, E.M.; Springer, M.V.; Cabral, H.L.; do Barbeito, D.F.C.; Souza, A.P.G.; da Moura, D.A.S.; Delgado, A.R.S. Hydrogen production from swine manure biogas via steam reforming of methane (SRM) and water gas shift (WGS): A ecological, technical, and economic analysis. Int. J. Hydrogen Energy 2021, 46, 8961–8971. [Google Scholar] [CrossRef]
- Singla, S.; Sharma, S.; Basu, S.; Shetti, N.P.; Aminabhavi, T.M. Photocatalytic water splitting hydrogen production via environmental benign carbon based nanomaterials. Int. J. Hydrogen Energy 2021, 46, 33696–33717. [Google Scholar] [CrossRef]
- Ramsay, R.R.; Dunford, C.; Gillman, P.K. Methylene blue and serotonin toxicity: Inhibition of monoamine oxidase A (MAO A) confirms a theoretical prediction: Methylene blue inhibits monoamine oxidase A. Br. J. Pharmacol. 2007, 152, 946–951. [Google Scholar] [CrossRef] [PubMed]
- Hejtmancik, M.R. Hematological effects in F344 rats and B6C3F1 mice during the 13-week gavage toxicity study of methylene blue trihydrate. Toxicol. Sci. 2002, 65, 126–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.-F.; Ying, G.-G.; Zhou, L.-J.; Liu, S.; Zhao, J.-L. Dissipation of oxytetracycline in soils under different redox conditions. Environ. Pollut. 2009, 157, 2704–2709. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yan, T.; Jiang, F. Adsorption of Cr(VI) from aqueous solution on mesoporous carbon nitride. J. Taiwan Inst. Chem. Eng. 2014, 45, 1842–1849. [Google Scholar] [CrossRef]
- Wei, D.; Li, B.; Huang, H.; Luo, L.; Zhang, J.; Yang, Y.; Guo, J.; Tang, L.; Zeng, G.; Zhou, Y. Biochar-based functional materials in the purification of agricultural wastewater: Fabrication, application and future research needs. Chemosphere 2018, 197, 165–180. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Liu, Y.; Pi, J.; Li, W.; Liao, Q.; Shang, J. Low-cost magnetic herbal biochar: Characterization and application for antibiotic removal. Environ. Sci. Pollut. Res. 2017, 24, 6679–6687. [Google Scholar] [CrossRef]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar physicochemical properties: Pyrolysis temperature and feedstock kind effects. Rev. Environ. Sci. Biotechnol. 2020, 19, 191–215. [Google Scholar] [CrossRef] [Green Version]
- Babel, S. Low-cost adsorbents for heavy metals uptake from contaminated water: A review. J. Hazard. Mater. 2003, 97, 219–243. [Google Scholar] [CrossRef]
- Babel, S.; Kurniawan, T.A. Cr(VI) removal from synthetic wastewater using coconut shell charcoal and commercial activated carbon modified with oxidizing agents and/or chitosan. Chemosphere 2004, 54, 951–967. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, L.; Liu, Y.; Feng, R.; Zou, T.; Zhang, Y.; Kang, Y.; Zhou, P. Efficient removal of methylene blue using the mesoporous activated carbon obtained from mangosteen peel wastes: Kinetic, equilibrium, and thermodynamic studies. Microporous Mesoporous Mater. 2021, 315, 110904. [Google Scholar] [CrossRef]
- Prusov, A.N.; Prusova, S.M.; Radugin, M.V.; Bazanov, A.V. Flax shive as a source of activated carbon for adsorption of methylene blue. Fuller. Nanotub. Carbon Nanostruct. 2021, 29, 685–694. [Google Scholar] [CrossRef]
- Araújo, L.K.F.; Albuquerque, A.A.; Ramos, W.C.O.; Santos, A.T.; Carvalho, S.H.V.; Soletti, J.I.; Bispo, M.D. Elaeis guineensis-activated carbon for methylene blue removal: Adsorption capacity and optimization using CCD-RSM. Environ. Dev. Sustain. 2021, 23, 11732–11750. [Google Scholar] [CrossRef]
- Yağmur, H.K.; Kaya, İ. Synthesis and characterization of magnetic ZnCl2-activated carbon produced from coconut shell for the adsorption of methylene blue. J. Mol. Struct. 2021, 1232, 130071. [Google Scholar] [CrossRef]
- Gu, S.; Zhang, D.; Gao, Y.; Qi, R.; Chen, W.; Xu, Z. Fabrication of porous carbon derived from cotton/polyester waste mixed with oyster shells: Pore-forming process and application for tetracycline removal. Chemosphere 2021, 270, 129483. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhao, B.; Guo, Y.; Guo, Y.; Pak, T.; Li, G. Preparation of mesoporous batatas biochar via soft-template method for high efficiency removal of tetracycline. Sci. Total Environ. 2021, 787, 147397. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. A Comparison of Chemisorption Kinetic Models Applied to Pollutant Removal on Various Sorbents. Process Saf. Environ. Prot. 1998, 76, 332–340. [Google Scholar] [CrossRef] [Green Version]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Xiao, L.; Bi, E.; Du, B.; Zhao, X.; Xing, C. Surface characterization of maize-straw-derived biochars and their sorption performance for MTBE and benzene. Environ. Earth Sci. 2014, 71, 5195–5205. [Google Scholar] [CrossRef]
- Ahmad, M.; Lee, S.S.; Dou, X.; Mohan, D.; Sung, J.-K.; Yang, J.E.; Ok, Y.S. Effects of pyrolysis temperature on soybean stover- and peanut shell-derived biochar properties and TCE adsorption in water. Bioresour. Technol. 2012, 118, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Unugul, T.; Nigiz, F.U. Preparation and Characterization an Active Carbon Adsorbent from Waste Mandarin Peel and Determination of Adsorption Behavior on Removal of Synthetic Dye Solutions. Water Air. Soil Pollut. 2020, 231, 538. [Google Scholar] [CrossRef]
- Bediako, J.K.; Lin, S.; Sarkar, A.K.; Zhao, Y.; Choi, J.-W.; Song, M.-H.; Cho, C.-W.; Yun, Y.-S. Evaluation of orange peel-derived activated carbons for treatment of dye-contaminated wastewater tailings. Environ. Sci. Pollut. Res. 2020, 27, 1053–1068. [Google Scholar] [CrossRef]
- Dod, R.; Banerjee, G.; Saini, S. Adsorption of methylene blue using green pea peels (Pisum sativum): A cost-effective option for dye-based wastewater treatment. Biotechnol. Bioprocess Eng. 2012, 17, 862–874. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Potential of jackfruit peel as precursor for activated carbon prepared by microwave induced NaOH activation. Bioresour. Technol. 2012, 112, 143–150. [Google Scholar] [CrossRef]
- Marzbali, M.H.; Esmaieli, M.; Abolghasemi, H.; Marzbali, M.H. Tetracycline adsorption by H3PO4-activated carbon produced from apricot nut shells: A batch study. Process Saf. Environ. Prot. 2016, 102, 700–709. [Google Scholar] [CrossRef]
- Cai, Y.; Liu, L.; Tian, H.; Yang, Z.; Luo, X. Adsorption and desorption performance and mechanism of tetracycline hydrochloride by activated carbon-based adsorbents derived from sugar cane bagasse activated with ZnCl2. Molecules 2019, 24, 4534. [Google Scholar] [CrossRef] [Green Version]









| Sample | Specific Surface Area (m2 g−1) | Aperture (nm) |
|---|---|---|
| OC | 0.90 | 147.57 |
| OAC-6 | 7.84 | 8.61 |
| OAC-7 | 723.68 | 3.49 |
| OAC-9 | 1046.51 | 3.30 |
| Sample | C (%) | O (%) |
|---|---|---|
| OC | 80.80 | 14.54 |
| OAC-6 | 87.05 | 10.80 |
| OAC-7 | 88.94 | 10.06 |
| OAC-9 | 84.78 | 11.32 |
| OC | OAC−6 | OAC−7 | OAC−9 | |
|---|---|---|---|---|
| MB | ||||
| qe (μg g−1) | 921 | 938 | 943 | 992 |
| First-order kinetic model | ||||
| k1 (min−1) | 0.146 | 0.144 | 0.161 | 0.160 |
| R2 | 0.971 | 0.975 | 0.975 | 0.976 |
| Second-order kinetic model | ||||
| k2 [g (mg min)−1] | 1.052 | 1.100 | 0.791 | 0.827 |
| R2 | 0.939 | 0.944 | 0.944 | 0.945 |
| TC | ||||
| qe (μg g−1) | 503 | 576 | 655 | 735 |
| First-order kinetic model | ||||
| k1 (min−1) | 0.081 | 0.068 | 0.060 | 0.058 |
| R2 | 0.968 | 0.967 | 0.970 | 0.969 |
| Second-order kinetic model | ||||
| k2 [g (mg min)−1] | 2.500 | 4.020 | 5.594 | 6.865 |
| R2 | 0.935 | 0.935 | 0.941 | 0.941 |
| NaFL | ||||
| qe (μg g−1) | 193 | 308 | 404 | 942 |
| First-order kinetic model | ||||
| k1 (min−1) | 0.080 | 0.092 | 0.109 | 0.131 |
| R2 | 0.942 | 0.952 | 0.957 | 0.960 |
| Second-order kinetic model | ||||
| k2 [g (mg h)−1] | 0.977 | 1.165 | 1.038 | 1.490 |
| R2 | 0.905 | 0.915 | 0.921 | 0.925 |
| Raw Material | Activating Agent | Processing Conditions | Specific Surface Area | Adsorbed Pollutants and Dosage | Adsorption Time | Adsorption Rate (%) | Ref. |
|---|---|---|---|---|---|---|---|
| Mandarin peel | NaOH | 4 h 105 °C | MB and MO (1, 3, 5, 10, 30 mg L−1) | 180 min | MB: 99.77% MO: 79.87% | [23] | |
| Orange peel | ZnCl2 | 800 °C | 1439.50 m2 g−1 | MB (0, 50, 100, 200, 400, 700, and 1100 mg L−1) | 24 h | 99% | [24] |
| Green pea peels | H2SO4 | 30 min 800 °C | 316.20 m2 g−1 | MB (50, 100, 150, and 200 mg L−1) | 10 h | 96–89.73% | [25] |
| Jack fruit peel | NaOH | 0.5 h 700 °C | 1286.7 m2 g−1 | MB (1000 mg L−1) | 25 h | 80% | [26] |
| Apricot nut shells | H3PO4 | 90 min 400 °C | 307.6 m2 g−1 | TC (100 mg L−1) | 24 h | 98% | [27] |
| Sugar cane bagasse | ZnCl2 | 120 min 600 °C | 831.23 m2 g−1 | TC (120–240 mg L−1) | 20 h | 96% | [28] |
| Orange peel | KOH | 1 h 800 °C | 1046 m2 g−1 | MB, TC and NaFL (10 mg L−1) | 30 min | MB: 99.17% TC: 73.5% NaFL: 94.24% | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Wang, Y.; Fan, L.; Liu, X.; Cao, W.; Ai, H.; Wang, Z.; Liu, X.; Jia, H. Sorbent Properties of Orange Peel-Based Biochar for Different Pollutants in Water. Processes 2022, 10, 856. https://doi.org/10.3390/pr10050856
Zhang W, Wang Y, Fan L, Liu X, Cao W, Ai H, Wang Z, Liu X, Jia H. Sorbent Properties of Orange Peel-Based Biochar for Different Pollutants in Water. Processes. 2022; 10(5):856. https://doi.org/10.3390/pr10050856
Chicago/Turabian StyleZhang, Weichao, Yuwei Wang, Liquan Fan, Xingmei Liu, Weiyan Cao, Honglin Ai, Ziteng Wang, Xijun Liu, and Hongge Jia. 2022. "Sorbent Properties of Orange Peel-Based Biochar for Different Pollutants in Water" Processes 10, no. 5: 856. https://doi.org/10.3390/pr10050856
APA StyleZhang, W., Wang, Y., Fan, L., Liu, X., Cao, W., Ai, H., Wang, Z., Liu, X., & Jia, H. (2022). Sorbent Properties of Orange Peel-Based Biochar for Different Pollutants in Water. Processes, 10(5), 856. https://doi.org/10.3390/pr10050856