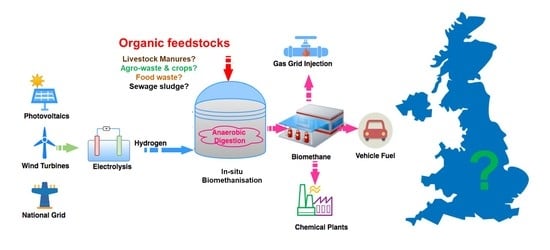
Potential for Biomethanisation of CO2 from Anaerobic Digestion of Organic Wastes in the United Kingdom
Abstract
:1. Introduction
2. Materials and Methods
2.1. Performance of CO2 Biomethanisation of Organic Feedstocks
2.2. AD Feedstock/Energy Production Data and Calculations
3. Results and Discussion
3.1. Performance of CO2 Biomethanisation by Feedstock Type
3.1.1. Livestock Manures
3.1.2. Crops and Agro-Wastes
3.1.3. Food Wastes
3.1.4. OFMSW
3.1.5. Sewage Sludges
3.1.6. Conclusions from Performance Analysis for CO2 Biomethanisation of Organic Feedstocks
3.2. UK Feedstock Data Extraction and Analysis of CO2 Biomethanisation Potential
3.2.1. Estimation of CO2 Biomethanisation Based on Ofgem RO Data
3.2.2. Estimation of CO2 Biomethanisation Uplift Based on Defra’s UK AD Feedstock Data
3.2.3. Potential for CO2 Biomethanisation Using Generation Derived from Wastewater Treatment
3.3. Advantages of CO2 Biomethanisation at Scale
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luo, G.; Angelidaki, I. Integrated biogas upgrading and hydrogen utilization in an anaerobic reactor containing enriched hydrogenotrophic methanogenic culture. Biotechnol. Bioeng. 2012, 109, 2729–2736. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Angelidaki, I.; Zhang, Y. In situ biogas upgrading by CO2-to-CH4 bioconversion. Trends Biotechnol. 2020, 39, 336–347. [Google Scholar] [CrossRef] [PubMed]
- Rafrafi, Y.; Laguillaumie, L.; Dumas, C. Biological Methanation of H2 and CO2 with Mixed Cultures: Current Advances, Hurdles and Challenges. Waste Biomass Valorization 2020, 12, 5259–5282. [Google Scholar] [CrossRef]
- Angelidaki, I.; Treu, L.; Tsapekos, P.; Luo, G.; Campanaro, S.; Wenzel, H.; Kougias, P.G. Biogas upgrading and utilization: Current status and perspectives. Biotechnol. Adv. 2018, 36, 452–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aryal, N.; Kvist, T.; Ammam, F.; Pant, D.; Ottosen, L.D. An overview of microbial biogas enrichment. Bioresour. Technol. 2018, 264, 359–369. [Google Scholar] [CrossRef]
- Lecker, B.; Illi, L.; Lemmer, A.; Oechsner, H. Biological hydrogen methanation—A review. Bioresour. Technol. 2017, 245, 1220–1228. [Google Scholar] [CrossRef]
- Zavarkó, M.; Imre, A.R.; Pörzse, G.; Csedő, Z. Past, Present and Near Future: An Overview of Closed, Running and Planned Biomethanation Facilities in Europe. Energies 2021, 14, 5591. [Google Scholar] [CrossRef]
- Sarker, S.; Lamb, J.J.; Hjelme, D.R.; Lien, K.M. Overview of recent progress towards in-situ biogas upgradation techniques. Fuel 2018, 226, 686–697. [Google Scholar] [CrossRef]
- CCC. The Sixth Carbon Budget; Committee on Climate Change: London, UK, 2020. [Google Scholar]
- D’Silva, T.C.; Isha, A.; Chandra, R.; Vijay, V.K.; Subbarao, P.M.V.; Kumar, R.; Chaudhary, V.P.; Singh, H.; Khan, A.A.; Tyagi, V.K. Enhancing methane production in anaerobic digestion through hydrogen assisted pathways–A state-of-the-art review. Renew. Sustain. Energy Rev. 2021, 151, 111536. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Y.; Dong, R. Recent progress towards in-situ biogas upgrading technologies. Sci. Total Environ. 2021, 800, 149667. [Google Scholar] [CrossRef]
- Tao, B.; Zhang, Y.; Heaven, S.; Banks, C.J. Predicting pH rise as a control measure for integration of CO2 biomethanisation with anaerobic digestion. Appl. Energy 2020, 277, 115535. [Google Scholar] [CrossRef]
- Ofgem. Renewables Obligation (RO) Annual Report 2019-20; Office of Gas and Electricity Markets: London, UK, 2021.
- BEIS. Sustainability Standards for Electricity Generation from Biomass; Department for Business, Energy & Industrial Strategy: London, UK, 2013; Volume 2022.
- Ofgem. Biomass Sustainability; Office of Gas and Electricity Markets: London, UK, 2021.
- ADAT. Anaerobic Digestion Assessment Tool: User Manual; University of Southampton: Southampton, UK, 2017. [Google Scholar]
- Defra. Official Statistics Section 3: Anaerobic Digestion; Department for Environment Food & Rural Affairs: London, UK, 2021.
- BEIS. Digest of UK Energy Statistics (DUKES); Department for Business, Energy & Industrial Strategy: London, UK, 2021.
- Khan, M.U.; Lee, J.T.E.; Bashir, M.A.; Dissanayake, P.D.; Ok, Y.S.; Tong, Y.W.; Shariati, M.A.; Wu, S.; Ahring, B.K. Current status of biogas upgrading for direct biomethane use: A review. Renew. Sustain. Energy Rev. 2021, 149, 111343. [Google Scholar] [CrossRef]
- Thema, M.; Weidlich, T.; Hörl, M.; Bellack, A.; Mörs, F.; Hackl, F.; Kohlmayer, M.; Gleich, J.; Stabenau, C.; Trabold, T. Biological CO2-methanation: An approach to standardization. Energies 2019, 12, 1670. [Google Scholar] [CrossRef] [Green Version]
- Bassani, I.; Kougias, P.G.; Treu, L.; Angelidaki, I. Biogas upgrading via hydrogenotrophic methanogenesis in two-stage continuous stirred tank reactors at mesophilic and thermophilic conditions. Environ. Sci. Technol. 2015, 49, 12585–12593. [Google Scholar] [CrossRef]
- Treu, L.; Kougias, P.; de Diego-Díaz, B.; Campanaro, S.; Bassani, I.; Fernández-Rodríguez, J.; Angelidaki, I. Two-year microbial adaptation during hydrogen-mediated biogas upgrading process in a serial reactor configuration. Bioresour. Technol. 2018, 264, 140–147. [Google Scholar] [CrossRef]
- Wahid, R.; Horn, S.J. The effect of mixing rate and gas recirculation on biological CO2 methanation in two-stage CSTR systems. Biomass Bioenergy 2021, 144, 105918. [Google Scholar] [CrossRef]
- Lebranchu, A.; Blanchard, F.; Fick, M.; Pacaud, S.; Olmos, E.; Delaunay, S. Pilot-scale biomethanation of cattle manure using dense membranes. Bioresour. Technol. 2019, 284, 430–436. [Google Scholar] [CrossRef]
- Zhu, X.; Cao, Q.; Chen, Y.; Sun, X.; Liu, X.; Li, D. Effects of mixing and sodium formate on thermophilic in-situ biogas upgrading by H2 addition. J. Clean. Prod. 2019, 216, 373–381. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, L.; Chen, Y.; Cao, Q.; Liu, X.; Li, D. Differences of methanogenesis between mesophilic and thermophilic in situ biogas-upgrading systems by hydrogen addition. J. Ind. Microbiol. Biotechnol. 2019, 46, 1569–1581. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, L.; Chen, Y.; Cao, Q.; Liu, X.; Li, D. Effect of H2 addition on the microbial community structure of a mesophilic anaerobic digestion system. Energy 2020, 198, 117368. [Google Scholar] [CrossRef]
- Jiang, H.; Wu, F.; Wang, Y.; Feng, L.; Zhou, H.; Li, Y. Characteristics of in-situ hydrogen biomethanation at mesophilic and thermophilic temperatures. Bioresour. Technol. 2021, 337, 125455. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Angelidaki, I. Co-digestion of manure and whey for in situ biogas upgrading by the addition of H2: Process performance and microbial insights. Appl. Microbiol. Biotechnol. 2013, 97, 1373–1381. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Angelidaki, I. Hollow fiber membrane based H2 diffusion for efficient in situ biogas upgrading in an anaerobic reactor. Appl. Microbiol. Biotechnol. 2013, 97, 3739–3744. [Google Scholar] [CrossRef] [PubMed]
- Wahid, R.; Horn, S.J. Impact of operational conditions on methane yield and microbial community composition during biological methanation in in situ and hybrid reactor systems. Biotechnol. Biofuels 2021, 14, 170. [Google Scholar] [CrossRef] [PubMed]
- Corbellini, V.; Kougias, P.G.; Treu, L.; Bassani, I.; Malpei, F.; Angelidaki, I. Hybrid biogas upgrading in a two-stage thermophilic reactor. Energy Convers. Manag. 2018, 168, 1–10. [Google Scholar] [CrossRef]
- Khan, A.; Akbar, S.; Okonkwo, V.; Smith, C.; Khan, S.; Shah, A.A.; Adnan, F.; Ijaz, U.Z.; Ahmed, S.; Badshah, M. Enrichment of the hydrogenotrophic methanogens for, in-situ biogas up-gradation by recirculation of gases and supply of hydrogen in methanogenic reactor. Bioresour. Technol. 2022, 345, 126219. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.; Angelidaki, I. Ammonia inhibition on hydrogen enriched anaerobic digestion of manure under mesophilic and thermophilic conditions. Water Res. 2016, 105, 314–319. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Zhu, X.; Yan, Q.; Zhang, Y.; Angelidaki, I. Microbial community response to ammonia levels in hydrogen assisted biogas production and upgrading process. Bioresour. Technol. 2020, 296, 122276. [Google Scholar] [CrossRef]
- Garcia-Robledo, E.; Ottosen, L.D.; Voigt, N.V.; Kofoed, M.; Revsbech, N.P. Micro-scale H2–CO2 dynamics in a hydrogenotrophic methanogenic membrane reactor. Front. Microbiol. 2016, 7, 1276. [Google Scholar] [CrossRef] [Green Version]
- Vechi, N.T.; Agneessens, L.M.; Feilberg, A.; Ottosen, L.D.M.; Kofoed, M.V.W. In situ biomethanation: Inoculum origin influences acetate consumption rate during hydrogen addition. Bioresour. Technol. Rep. 2021, 14, 100656. [Google Scholar] [CrossRef]
- Lukehurst, C.; Bywater, A. Exploring the Viability of Small Scale Anaerobic Digesters in Livestock Farming; IEA Bioenergy: Paris, France, 2015. [Google Scholar]
- Jensen, M.B.; Jensen, B.; Ottosen, L.D.M.; Kofoed, M.V.W. Integrating H2 injection and reactor mixing for low-cost H2 gas-liquid mass transfer in full-scale in situ biomethanation. Biochem. Eng. J. 2021, 166, 107869. [Google Scholar] [CrossRef]
- Jensen, M.B.; Kofoed, M.V.W.; Fischer, K.; Voigt, N.V.; Agneessens, L.M.; Batstone, D.J.; Ottosen, L.D.M. Venturi-type injection system as a potential H2 mass transfer technology for full-scale in situ biomethanation. Appl. Energy 2018, 222, 840–846. [Google Scholar] [CrossRef]
- Nock, W.J.; Serna-Maza, A.; Heaven, S.; Banks, C.J. Evaluation of microporous hollow fibre membranes for mass transfer of H2 into anaerobic digesters for biomethanization. J. Chem. Technol. Biotechnol. 2019, 94, 2693–2701. [Google Scholar] [CrossRef] [Green Version]
- Voelklein, M.; Rusmanis, D.; Murphy, J. Biological methanation: Strategies for in-situ and ex-situ upgrading in anaerobic digestion. Appl. Energy 2019, 235, 1061–1071. [Google Scholar] [CrossRef]
- Illi, L.; Lecker, B.; Lemmer, A.; Müller, J.; Oechsner, H. Biological methanation of injected hydrogen in a two-stage anaerobic digestion process. Bioresour. Technol. 2021, 333, 125126. [Google Scholar] [CrossRef]
- Schönberg, V.; Busch, G. Steigerung des Methangehaltes durch biologische Wasserstoffumsetzung. Bornim Agrar. Ber 2012, 79, 66–75. [Google Scholar]
- Agneessens, L.M.; Ottosen, L.D.M.; Voigt, N.V.; Nielsen, J.L.; de Jonge, N.; Fischer, C.H.; Kofoed, M.V.W. In-situ biogas upgrading with pulse H2 additions: The relevance of methanogen adaption and inorganic carbon level. Bioresour. Technol. 2017, 233, 256–263. [Google Scholar] [CrossRef]
- Frydendal-Nielsen, S.; Jørgensen, U.; Hjorth, M.; Felby, C.; Gislum, R. Comparing methods for measuring the digestibility of miscanthus in bioethanol or biogas processing. GCB Bioenergy 2017, 9, 168–175. [Google Scholar] [CrossRef] [Green Version]
- Agneessens, L.M.; Ottosen, L.D.M.; Andersen, M.; Olesen, C.B.; Feilberg, A.; Kofoed, M.V.W. Parameters affecting acetate concentrations during in-situ biological hydrogen methanation. Bioresour. Technol. 2018, 258, 33–40. [Google Scholar] [CrossRef]
- Mulat, D.G.; Mosbæk, F.; Ward, A.J.; Polag, D.; Greule, M.; Keppler, F.; Nielsen, J.L.; Feilberg, A. Exogenous addition of H2 for an in situ biogas upgrading through biological reduction of carbon dioxide into methane. Waste Manag. 2017, 68, 146–156. [Google Scholar] [CrossRef]
- Szuhaj, M.; Ács, N.; Tengölics, R.; Bodor, A.; Rákhely, G.; Kovács, K.L.; Bagi, Z. Conversion of H2 and CO2 to CH4 and acetate in fed-batch biogas reactors by mixed biogas community: A novel route for the power-to-gas concept. Biotechnol. Biofuels 2016, 9, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarker, S.; Wijnsma, S.N.; Lien, K.M. Lessons Learned from an Experimental Campaign on Promoting Energy Content of Renewable Biogas by Injecting H2 during Anaerobic Digestion. Energies 2020, 13, 3542. [Google Scholar] [CrossRef]
- Song, H.; Zhang, Y.; Kusch-Brandt, S.; Banks, C.J. Comparison of variable and constant loading for mesophilic food waste digestion in a long-term experiment. Energies 2020, 13, 1279. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Alessi, A.M.; Heaven, S.; Chong, J.P.; Banks, C.J. Dynamic changes in anaerobic digester metabolic pathways and microbial populations during acclimatisation to increasing ammonium concentrations. Waste Manag. 2021, 135, 409–419. [Google Scholar] [CrossRef]
- Zhang, Y. Unpublished Work on CO2 Biomethanisation of Organic Feedstocks; Univerity of Southampton: Southampton, UK, 2022. [Google Scholar]
- Kim, S.; Mostafa, A.; Im, S.; Lee, M.-K.; Kang, S.; Na, J.-G.; Kim, D.-H. Production of high-calorific biogas from food waste by integrating two approaches: Autogenerative high-pressure and hydrogen injection. Water Res. 2021, 194, 116920. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, Y.; Zhang, J.; Mao, K.; Kurbonova, M.; Liu, G.; Zhang, R.; Wang, W. Improvement of biofuel recovery from food waste by integration of anaerobic digestion, digestate pyrolysis and syngas biomethanation under mesophilic and thermophilic conditions. J. Clean. Prod. 2020, 256, 120594. [Google Scholar] [CrossRef]
- Zhang, W.; Heaven, S.; Banks, C.J. Thermophilic digestion of food waste by dilution: Ammonia limit values and energy considerations. Energy Fuels 2017, 31, 10890–10900. [Google Scholar] [CrossRef]
- Thapa, A.; Park, J.-G.; Yang, H.-M.; Jun, H.-B. In-situ biogas upgrading in an anaerobic trickling filter bed reactor treating a thermal post-treated digestate. J. Environ. Chem. Eng. 2021, 9, 106780. [Google Scholar] [CrossRef]
- Okoro-Shekwaga, C.K.; Ross, A.B.; Camargo-Valero, M.A. Improving the biomethane yield from food waste by boosting hydrogenotrophic methanogenesis. Appl. Energy 2019, 254, 113629. [Google Scholar] [CrossRef]
- Okoro-Shekwaga, C.K.; Ross, A.B.; Camargo-Valero, M.A. Enhanced in-situ biomethanation of food waste by sequential inoculum acclimation: Energy efficiency and carbon savings analysis. Waste Manag. 2021, 130, 12–22. [Google Scholar] [CrossRef]
- Okoro-Shekwaga, C.K.; Ross, A.; Camargo-Valero, M.A. Enhancing bioenergy production from food waste by in situ biomethanation: Effect of the hydrogen injection point. Food Energy Secur. 2021, 10, e288. [Google Scholar] [CrossRef]
- Treu, L.; Tsapekos, P.; Peprah, M.; Campanaro, S.; Giacomini, A.; Corich, V.; Kougias, P.G.; Angelidaki, I. Microbial profiling during anaerobic digestion of cheese whey in reactors operated at different conditions. Bioresour. Technol. 2019, 275, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Fontana, A.; Kougias, P.G.; Treu, L.; Kovalovszki, A.; Valle, G.; Cappa, F.; Morelli, L.; Angelidaki, I.; Campanaro, S. Microbial activity response to hydrogen injection in thermophilic anaerobic digesters revealed by genome-centric metatranscriptomics. Microbiome 2018, 6, 194. [Google Scholar] [CrossRef] [PubMed]
- Lovato, G.; Alvarado-Morales, M.; Kovalovszki, A.; Peprah, M.; Kougias, P.G.; Rodrigues, J.A.D.; Angelidaki, I. In-situ biogas upgrading process: Modeling and simulations aspects. Bioresour. Technol. 2017, 245, 332–341. [Google Scholar] [CrossRef] [Green Version]
- Palù, M.; Peprah, M.; Tsapekos, P.; Kougias, P.; Campanaro, S.; Angelidaki, I.; Treu, L. In-situ biogas upgrading assisted by bioaugmentation with hydrogenotrophic methanogens during mesophilic and thermophilic co-digestion. Bioresour. Technol. 2022, 348, 126754. [Google Scholar] [CrossRef]
- Bassani, I.; Kougias, P.G.; Angelidaki, I. In-situ biogas upgrading in thermophilic granular UASB reactor: Key factors affecting the hydrogen mass transfer rate. Bioresour. Technol. 2016, 221, 485–491. [Google Scholar] [CrossRef] [Green Version]
- Deschamps, L.; Imatoukene, N.; Lemaire, J.; Mounkaila, M.; Filali, R.; Lopez, M.; Theoleyre, M.-A. In-situ biogas upgrading by bio-methanation with an innovative membrane bioreactor combining sludge filtration and H2 injection. Bioresour. Technol. 2021, 337, 125444. [Google Scholar] [CrossRef]
- Tao, B.; Alessi, A.M.; Zhang, Y.; Chong, J.P.; Heaven, S.; Banks, C.J. Simultaneous biomethanisation of endogenous and imported CO2 in organically loaded anaerobic digesters. Appl. Energy 2019, 247, 670–681. [Google Scholar] [CrossRef]
- Wahid, R.; Mulat, D.G.; Gaby, J.C.; Horn, S.J. Effects of H2: CO2 ratio and H2 supply fluctuation on methane content and microbial community composition during in-situ biological biogas upgrading. Biotechnol. Biofuels 2019, 12, 104. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Wang, K.; Zhang, X.; Gong, H.; Xia, Y.; Holmes, D.E. Application of in-situ H2-assisted biogas upgrading in high-rate anaerobic wastewater treatment. Bioresour. Technol. 2020, 299, 122598. [Google Scholar] [CrossRef]
- Jing, Y.; Campanaro, S.; Kougias, P.; Treu, L.; Angelidaki, I.; Zhang, S.; Luo, G. Anaerobic granular sludge for simultaneous biomethanation of synthetic wastewater and CO with focus on the identification of CO-converting microorganisms. Water Res. 2017, 126, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Banks, C.; Heaven, S.; Zhang, Y.; Baier, U. Food Waste Digestion: Anaerobic Digestion of Food Waste for a Circular Economy; IEA Bioenergy: Paris, France, 2018. [Google Scholar]
- Chen, A.C.; Ohashi, A.; Harada, H. Acetate synthesis from H2/CO2 in simulated and actual landfill samples. Environ. Technol. 2003, 24, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Sekoai, P.T.; Engelbrecht, N.; du Preez, S.P.; Bessarabov, D. Thermophilic Biogas Upgrading via ex Situ Addition of H2 and CO2 Using Codigested Feedstocks of Cow Manure and the Organic Fraction of Solid Municipal Waste. ACS Omega 2020, 5, 17367–17376. [Google Scholar] [CrossRef] [PubMed]
- Alfaro, N.; Fdz-Polanco, M.; Fdz-Polanco, F.; Díaz, I. H2 addition through a submerged membrane for in-situ biogas upgrading in the anaerobic digestion of sewage sludge. Bioresour. Technol. 2019, 280, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Corbellini, V.; Catenacci, A.; Malpei, F. Hydrogenotrophic biogas upgrading integrated into WWTPs: Enrichment strategy. Water Sci. Technol. 2019, 79, 759–770. [Google Scholar] [CrossRef] [PubMed]
- Corbellini, V.; Feng, C.; Bellucci, M.; Catenacci, A.; Stella, T.; Espinoza-Tofalos, A.; Malpei, F. Performance Analysis and Microbial Community Evolution of In Situ Biological Biogas Upgrading with Increasing H2/CO2 Ratio. Archaea 2021, 8894455. [Google Scholar] [CrossRef]
- Díaz, I.; Fdz-Polanco, F.; Mutsvene, B.; Fdz-Polanco, M. Effect of operating pressure on direct biomethane production from carbon dioxide and exogenous hydrogen in the anaerobic digestion of sewage sludge. Appl. Energy 2020, 280, 115915. [Google Scholar] [CrossRef]
- Luo, G.; Wang, W.; Angelidaki, I. Anaerobic digestion for simultaneous sewage sludge treatment and CO biomethanation: Process performance and microbial ecology. Environ. Sci. Technol. 2013, 47, 10685–10693. [Google Scholar] [CrossRef]
- Wang, W.; Xie, L.; Luo, G.; Zhou, Q.; Angelidaki, I. Performance and microbial community analysis of the anaerobic reactor with coke oven gas biomethanation and in situ biogas upgrading. Bioresour. Technol. 2013, 146, 234–239. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Hao, X.; Zhao, D.; Fu, K. Enhancing the CH4 yield of anaerobic digestion via endogenous CO2 fixation by exogenous H2. Chemosphere 2015, 140, 34–39. [Google Scholar] [CrossRef]
- Braga Nan, L.; Trably, E.; Santa-Catalina, G.; Bernet, N.; Delgenès, J.-P.; Escudié, R. Biomethanation processes: New insights on the effect of a high H2 partial pressure on microbial communities. Biotechnol. Biofuels 2020, 13, 141. [Google Scholar] [CrossRef] [PubMed]
- Alfaro, N.; Fdz-Polanco, M.; Fdz-Polanco, F.; Díaz, I. Evaluation of process performance, energy consumption and microbiota characterization in a ceramic membrane bioreactor for ex-situ biomethanation of H2 and CO2. Bioresour. Technol. 2018, 258, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Fernández, Y.B.; Soares, A.; Villa, R.; Vale, P.; Cartmell, E. Carbon capture and biogas enhancement by carbon dioxide enrichment of anaerobic digesters treating sewage sludge or food waste. Bioresour. Technol. 2014, 159, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Mikucka, W.; Zielińska, M. Distillery stillage: Characteristics, treatment, and valorization. Appl. Biochem. Biotechnol. 2020, 192, 770–793. [Google Scholar] [CrossRef] [PubMed]
- Ofgem. Renewables Obligation (RO) Annual Report 2018–19; Office of Gas and Electricity Markets: London, UK, 2020.
- NNFCC. Anaerobic Digestion Deployment in the UK; National Non-Food Crops Centre: York, UK, 2021. [Google Scholar]
- Ofwat. Bioresources Market Review: Jacobs Report; Office of Water Services: Birmingham, UK, 2021.
- ADBA. ADBA Policy Report October 2021; Anaerobic Digestion and Bioresources Association: London, UK, 2021. [Google Scholar]
- UKWIR. BQ10: How Do We Remove More Carbon than We Emit by 2050? UK Water Industry Research: London, UK, 2022. [Google Scholar]
- EA. Renewable Energy Potential for the Water Industry; Environment Agency-Evidence Directorate: Bristol, UK, 2009.
- Ofgem. Decision on Revised Typical Domestic Consumption Values for Gas and Electricity and Economy 7 Consumption Split; Office of Gas and Electricity Markets: London, UK, 2020.
- Defra. Key Messages for 2021; Department for Environment & Rural Affairs: London, UK, 2021.
- DfT. Transport and Environment Statistics: Autumn 2021; Department for Transport: London, UK, 2021.
- BEIS. Greenhouse Gas Reporting: Conversion Factors 2018; Department for Business, Energy & Industrial Strategy: London, UK, 2018.
- Zhang, L.; Kuroki, A.; Tong, Y.W. A mini-review on in situ biogas upgrading technologies via enhanced hydrogenotrophic methanogenesis to improve the quality of biogas from anaerobic digesters. Front. Energy Res. 2020, 8, 69. [Google Scholar] [CrossRef]
- BEIS. Biomass Policy Statement; Department for Business, Energy & Industrial Strategy: London, UK, 2021.
| Without CO2 Biometh | With CO2 Biometh | ||||||
|---|---|---|---|---|---|---|---|
| Substrate Type | SMP a | CH4 a | pH | ab | Assumed Max pH a | Predicted Max CH4 | Predicted Max SMP |
| L CH4 g−1 VS | % | % | L CH4 g−1 VS | ||||
| Livestock manures | 0.190 | 60 | 7.5 | 7.60 × 10−8 | 8.2 | 93 | 0.295 |
| Crop & agro-wastes | 0.350 | 55 | 7.5 | 6.75 × 10−8 | 8.2 | 92 | 0.587 |
| Post-consumer food wastes | 0.450 | 55 | 7.9 | 2.54 × 10−8 | 8.2 | 79 | 0.649 |
| OFMSW | 0.300 | 55 | 7.5 | 6.75 × 10−8 | 8.2 | 92 | 0.503 |
| Sewage sludge | 0.260 | 65 | 7.5 | 8.68 × 10−8 | 8.0 | 90 | 0.359 |
| Feedstock Category 1 | Biogas Produced 1 m3 | Without CO2 Biometh | With CO2 Biometh | TS 2 %FM | VS 2 %TS | Feedstock 7 Tonnes FM | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| CH4 2 % vol | SMP 2 L CH4 kg−1 VS | Gross Energetic Value 3 GWh | CH4 4 % vol | SMP 5 L CH4 kg−1 VS | Gross Energetic Value 6 GWh | |||||
| (a) | (b) | (c) | (d) | (e) | (f) | (g) | (h) | (i) | (j) | (k) |
| Silage | 219,462,510 | 55 | 350 | 1201 | 92 | 585 | 2009 | 30 | 94 | 1,222,942 |
| Food, garden and plant waste | 165,291,843 | 55 | 450 | 905 | 79 | 646 | 1299 | 24 | 92 | 914,961 |
| Manures and slurries | 64,654,159 | 60 | 190 | 386 | 93 | 295 | 598 | 9 | 83 | 2,733,213 |
| Distillery waste | 16,055,060 | 60 | 300 | 96 | 90 | 450 | 144 | 7.1 | 73 | 619,528 |
| DAF sludge/wastewater | 14,960,483 | 65 | 260 | 97 | 90 | 360 | 134 | 6 | 65 | 959,005 |
| Crops | 14,141,474 | 55 | 350 | 77 | 92 | 585 | 129 | 60 | 93 | 39,825 |
| Glycerol | 9,919,812 | 60 | 425 | 59 | 90 | 638 | 89 | 99.5 | 99.5 | 14,146 |
| Dairy waste | 7,921,015 | 51 | 450 | 40 | 90 | 794 | 71 | 6.1 | 90 | 163,518 |
| Municipal waste | 6,271,886 | 55 | 300 | 34 | 92 | 502 | 57 | 24 | 92 | 52,076 |
| Other | 6,513,539 | 50 | 350 | 32 | 90 | 630 | 58 | 24 | 92 | 42,142 |
| TOTAL | 525,191,781 | 2928 | 4589 | 6,761,357 | ||||||
| Feedstock Category 1 | Feedstock 2 Tonnes FM | TS 3 %FM | VS 3 %TS | Without CO2 Biometh | With CO2 Biometh | ||||
|---|---|---|---|---|---|---|---|---|---|
| CH4 4 % vol | SMP 4 L CH4 kg−1 VS | Gross Energetic Value 5 GWh | CH4 4 % vol | SMP 4 L CH4 kg−1 VS | Gross Energetic Value 6 GWh | ||||
| (a) | (b) | (c) | (d) | (e) | (f) | (g) | (h) | (i) | (j) |
| Crops (treated as silage) | 4,163,000 | 30 | 94 | 55 | 350 | 4088 | 92 | 585 | 6839 |
| Food waste | 4,084,000 | 24 | 92 | 55 | 450 | 4038 | 79 | 646 | 5799 |
| Manures and slurries | 2,359,000 | 9 | 83 | 60 | 190 | 333 | 93 | 295 | 516 |
| Crop waste | 525,000 | 60 | 93 | 55 | 350 | 1020 | 92 | 585 | 1707 |
| Other | 2,727,000 | 24 | 92 | 50 | 350 | 2097 | 90 | 630 | 3774 |
| TOTAL | 13,858,000 | 11,576 | 18,635 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bywater, A.; Heaven, S.; Zhang, Y.; Banks, C.J. Potential for Biomethanisation of CO2 from Anaerobic Digestion of Organic Wastes in the United Kingdom. Processes 2022, 10, 1202. https://doi.org/10.3390/pr10061202
Bywater A, Heaven S, Zhang Y, Banks CJ. Potential for Biomethanisation of CO2 from Anaerobic Digestion of Organic Wastes in the United Kingdom. Processes. 2022; 10(6):1202. https://doi.org/10.3390/pr10061202
Chicago/Turabian StyleBywater, Angela, Sonia Heaven, Yue Zhang, and Charles J. Banks. 2022. "Potential for Biomethanisation of CO2 from Anaerobic Digestion of Organic Wastes in the United Kingdom" Processes 10, no. 6: 1202. https://doi.org/10.3390/pr10061202
APA StyleBywater, A., Heaven, S., Zhang, Y., & Banks, C. J. (2022). Potential for Biomethanisation of CO2 from Anaerobic Digestion of Organic Wastes in the United Kingdom. Processes, 10(6), 1202. https://doi.org/10.3390/pr10061202