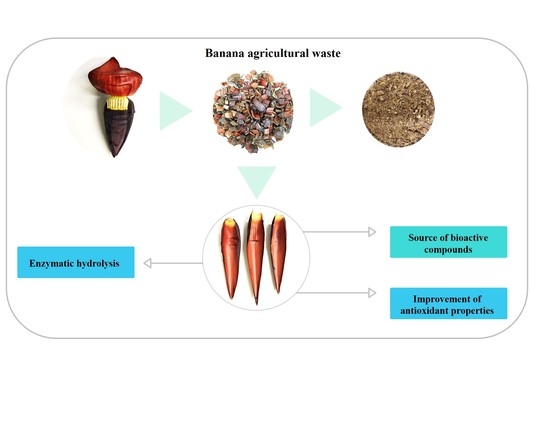
Synergistic Action of Multiple Enzymes Resulting in Efficient Hydrolysis of Banana Bracts and Products with Improved Antioxidant Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material and Reagents
2.2. Enzymatic Hydrolysis and Obtaining Banana Bract Extracts
2.2.1. Determination of Total Phenolic Compounds (TPC)
2.2.2. Measurement of the Antioxidant Properties
ABTS-Radical Cation Scavenging Activity
DPPH-Radical Scavenging Activity
Ferric Reducing Antioxidant Power (FRAP)
2.2.3. Identification of Phenolic Compounds by HPLC
2.2.4. Calculations and Statistics
3. Results and Discussion
3.1. Effect of Enzymatic Hydrolysis on the Recovery of Antioxidant Compounds
3.2. Identification of Phenolic Compounds by HPLC
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- FAO. Food and Agriculture Organization of the United Nations. Banana—Production Quantity. 2022. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 17 March 2022).
- Mathew, N.S.; Negi, P.S. Traditional Uses, Phytochemistry and Pharmacology of Wild Banana (Musa acuminata Colla): A Review. J. Ethnopharmacol. 2017, 196, 124–140. [Google Scholar] [CrossRef] [PubMed]
- Sitthiya, K.; Devkota, L.; Sadiq, M.B.; Anal, A.K. Extraction and Characterization of Proteins from Banana (Musa sapientum L.) Flower and Evaluation of Antimicrobial Activities. J. Food Sci. Technol. 2018, 55, 658–666. [Google Scholar] [CrossRef]
- Basumatary, S.; Nath, N. Assessment of Chemical Compositions and in Vitro Antioxidant Properties of Musa balbisiana Colla Inflorescence. Int. J. Pharm. Res. 2018, 10, 80–85. [Google Scholar]
- Ramu, R.; Shirahatti, P.S.; Anilakumar, K.R.; Nayakavadi, S.; Zameer, F.; Dhananjaya, B.L.; Nagendra Prasad, M.N. Assessment of Nutritional Quality and Global Antioxidant Response of Banana (Musa sp. CV. Nanjangud Rasa Bale) Pseudostem and Flower. Pharmacogn. Res. 2017, 9 (Suppl 1), S74–S83. [Google Scholar] [CrossRef]
- Bhaskar, J.J.; Chilkunda, N.D.; Salimath, P.V. Banana (Musa Sp. Var. Elakki Bale) Flower and Pseudostem: Dietary Fiber and Associated Antioxidant Capacity. J. Agric. Food Chem. 2012, 60, 427–432. [Google Scholar] [CrossRef]
- Padam, B.S.; Tin, H.S.; Chye, F.Y.; Abdullah, M.I. Antibacterial and Antioxidative Activities of the Various Solvent Extracts of Banana (Musa Paradisiaca Cv. Mysore) Inflorescences. J. Biol. Sci. 2012, 12, 62–73. [Google Scholar] [CrossRef]
- Prakasan, N.; Saraswathy, M. Flavanoid Rich Ethyl Acetate Fraction of Musa Paradisiaca Inflorescence Down-Regulates the Streptozotocin Induced Oxidative Stress, Hyperglycaemia and MRNA Levels of Selected Inflammatory Genes in Rats. J. Funct. Foods 2013, 5, 1838–1847. [Google Scholar] [CrossRef]
- Arun, K.B.; Thomas, S.; Reshmitha, T.R.; Akhil, G.C.; Nisha, P. Dietary Fibre and Phenolic-Rich Extracts from Musa Paradisiaca Inflorescence Ameliorates Type 2 Diabetes and Associated Cardiovascular Risks. J. Funct. Foods 2017, 31, 198–207. [Google Scholar] [CrossRef]
- Sheng, Z.; Dai, H.; Pan, S.; Wang, H.; Hu, Y.; Ma, W. Isolation and Characterization of an α-Glucosidase Inhibitor from Musa spp. (Baxijiao) Flowers. Molecules 2014, 19, 10563–10573. [Google Scholar] [CrossRef] [PubMed]
- Bandara, N.; Chalamaiah, M. Encyclopedia of Food Chemistry. In Encyclopedia of Food Chemistry; Varelis, P., Melton, L., Shahidi, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; p. 2194. [Google Scholar]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef]
- Marathe, S.J.; Jadhav, S.B.; Bankar, S.B.; Singhal, R.S. Enzyme-Assisted Extraction of Bioactives. In Food Bioactives: Extraction and Biotecnhonology Applications; Elsevier: Chatswood, Australia, 2017; p. 326. [Google Scholar]
- Olszowy, M. What Is Responsible for Antioxidant Properties of Polyphenolic Compounds from Plants? Plant Physiol. Biochem. 2019, 144, 135–143. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for Extraction of Bioactive Compounds from Plant Materials: A Review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Soquetta, M.B.; Terra, L.D.M.; Bastos, C.P. Green Technologies for the Extraction of Bioactive Compounds in Fruits and Vegetables. CYTA—J. Food 2018, 16, 400–412. [Google Scholar] [CrossRef]
- De la Peña-Armada, R.; Villanueva-Suárez, M.J.; Rupérez, P.; Mateos-Aparicio, I. High Hydrostatic Pressure Assisted by Celluclast® Releases Oligosaccharides from Apple By-Product. Foods 2020, 9, 1058. [Google Scholar] [CrossRef] [PubMed]
- Nadar, S.S.; Rao, P.; Rathod, V.K. Enzyme Assisted Extraction of Biomolecules as an Approach to Novel Extraction Technology: A Review. Food Res. Int. 2018, 108, 309–330. [Google Scholar] [CrossRef] [PubMed]
- Lau, B.F.; Kong, K.W.; Leong, K.H.; Sun, J.; He, X.; Wang, Z.; Mustafa, M.R.; Ling, T.C.; Ismail, A. Banana Inflorescence: Its Bio-Prospects as an Ingredient for Functional Foods. Trends Food Sci. Technol. 2020, 97, 14–28. [Google Scholar] [CrossRef]
- Gligor, O.; Mocan, A.; Moldovan, C.; Locatelli, M.; Crișan, G.; Ferreira, I.C.F.R. Enzyme-Assisted Extractions of Polyphenols—A Comprehensive Review. Trends Food Sci. Technol. 2019, 88, 302–315. [Google Scholar] [CrossRef]
- Casas, M.P.; Domínguez González, H. Enzyme-Assisted Aqueous Extraction Processes. In Water Extraction of Bioactive Compounds: From Plants to Drug Development; Elsevier: Amsterdam, The Netherlands, 2017; pp. 333–368. [Google Scholar] [CrossRef]
- Danalache, F.; Mata, P.; Alves, V.D.; Moldão-Martins, M. Enzyme-Assisted Extraction of Fruit Juices. In Fruit Juices: Extraction, Composition, Quality and Analysis; Academic Press: Cambridge, MA, USA, 2018; pp. 183–200. [Google Scholar] [CrossRef]
- Mu, R.; Wang, Z.; Wamsley, M.C.; Duke, C.N.; Lii, P.H.; Epley, S.E.; Todd, L.C.; Roberts, P.J. Application of Enzymes in Regioselective and Stereoselective Organic Reactions. Catalysts 2020, 10, 832. [Google Scholar] [CrossRef]
- Liu, J.J.; Gasmalla, M.A.A.; Li, P.; Yang, R. Enzyme-Assisted Extraction Processing from Oilseeds: Principle, Processing and Application. Innov. Food Sci. Emerg. Technol. 2016, 35, 184–193. [Google Scholar] [CrossRef]
- Neto, B.B.; Scarminio, I.S.; Bruns, R.E. Como Fazer Experimentos: Aplicações Na Ciência e Na Indústria, 4th ed.; Bookman: Porto Alegre, Brazil, 2010. [Google Scholar]
- de Castro, R.J.S.; Cason, V.G.; Sato, H.H. Binary Mixture of Proteases Increases the Antioxidant Properties of White Bean (Phaseolus vulgaris L.) Protein-Derived Peptides Obtained by Enzymatic Hydrolysis. Biocatal. Agric. Biotechnol. 2017, 10, 291–297. [Google Scholar] [CrossRef]
- Magro, A.E.A.; de Castro, R.J.S. Effects of Solid-State Fermentation and Extraction Solvents on the Antioxidant Properties of Lentils. Biocatal. Agric. Biotechnol. 2020, 28, 101753. [Google Scholar] [CrossRef]
- Neta, I.M.R.; de Castro, R.J.S. Enzyme-Assisted Extraction of Biocomponents of Lentils (Lens Culinaris L.): Effect of Process Parameters on the Recovery of Compounds with Antioxidant Properties. Biocatal. Biotransform. 2020, 38, 15–23. [Google Scholar] [CrossRef]
- Rasera, G.B.; Hilkner, M.H.; de Alencar, S.M.; de Castro, R.J.S. Biologically Active Compounds from White and Black Mustard Grains: An Optimization Study for Recovery and Identification of Phenolic Antioxidants. Ind. Crops Prod. 2019, 135, 294–300. [Google Scholar] [CrossRef]
- Firuzi, O.; Lacanna, A.; Petrucci, R.; Marrosu, G.; Saso, L. Evaluation of the Antioxidant Activity of Flavonoids by “Ferric Reducing Antioxidant Power” Assay and Cyclic Voltammetry. Biochim. Biophys. Acta 2005, 1721, 174–184. [Google Scholar] [CrossRef]
- Aguilar, J.G.; de Castro, R.J.S.; Sato, H.H. Optimization of the Enzymatic Hydrolysis of Rice Protein by Different Enzymes Using the Response Surface Methodology. 3 Biotech 2018, 8, 372. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.P.; Rosalen, P.L.; de Camargo, A.C.; Lazarini, J.G.; Rocha, G.; Shahidi, F.; Franchin, M.; de Alencar, S.M. Inajá Oil Processing By-Product: A Novel Source of Bioactive Catechins and Procyanidins from a Brazilian Native Fruit. Food Res. Int. 2021, 144, 110353. [Google Scholar] [CrossRef]
- Marathe, S.J.; Jadhav, S.B.; Bankar, S.B.; Kumari Dubey, K.; Singhal, R.S. Improvements in the Extraction of Bioactive Compounds by Enzymes. Curr. Opin. Food Sci. 2019, 25, 62–72. [Google Scholar] [CrossRef]
- Patil, P.D.; Patil, S.P.; Kelkar, R.K.; Patil, N.P.; Pise, P.V.; Nadar, S.S. Enzyme-Assisted Supercritical Fluid Extraction: An Integral Approach to Extract Bioactive Compounds. Trends Food Sci. Technol. 2021, 116, 357–369. [Google Scholar] [CrossRef]
- Costa, J.R.; Tonon, R.V.; Cabral, L.; Gottschalk, L.; Pastrana, L.; Pintado, M.E. Valorization of Agricultural Lignocellulosic Plant Byproducts through Enzymatic and Enzyme-Assisted Extraction of High-Value-Added Compounds: A Review. ACS Sustain. Chem. Eng. 2020, 8, 13112–13125. [Google Scholar] [CrossRef]
- Liu, X.; Kokare, C. Microbial Enzymes of Use in Industry. In Biotechnology of Microbial Enzymes: Production, Biocatalysis and Industrial Applications; Academic Press: Cambridge, MA, USA, 2017; pp. 267–298. [Google Scholar] [CrossRef]
- Mehta, P.K.; Sehgal, S. Microbial Enzymes in Food Processing. In Biocatalysis: Enzymatic Basics and Applications; Springer: Singapore, 2019; pp. 255–275. [Google Scholar] [CrossRef]
- Ohara, A.; Cason, V.G.; Nishide, T.G.; Miranda de Matos, F.; de Castro, R.J.S. Improving the Antioxidant and Antidiabetic Properties of Common Bean Proteins by Enzymatic Hydrolysis Using a Blend of Proteases. Biocatal. Biotransform. 2021, 39, 100–108. [Google Scholar] [CrossRef]
- Phirom-on, K.; Apiraksakorn, J. Development of Cellulose-Based Prebiotic Fiber from Banana Peel by Enzymatic Hydrolysis. Food Biosci. 2021, 41, 101083. [Google Scholar] [CrossRef]
- de Freitas, C.; Terrone, C.C.; Masarin, F.; Carmona, E.C.; Brienzo, M. In Vitro Study of the Effect of Xylooligosaccharides Obtained from Banana Pseudostem Xylan by Enzymatic Hydrolysis on Probiotic Bacteria. Biocatal. Agric. Biotechnol. 2021, 33, 101973. [Google Scholar] [CrossRef]
- Baruah, J.; Bardhan, P.; Mukherjee, A.K.; Deka, R.C.; Mandal, M.; Kalita, E. Integrated Pretreatment of Banana Agrowastes: Structural Characterization and Enhancement of Enzymatic Hydrolysis of Cellulose Obtained from Banana Peduncle. Int. J. Biol. Macromol. 2022, 201, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Leichtweis, M.G.; Oliveira, M.B.P.P.; Ferreira, I.C.F.R.; Pereira, C.; Barros, L. Sustainable Recovery of Preservative and Bioactive Compounds from Food Industry Bioresidues. Antioxidants 2021, 10, 1827. [Google Scholar] [CrossRef] [PubMed]
- Torrisi, C.; Morgante, A.; Malfa, G.; Acquaviva, R.; Castelli, F.; Pignatello, R.; Sarpietro, M.G. Sinapic Acid Release at the Cell Level by Incorporation into Nanoparticles: Experimental Evidence Using Biomembrane Models. Micro 2021, 1, 120–128. [Google Scholar] [CrossRef]
- Ullah, R.; Ikram, M.; Park, T.J.; Ahmad, R.; Saeed, K.; Alam, S.I.; Rehman, I.U.; Khan, A.; Khan, I.; Jo, M.G.; et al. Vanillic Acid, a Bioactive Phenolic Compound, Counteracts LPS-Induced Neurotoxicity by Regulating c-Jun N-Terminal Kinase in Mouse Brain. Int. J. Mol. Sci. 2021, 22, 361. [Google Scholar] [CrossRef]
- Punvittayagul, C.; Chariyakornkul, A.; Jarukamjorn, K.; Wongpoomchai, R. Protective Role of Vanillic Acid against Diethylnitrosamine- and 1,2-Dimethylhydrazine-Induced Hepatocarcinogenesis in Rats. Molecules 2021, 26, 2718. [Google Scholar] [CrossRef]
- Gullón, B.; Lú-Chau, T.A.; Moreira, M.T.; Lema, J.M.; Eibes, G. Rutin: A Review on Extraction, Identification and Purification Methods, Biological Activities and Approaches to Enhance Its Bioavailability. Trends Food Sci. Technol. 2017, 67, 220–235. [Google Scholar] [CrossRef]
- Chua, L.S. A Review on Plant-Based Rutin Extraction Methods and Its Pharmacological Activities. J. Ethnopharmacol. 2013, 150, 805–817. [Google Scholar] [CrossRef] [PubMed]
- Ramu, R.; Shirahatti, P.S.; Zameer, F.; Ranganatha, L.V.; Nagendra Prasad, M.N. Inhibitory Effect of Banana (Musa sp. Var. Nanjangud Rasa Bale) Flower Extract and Its Constituents Umbelliferone and Lupeol on α-Glucosidase, Aldose Reductase and Glycation at Multiple Stages. S. Afr. J. Bot. 2014, 95, 54–63. [Google Scholar] [CrossRef]
- De Camargo, A.C.; Biasoto, A.C.T.; Schwember, A.R.; Granato, D.; Rasera, G.B.; Franchin, M.; Rosalen, P.L.; Alencar, S.M.; Shahidi, F. Should We Ban Total Phenolics and Antioxidant Screening Methods? The Link Between Antioxidant Potential and Activation of NF-Κb Using Phenolic Compounds from Grape By-Products. Food Chem. 2019, 290, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Granato, D.; Shahidi, F.; Wrolstad, R.; Kilmartin, P.; Melton, L.D.; Hidalgo, F.J.; Miyashita, K.; van Camp, J.; Alasalvar, C.; Ismail, A.B. Antioxidant activity, total phenolics and flavonoids contents: Should we ban in vitro screening methods? Food Chem. 2018, 264, 471–475. [Google Scholar] [CrossRef] [PubMed]


| Run | Independent Variables | |||||
|---|---|---|---|---|---|---|
| Coded Variables | Real Variables (mL) | |||||
| x1 | x2 | x3 | Pectinex™ | Flavourzyme™ | Celluclast™ | |
| Control | 0 | 0 | 0 | 0 | 0 | 0 |
| 1 | 1 | 0 | 0 | 0.250 | 0 | 0 |
| 2 | 0 | 1 | 0 | 0 | 0.250 | 0 |
| 3 | 0 | 0 | 1 | 0 | 0 | 0.250 |
| 4 | 1/2 | 1/2 | 0 | 0.125 | 0.125 | 0 |
| 5 | 1/2 | 0 | 1/2 | 0.125 | 0 | 0.125 |
| 6 | 0 | 1/2 | 1/2 | 0 | 0.125 | 0.125 |
| 7 | 1/3 | 1/3 | 1/3 | 0.083 | 0.083 | 0.083 |
| 8 | 2/3 | 1/6 | 1/6 | 0.166 | 0.042 | 0.042 |
| 9 | 1/6 | 2/3 | 1/6 | 0.042 | 0.166 | 0.042 |
| 10 | 1/6 | 1/6 | 2/3 | 0.042 | 0.042 | 0.166 |
| Run | TPC (mg GAE g−1) | ABTS (µmol TE g−1) | DPPH (µmol TE g−1) | FRAP (µmol TE g−1) |
|---|---|---|---|---|
| Maçã Variety | ||||
| Control | 2.69 ± 0.76 f | Not detected | 17.43 ± 0.09 ef | 3.38 ± 0.25 f |
| 1 | 5.49 ± 0.71 de | 9.45 ± 3.97 d | 23.65 ± 2.19 bc | 3.70 ± 0.31 def |
| 2 | 8.89 ± 0.33 c | 3.50 ± 0.38 d | 18.71 ± 1.39 def | 3.67 ± 0.28 def |
| 3 | 10.24 ± 0.24 c | 37.30 ± 0.31 abc | 15.55 ± 1.66 f | 3.64 ± 0.30 ef |
| 4 | 6.83 ± 0.13 d | 8.84 ± 0.74 d | 20.03 ± 1.57 cde | 3.91 ± 0.46 cdef |
| 5 | 4.68 ± 0.05 e | 47.25 ± 3.33 a | 20.01 ± 1.03 cde | 3.75 ± 0.27 def |
| 6 | 6.55 ± 0.29 d | 31.05 ± 1.29 bc | 24.88 ± 0.68 b | 5.10 ± 0.43 b |
| 7 | 15.00 ± 0.36 a | 37.58 ± 2.60 abc | 30.52 ± 1.86 a | 4.40 ± 0.03 bcde |
| 8 | 12.98 ± 0.57 b | 39.80 ± 1.59 ab | 22.26 ± 0.75 bcd | 4.48 ± 0.19 bcd |
| 9 | 6.54 ± 0.17 d | 36.11 ± 3.13 abc | 20.46 ± 1.58 cde | 4.66 ± 0.08 bc |
| 10 | 15.87 ± 0.59 a | 24.48 ± 4.74 c | 19.66 ± 0.47 de | 7.42 ± 0.09 a |
| Nanica variety | ||||
| Control | 0.77 ± 0.22 d | 14.54 ± 0.65 f | 14.61 ± 0.14 b | 18.21 ± 1.23 abc |
| 1 | 2.03 ± 0.06 d | 34.73 ± 1.44 de | 15.87 ± 1.47 ab | 16.02 ± 0.18 c |
| 2 | 3.76 ± 0.17 c | 49.16 ± 0.23 bc | 16.72 ± 0.75 bc | 16.70 ± 0.72 bc |
| 3 | 4.77 ± 0.25 abc | 30.15 ± 1.45 ef | 10.63 ± 0.75 ab | 18.30 ± 2.28 abc |
| 4 | 4.66 ± 0.04 bc | 49.56 ± 1.37 bc | 12.62 ± 2.42 b | 17.24 ± 0.84 abc |
| 5 | 4.28 ± 0.52 c | 44.94 ± 1.06 cd | 14.96 ± 0.54 ab | 17.97 ± 0.33 abc |
| 6 | 4.59 ± 0.17 bc | 53.02 ± 1.89 abc | 13.08 ± 0.10 ab | 16.64 ± 0.68 bc |
| 7 | 5.00 ± 0.27 abc | 59.73 ± 2.60 ab | 14.52 ± 0.04 ab | 19.53 ± 0.35 abc |
| 8 | 3.81 ± 0.36 c | 49.47 ± 0.90 bc | 22.02 ± 3.05 ab | 17.47 ± 0.50 abc |
| 9 | 5.99 ± 0.80 a | 62.44 ± 4.43 a | 24.10 ± 0.43 a | 20.62 ± 1.18 a |
| 10 | 5.67 ± 0.39 ab | 51.46 ± 0.78 bc | 13.37 ± 0.51 ab | 19.85 ± 1.02 ab |
| Prata variety | ||||
| Control | Not detected | 20.77 ± 2.41 d | 11.81 ± 1.01 d | 28.06 ± 3.84 bcd |
| 1 | 8.51 ± 0.38 e | 43.41 ± 3.58 cd | 18.54 ± 1.63 abc | 43.15 ± 4.72 ab |
| 2 | 14.70 ± 0.23 a | 77.92 ± 0.89 ab | 17.30 ± 0.53 abc | 34.43 ± 2.83 abc |
| 3 | 1.68 ± 0.25 g | 52.95 ± 6.84 bc | 16.60 ± 1.34 bcd | 13.37 ± 3.07 d |
| 4 | 1.81 ± 0.15 g | 80.18 ± 2.51 ab | 4.29 ± 3.43 e | 47.09 ± 0.79 a |
| 5 | 0.75 ± 0.12 h | 63.78 ± 2.81 abc | 5.36 ± 0.75 e | 38.27 ± 5.07 ab |
| 6 | 12.56 ± 0.44 b | 79.17 ± 7.07 ab | 17.85 ± 1.98 abc | 19.85 ± 1.78 cd |
| 7 | 9.51 ± 0.22 d | 66.22 ± 8.39 abc | 22.26 ± 0.92 a | 46.29 ± 3.60 a |
| 8 | 9.40 ± 0.41 de | 73.93 ± 1.34 ab | 20.51 ± 1.58 ab | 38.08 ± 4.02 ab |
| 9 | 5.20 ± 0.06 f | 82.57 ± 1.21 a | 15.32 ± 1.27 cd | 18.39 ± 5.64 cd |
| 10 | 10.85 ± 0.13 c | 76.76 ± 11.87 ab | 19.44 ± 1.14 abc | 33.28 ± 3.01 abc |
| Responses | Model | Equations | Fcalculated | Ftabulated | R2 | p-Value |
|---|---|---|---|---|---|---|
| Nanica Variety | ||||||
| TPC | Quadratic | Y = 2.20x1 + 4.18x2 + 5.51x3 + 7.95x1x2 | 5.67 | 3.29 | 0.74 | 0.035 |
| ABTS | Cubic | Y = 34.18x1 + 50.10x2 + 30.25x3 + 31.25x1x2 + 49.10x1x3 + 55.54x2x3 + 214.21x1x2x3 | 24.49 | 5.28 | 0.98 | 0.012 |
| Prata variety | ||||||
| ABTS | Quadratic | Y = 50.46x1 + 81.48x2 + 64.86x3 + 66.92x1x2 | 4.09 | 3.26 | 0.77 | 0.070 |
| DPPH | Cubic | Y= 19.91x1 + 16.38x2 + 16.77x3 -54.14x1x2 − 46.28x1x3 + 423.52x1x2x3 | 6.67 | 4.05 | 0.89 | 0.040 |
| Responses | Fcalculated | Ftabulated | R2 | p-Value |
|---|---|---|---|---|
| Maçã variety | ||||
| TPC | 3.40 | 3.29 | 0.63 | 0.09 |
| ABTS | 1.13 | 5.28 | 0.68 | 0.49 |
| DPPH | 2.74 | 3.29 | 0.58 | 0.13 |
| FRAP | 0.46 | 3.26 | 0.11 | 0.65 |
| Nanica variety | ||||
| DPPH | 1.28 | 3.26 | 0.26 | 0.330 |
| FRAP | 0.66 | 3.26 | 0.15 | 0.540 |
| Prata variety | ||||
| TPC | 1.64 | 3.29 | 0.45 | 0.270 |
| FRAP | 4.17 | 3.26 | 0.54 | 0.070 |
| Compound | Variety | Concentration (µg g−1) | Validation Parameters | |||
|---|---|---|---|---|---|---|
| Non-Hydrolyzed Samples (Control) | Hydrolyzed Samples | LOD (µg) 1 | LOQ (µg) 2 | Linearity (R2) | ||
| Coumaric acid | Maçã | n.d. | n.d. | 0.0023 | 0.0070 | 0.9994 |
| Nanica | n.d. | 76.46 ± 5.38 | ||||
| Prata | n.d. | 46.97 ± 0.09 | ||||
| Ferulic acid | Maçã | n.d. | 36.17 ± 0.60 | 0.0015 | 0.0046 | 0.9985 |
| Nanica | 7.20 ± 0.18 b | 44.22 ± 2.48 a | ||||
| Prata | n.d. | 78.98 ± 0.24 | ||||
| Sinapic acid | Maçã | n.d. | n.d. | 0.0262 | 0.0792 | 0.9907 |
| Nanica | n.d. | 22.77 ± 0.13 | ||||
| Prata | n.d. | n.d. | ||||
| Vanillic acid | Maçã | 4.64 ± 0.31 b | 6.81 ± 0.62 a | 0.0022 | 0.0066 | 0.9979 |
| Nanica | n.d. | n.d. | ||||
| Prata | 5.85 ± 0.11 b | 13.07 ± 0.18 a | ||||
| Rutin | Maçã | n.d. | n.d. | 0.0014 | 0.0042 | 0.9745 |
| Nanica | n.d. | n.d. | ||||
| Prata | 27.01 ± 0.01 b | 33.56 ± 0.01 a | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, K.L.d.O.; Daccache, I.S.C.; de Castro, R.J.S. Synergistic Action of Multiple Enzymes Resulting in Efficient Hydrolysis of Banana Bracts and Products with Improved Antioxidant Properties. Processes 2022, 10, 1807. https://doi.org/10.3390/pr10091807
Santos KLdO, Daccache ISC, de Castro RJS. Synergistic Action of Multiple Enzymes Resulting in Efficient Hydrolysis of Banana Bracts and Products with Improved Antioxidant Properties. Processes. 2022; 10(9):1807. https://doi.org/10.3390/pr10091807
Chicago/Turabian StyleSantos, Karen Linelle de Oliveira, Isabella Shara Cortez Daccache, and Ruann Janser Soares de Castro. 2022. "Synergistic Action of Multiple Enzymes Resulting in Efficient Hydrolysis of Banana Bracts and Products with Improved Antioxidant Properties" Processes 10, no. 9: 1807. https://doi.org/10.3390/pr10091807
APA StyleSantos, K. L. d. O., Daccache, I. S. C., & de Castro, R. J. S. (2022). Synergistic Action of Multiple Enzymes Resulting in Efficient Hydrolysis of Banana Bracts and Products with Improved Antioxidant Properties. Processes, 10(9), 1807. https://doi.org/10.3390/pr10091807