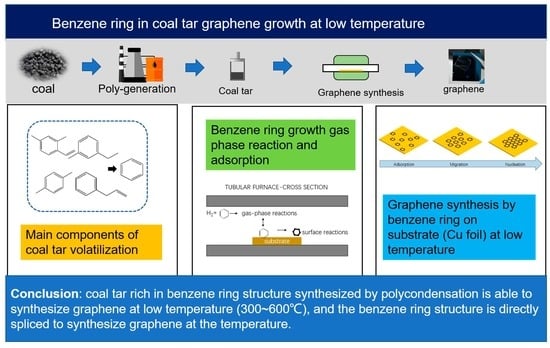
Characteristics of Graphene Growth at Different Temperatures from the Benzene Ring Structure in Coal Tar
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Graphene Synthesis
2.3. Reaction Gas and Liquids Collection
2.4. Characterization
3. Results and Discussion
3.1. Mechanism of Graphene Growth by Benzene at Different Temperatures
3.2. Synthesis of Graphene from Coal Tar at 500 °C
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Bonaccorso, F.; Sun, Z.; Hasan, T.A. Graphene photonics and optoelectronics. Nat. Photonics 2010, 4, 611–622. [Google Scholar] [CrossRef] [Green Version]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V. Two-dimensional gas of massless Dirac fermions in graphene. Nature 2005, 438, 197–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bunch, J.S.; Verbridge, S.S.; Alden, J.S. Impermeable atomic membranes from graphene sheets. Nano Lett. 2008, 8, 2458–2462. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Colombo, L.; Ruoff, R.S. Synthesis of graphene films on copper foils by chemical vapor deposition. Adv. Mater. 2016, 28, 6247–6252. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [Green Version]
- Parvez, K.; Yang, S.; Feng, X. Exfoliation of graphene via wet chemical routes. Synth. Met. 2015, 210, 123–132. [Google Scholar] [CrossRef] [Green Version]
- Yazdi, G.R.; Iakimov, T.; Yakimova, R. Epitaxial graphene on SiC: A review of growth and characterization. Crystals 2016, 6, 53. [Google Scholar] [CrossRef] [Green Version]
- Emiru, T.F.; Ayele, D.W. Controlled synthesis, characterization and reduction of graphene oxide: A convenient method for large scale production. Egypt. J. Basic Appl. Sci. 2017, 4, 74–79. [Google Scholar] [CrossRef] [Green Version]
- Reina, A.; Thiele, S.; Jia, X. Growth of large-area single-and bi-layer graphene by controlled carbon precipitation on polycrystalline Ni surfaces. Nano Res. 2009, 2, 509–516. [Google Scholar] [CrossRef] [Green Version]
- Luong, D.X.; Bets, K.V.; Algozeeb, W.A. Gram-scale bottom-up flash graphene synthesis. Nature 2020, 577, 647–651. [Google Scholar] [CrossRef]
- Li, X.; Cai, W.; An, J. Large-area synthesis of high-quality and uniform graphene films on copper foils. Science 2009, 324, 1312–1314. [Google Scholar] [CrossRef] [Green Version]
- Mafra, D.L.; Olmos-Asar, J.A.; Negreiros, F.R. Ambient-pressure CVD of graphene on low-index Ni surfaces using methane: A combined experimental and first-principles study. Phys. Rev. Mater. 2018, 2, 073404. [Google Scholar] [CrossRef]
- Chen, C.S.; Hsieh, C.K. Effects of acetylene flow rate and processing temperature on graphene films grown by thermal chemical vapor deposition. Thin Solid Films 2015, 584, 265–269. [Google Scholar] [CrossRef]
- Li, Z.; Wu, P.; Wang, C. Low-temperature growth of graphene by chemical vapor deposition using solid and liquid carbon sources. ACS Nano 2011, 5, 3385–3390. [Google Scholar] [CrossRef]
- Guermoune, A.; Chari, T.; Popescu, F. Chemical vapor deposition synthesis of graphene on copper with methanol, ethanol, and propanol precursors. Carbon 2011, 49, 4204–4210. [Google Scholar] [CrossRef]
- Kumar, E.S.; Sivasankar, V.; Sureshbabu, R. Facile synthesis of few layer graphene from bituminous coal and its application towards electrochemical sensing of caffeine. Adv. Mater. Lett. 2017, 8, 239–245. [Google Scholar] [CrossRef]
- Wang, D.; Vijapur, S.H.; Botte, G.G. Coal char derived few-layer graphene anodes for lithiumion batteries. Photonics 2014, 1, 251–259. [Google Scholar] [CrossRef] [Green Version]
- Primo, A.; Atienzar, P.; Sanchez, E. From biomass wastes to large-area, high-quality, N-doped graphene: Catalyst-free carbonization of chitosan coatings on arbitrary substrates. Chem. Commun. 2012, 48, 9254–9256. [Google Scholar] [CrossRef]
- Ruan, G.; Sun, Z.; Peng, Z. Growth of graphene from food, insects, and waste. ACS Nano 2011, 5, 7601–7607. [Google Scholar] [CrossRef]
- Vijapur, S.H.; Wang, D.; Ingram, D.C. An investigation of growth mechanism of coal derived graphene films. Mater. Today Commun. 2017, 11, 147–155. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Lin, Q.; Zhou, T. Facile preparation of graphene nanosheets by pyrolysis of coal-tar pitch with the presence of aluminum. J. Anal. Appl. Pyrolysis 2014, 110, 481–485. [Google Scholar] [CrossRef]
- Wan, X.; Chen, K.; Liu, D. High-quality large-area graphene from dehydrogenated polycyclic aromatic hydrocarbons. Chem. Mater. 2012, 24, 3906–3915. [Google Scholar] [CrossRef]
- Yu, P.; Luo, Z.; Wang, Q. Life cycle assessment of transformation from a sub-critical power plant into a polygeneration plant. Energy Convers. Manag. 2019, 198, 111801. [Google Scholar] [CrossRef]
- Rane, K.; Adams, J.J.; Thode, J.M. Multistep Fractionation of Coal and Application for Graphene Synthesis. ACS Omega 2021, 6, 16573–16583. [Google Scholar] [CrossRef]
- Kang, C.; Jung, D.H.; Lee, J.S. Atmospheric pressure chemical vapor deposition of graphene using a liquid benzene precursor. J. Nanosci. Nanotechnol. 2015, 15, 9098–9103. [Google Scholar] [CrossRef]
- Zhang, B.; Lee, W.H.; Piner, R. Low-temperature chemical vapor deposition growth of graphene from toluene on electropolished copper foils. ACS Nano 2012, 6, 2471–2476. [Google Scholar] [CrossRef]
- Sun, X.; Lin, L.; Sun, L. Low-temperature and rapid growth of large single-crystalline graphene with ethane. Small 2018, 14, 1702916. [Google Scholar] [CrossRef]
- Liu, Z.; Tu, Z.; Li, Y. Synthesis of three-dimensional graphene from petroleum asphalt by chemical vapor deposition. Mater. Lett. 2014, 122, 285–288. [Google Scholar] [CrossRef]
- Safron, N.S.; Arnold, M.S. Experimentally determined model of atmospheric pressure CVD of graphene on Cu. J. Mater. Chem. C 2014, 2, 744–755. [Google Scholar] [CrossRef]
- Brooks, C.T.; Peacock, S.J.; Reuben, B.G. Pyrolysis of benzene. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1979, 75, 652–662. [Google Scholar] [CrossRef]
- Lewis, A.M.; Derby, B.; Kinloch, I.A. Influence of gas phase equilibria on the chemical vapor deposition of graphene. ACS Nano 2013, 7, 3104–3117. [Google Scholar] [CrossRef]
- Frusteri, L.; Cannilla, C.; Barbera, K. Carbon growth evidences as a result of benzene pyrolysis. Carbon 2013, 59, 296–307. [Google Scholar] [CrossRef]
- Becker, A.; Hüttinger, K.J. Chemistry and kinetics of chemical vapor deposition of pyrocarbon—III pyrocarbon deposition from propylene and benzene in the low temperature regime. Carbon 1998, 36, 201–211. [Google Scholar] [CrossRef]
- Shazni Mohammad Haniff, M.A.; Zainal Ariffin, N.H.; Ooi, P.C. Practical route for the low-temperature growth of large-area bilayer graphene on polycrystalline nickel by cold-wall chemical vapor deposition. ACS Omega 2021, 6, 12143–12154. [Google Scholar] [CrossRef]
- Wu, P.; Zhang, Y.; Cui, P. Carbon dimers as the dominant feeding species in epitaxial growth and morphological phase transition of graphene on different Cu substrates. Phys. Rev. Lett. 2015, 114, 216102. [Google Scholar] [CrossRef]
- Zhou, Y.; Tong, L.; Wu, L. Review on the research of graphene from coal tar pitch. In Proceedings of the 2022 International Conference on Optoelectronic Information and Functional Materials (OIFM 2022), Chongqing, China, 18–20 March 2022; Volume 12255, pp. 458–462. [Google Scholar] [CrossRef]
- Kong, X.; Chen, Q. The positive influence of boron-doped graphene with pyridine as a probe molecule on SERS: A density functional theory study. J. Mater. Chem. 2012, 22, 15336–15341. [Google Scholar] [CrossRef]
- Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S. Studying disorder in graphite-based systems by Raman spectroscopy. Phys. Chem. Chem. Phys. 2007, 9, 1276–1290. [Google Scholar] [CrossRef]
- Reina, A.; Jia, X.; Ho, J. Large area, few-layer graphene films on arbitrary substrates by chemical vapor deposition. Nano Lett. 2009, 9, 30–35. [Google Scholar] [CrossRef]
- Das, A.; Pisana, S.; Chakraborty, B. Monitoring dopants by Raman scattering in an electrochemically top-gated graphene transistor. Nat. Nanotechnol. 2008, 3, 210–215. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrari, A.C. Raman spectroscopy of graphene and graphite: Disorder, electron–phonon coupling, doping and nonadiabatic effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]








| Coal Tar Components | Content (%) |
|---|---|
| Phenols | 2.52 |
| Chain hydrocarbons | 0.32 |
| Cyclic hydrocarbons | 0.57 |
| Monocyclic aromatic hydrocarbons | 13.51 |
| Polycyclic aromatic hydrocarbons | 71.72 |
| Other oxygenated compounds | 8.12 |
| Nitrogen-containing compounds | 1.68 |
| Oxygen and nitrogen compounds | 0.69 |
| Nitrogenous sulfur compounds | 0.19 |
| Metal-containing compounds | 0.69 |
| T (°C) | CH4 | C2H4 | C2H6 | C3H8 | C3H6 |
|---|---|---|---|---|---|
| 300 | 3.2 × 10−3 * | 1.2 × 10−4 | 3.4 × 10−3 | 2.8 × 10−4 | 5.5 × 10−4 |
| 400 | 5.7 × 10−4 | 2.9 × 10−5 | 9.9 × 10−4 | 1.4 × 10−4 | 3.4 × 10−4 |
| 500 | 8.4 × 10−4 | 3.6 × 10−5 | 1.6 × 10−3 | 1.1 × 10−4 | 2.7 × 10−4 |
| 600 | 2.3 × 10−3 | 5.5 × 10−5 | 4.2 × 10−3 | 7.1 × 10−5 | 2.0 × 10−4 |
| 700 | 1.5 × 10−1 | 7.8 × 10−3 | 2.5 × 10−1 | 4.2 × 10−4 | 9.6 × 10−4 |
| 800 | 1.6 × 10−1 | 4.7 × 10−3 | 5.0 × 10−1 | 4.8 × 10−5 | 2.0 × 10−4 |
| 900 | 4.8 × 10−1 | 3.2 × 10−3 | 8.7 × 10−1 | 0 | 1.1 × 10−4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, S.; Luo, Z.; Fang, M.; Wang, Q.; Cen, J. Characteristics of Graphene Growth at Different Temperatures from the Benzene Ring Structure in Coal Tar. Processes 2023, 11, 593. https://doi.org/10.3390/pr11020593
Zhao S, Luo Z, Fang M, Wang Q, Cen J. Characteristics of Graphene Growth at Different Temperatures from the Benzene Ring Structure in Coal Tar. Processes. 2023; 11(2):593. https://doi.org/10.3390/pr11020593
Chicago/Turabian StyleZhao, Shuhan, Zhongyang Luo, Mengxiang Fang, Qinhui Wang, and Jianmeng Cen. 2023. "Characteristics of Graphene Growth at Different Temperatures from the Benzene Ring Structure in Coal Tar" Processes 11, no. 2: 593. https://doi.org/10.3390/pr11020593
APA StyleZhao, S., Luo, Z., Fang, M., Wang, Q., & Cen, J. (2023). Characteristics of Graphene Growth at Different Temperatures from the Benzene Ring Structure in Coal Tar. Processes, 11(2), 593. https://doi.org/10.3390/pr11020593