Interfacial Adsorption Mechanism of Diethyldithiocarbamate in High-Sulfur Residue Flotation
Abstract
:1. Introduction
2. Methods
2.1. DFT Calculation
2.2. Material and Reagents
2.2.1. High-Sulfur Residue
2.2.2. Pyrite
2.2.3. Reagents
2.3. Mineral Flotation Evaluation
2.3.1. Pure Mineral Flotation
2.3.2. Adsorption Behavior of DDTC on Pure Minerals
2.3.3. High-Sulfur Residue Flotation
2.3.4. Analysis and Characterization
3. Results and Discussion
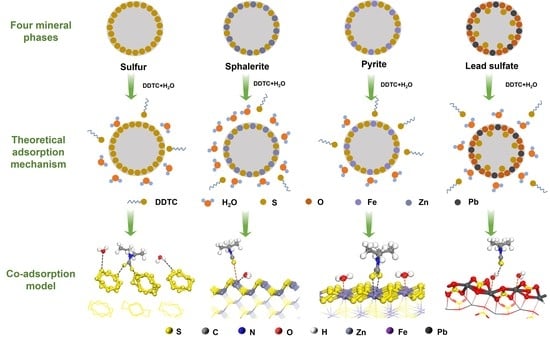
3.1. Coadsorption Model of DDTC and H2O
3.1.1. Adsorption Behavior of DDTC
3.1.2. Adsorption Behavior of H2O
3.1.3. Coadsorption Model of DDTC and H2O
3.2. Model Validation
3.3. Practical Bench-Scale Operation of High-Sulfur Residue Flotation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, G.Q.; Zhang, B.S.; Dong, Z.L.; Zhang, F.; Wan, F.; Jiang, T.; Xu, B. Flotation Performance, Structure-Activity Relationship and Adsorption Mechanism of O-Isopropyl-N-Ethyl Thionocarbamate Collector for Elemental Sulfur in a High-Sulfur Residue. Metals 2021, 11, 727. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, Y.; Niu, L.; Jing, T.; Zhang, T.-a. Separation and purification of elemental sulfur from sphalerite concentrate direct leaching residue by liquid paraffin. Hydrometallurgy 2019, 186, 162–169. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, G.; Zhang, L.; Zhou, C. Mineralogical and morphological factors affecting the separation of copper and arsenic in flash copper smelting slag flotation beneficiation process. J. Hazard. Mater. 2021, 401, 123293. [Google Scholar] [CrossRef]
- Jorjani, E.; Ghahreman, A. Challenges with elemental sulfur removal during the leaching of copper and zinc sulfides, and from the residues; a review. Hydrometallurgy 2017, 171, 333–343. [Google Scholar] [CrossRef]
- Halfyard, J.E.; Hawboldt, K. Separation of elemental sulfur from hydrometallurgical residue: A review. Hydrometallurgy 2011, 109, 80–89. [Google Scholar] [CrossRef]
- Wang, Z.-y.; Cai, X.-l.; Zhang, Z.-b.; Zhang, L.-b.; Wang, S.-x.; Peng, J.-h. Separation and enrichment of elemental sulfur and mercury from hydrometallurgical zinc residue using sodium sulfide. Trans. Nonferrous Met. Soc. China 2015, 25, 640–646. [Google Scholar] [CrossRef]
- Li, H.; Wu, X.; Wang, M.; Wang, J.; Wu, S.; Yao, X.; Li, L. Separation of elemental sulfur from zinc concentrate direct leaching residue by vacuum distillation. Sep. Purif. Technol. 2014, 138, 41–46. [Google Scholar] [CrossRef]
- Liu, G.; Yang, X.; Zhong, H. Molecular design of flotation collectors: A recent progress. Adv. Colloid Interface Sci. 2017, 246, 181–195. [Google Scholar] [CrossRef]
- Silva, L.A.; Garrot, T.G.; Pereira, A.M.; Correia, J.C.G. Historical perspective and bibliometric analysis of molecular modeling applied in mineral flotation systems. Miner. Eng. 2021, 170, 107062. [Google Scholar] [CrossRef]
- Ngobeni, W.A.; Hangone, G. The effect of using sodium di-methyl-dithiocarbamate as a co-collector with xanthates in the froth flotation of pentlandite containing ore from Nkomati mine in South Africa. Miner. Eng. 2013, 54, 94–99. [Google Scholar] [CrossRef]
- Hu, Y.; Wu, M.; Liu, R.; Sun, W. A review on the electrochemistry of galena flotation. Miner. Eng. 2020, 150, 106272. [Google Scholar] [CrossRef]
- Niu, X.; Ruan, R.; Xia, L.; Li, L.; Sun, H.; Jia, Y.; Tan, Q. Correlation of Surface Adsorption and Oxidation with a Floatability Difference of Galena and Pyrite in High-Alkaline Lime Systems. Langmuir 2018, 34, 2716–2724. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Hu, Y.H.; Xu, J.; Chen, T.J. FTIR spectroscopic study of electrochemical flotation of jamesonite-diethyldithiocarbamate system. Trans. Nonferrous Met. Soc. China 2006, 16, 493–496. [Google Scholar] [CrossRef]
- Cui, W.Y.; Zhang, J.J.; Liu, Z.R.; Chen, J.H. Selective enhancement of jamesonite flotation using Aerophine 3418A/DDTC mixture. Miner. Eng. 2023, 191, 107934. [Google Scholar] [CrossRef]
- Qiu, H.X.; Wu, B.Z.; Deng, J.S.; Sun, X.H.; Hu, M.Z.; Cai, J.Z.; Zheng, C. The effect of collectors on froth stability of frother: Atomic-scale study by experiments and molecular dynamics simulations. J. Mol. Liq. 2022, 364, 120035. [Google Scholar] [CrossRef]
- Zhang, L.M.; Gao, J.D.; Khoso, S.A.; Wang, L.; Liu, Y.L.; Ge, P.; Tian, M.J.; Sun, W. A reagent scheme for galena/sphalerite flotation separation: Insights from first-principles calculations. Miner. Eng. 2021, 167, 106885. [Google Scholar] [CrossRef]
- Chen, J.H.; Lan, L.H.; Chen, Y. Computational simulation of adsorption and thermodynamic study of xanthate, dithiophosphate and dithiocarbamate on galena and pyrite surfaces. Miner. Eng. 2013, 46–47, 136–143. [Google Scholar] [CrossRef]
- Dai, P.L.; Wei, Z.C.; Chen, L.Z.; Liu, Y. Adsorption of butyl xanthate on arsenopyrite (001) and Cu2+-activated arsenopyrite (001) surfaces: A DFT study. Chem. Phys. 2022, 562, 111668. [Google Scholar] [CrossRef]
- Huang, X.P.; Jia, Y.; Wang, S.; Ma, X.; Cao, Z.F.; Zhong, H. Novel Sodium O-Benzythioethyl Xanthate Surfactant: Synthesis, DFT Calculation and Adsorption Mechanism on Chalcopyrite Surface. Langmuir 2019, 35, 15106–15113. [Google Scholar] [CrossRef] [PubMed]
- Kohn, J.; Spicher, S.; Bursch, M.; Grimme, S. Quickstart guide to model structures and interactions of artificial molecular muscles with efficient computational methods. Chem. Commun. 2021, 58, 258–261. [Google Scholar] [CrossRef]
- Pracht, P.; Bohle, F.; Grimme, S. Automated exploration of the low-energy chemical space with fast quantum chemical methods. Phys. Chem. Chem. Phys. 2020, 22, 7169–7192. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F.W. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Mkhonto, P.P.; Zhang, X.R.; Lu, L.; Xiong, W.; Zhu, Y.G.; Han, L.; Ngoepe, P.E. Adsorption mechanisms and effects of thiocarbamate collectors in the separation of chalcopyrite from pyrite minerals: DFT and experimental studies. Miner. Eng. 2022, 176, 107318. [Google Scholar] [CrossRef]
- Sahraei, A.A.; Larachi, F. How Do Surface Defects Change Local Wettability of the Hydrophilic ZnS Surface? Insights into Sphalerite Flotation from Density Functional Theory Calculations. J. Phys. Chem. C 2021, 125, 998–1009. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F.W. Meaning and Functional Form of the Electron Localization Function. Acta Phys.-Chim. Sin. 2011, 27, 2786–2792. [Google Scholar]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Vakylabad, A.B. Treatment of highly concentrated formaldehyde effluent using adsorption and ultrasonic dissociation on mesoporous copper iodide (CuI) nano-powder. J. Environ. Manag. 2021, 285, 112085. [Google Scholar] [CrossRef]
- Sit, P.H.L.; Cohen, M.H.; Selloni, A. Interaction of Oxygen and Water with the (100) Surface of Pyrite: Mechanism of Sulfur Oxidation. J. Phys. Chem. Lett. 2012, 3, 2409–2414. [Google Scholar] [CrossRef]
- Pollet, R.; Boehme, C.; Marx, D. Ab initio simulations of desorption and reactivity of glycine at a water-pyrite interface at “iron-sulfur world” prebiotic conditions. Orig. Life Evol. Biosph. 2006, 36, 363–379. [Google Scholar] [CrossRef]










| Element | S * | Fe | Zn | Mg | Mn | Pb | Al | Ca | S # |
|---|---|---|---|---|---|---|---|---|---|
| Content | 38.20 | 16.90 | 4.72 | 0.68 | 0.35 | 2.65 | 0.32 | 1.37 | 32.98 |
| Element | Fe | S * | Others |
|---|---|---|---|
| Content | 46.23 | 52.49 | 1.28 |
| Reagents | Molecular Formula | Standard | Manufacturer |
|---|---|---|---|
| DDTC | C4H12NCSSNa | AR | Tianjin Kemiou Chemical Reagent Co., Ltd., Tianjin, China |
| Sulfur | S | AR | Tianjin Kemiou Chemical Reagent Co., Ltd., Tianjin, China |
| Lead sulfate | PbSO4 | AR | Tianjin Kemiou Chemical Reagent Co., Ltd., Tianjin, China |
| Sphalerite | ZnS | AR | Tianjin Kemiou Chemical Reagent Co., Ltd., Tianjin, China |
| Terpineol | C10H11OH | Industrial | Tianjin Kemiou Chemical Reagent Co., Ltd., Tianjin, China |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; He, J.; Luo, T.; Dai, J.; Cao, S.; Yang, S.; Tang, C.; Wang, C.; Chen, Y. Interfacial Adsorption Mechanism of Diethyldithiocarbamate in High-Sulfur Residue Flotation. Processes 2023, 11, 1568. https://doi.org/10.3390/pr11051568
Liu H, He J, Luo T, Dai J, Cao S, Yang S, Tang C, Wang C, Chen Y. Interfacial Adsorption Mechanism of Diethyldithiocarbamate in High-Sulfur Residue Flotation. Processes. 2023; 11(5):1568. https://doi.org/10.3390/pr11051568
Chicago/Turabian StyleLiu, Hong, Jing He, Tao Luo, Jie Dai, Shuqiong Cao, Shenghai Yang, Chaobo Tang, Changhong Wang, and Yongming Chen. 2023. "Interfacial Adsorption Mechanism of Diethyldithiocarbamate in High-Sulfur Residue Flotation" Processes 11, no. 5: 1568. https://doi.org/10.3390/pr11051568
APA StyleLiu, H., He, J., Luo, T., Dai, J., Cao, S., Yang, S., Tang, C., Wang, C., & Chen, Y. (2023). Interfacial Adsorption Mechanism of Diethyldithiocarbamate in High-Sulfur Residue Flotation. Processes, 11(5), 1568. https://doi.org/10.3390/pr11051568