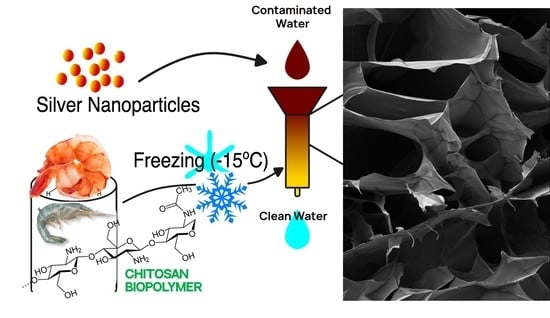
Chitosan Glutaraldegyde Cryogels for Wastewater Treatment and Extraction of Silver Nanoparticles
Abstract
:Highlights
- Covalently crosslinked chitosan-based cryogel for removal of AgNPs
- AgNPs suspension stabilized by plant extract a model water contaminant having complex composition
- Water permeability of cryogels significantly affects AgNP adsorption efficiency and adsorption capacity
- Maximum capacity for chitosan based cryogel prepared at −15 °C is 82 mg/g
- Low-cost adsorbent with high adsorption capacities to metal nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of CHI–GA Cryogel
2.2. Characterization of AgNPs
2.3. Cryogel Characterization
2.3.1. Scanning Electron Microscopy (SEM)
2.3.2. Transmission Electron Microscopy (TEM)
2.3.3. FTIR Spectroscopy
2.3.4. Study of Mechanical Properties (Stress–Strain Regime and Young’s Modulus)
2.3.5. Surface Area Evaluation (Nitrogen Adsorption)
2.3.6. Thermogravimetric Analysis (TGA)
2.4. AgNP Adsorption Experiments
2.5. Adsorption Capacity
2.6. AgNP Concentration Evaluation
2.7. Freundlich Isotherm
2.8. Langmuir Isotherm
- The energy is uniform over the entire area.
- Deposited molecules are inert with respect to each other.
- Adsorbed molecules are fixed at the adsorption sites.
- The monolayer is formed only at the point of peak adsorption.
- The molecules of the sorbate are deposited only on the free surface of the adsorbent.
2.9. Adsorption Kinetics: Pseudo-First-Order Equation
2.10. Thomas Isotherm
3. Results
3.1. Cryogel Characterization
3.2. AgNP Characterization
3.3. AgNP Adsorption Kinetics
3.4. AgNP Adsorption Mechanism
3.5. Effect of Flow Rate on Adsorption Efficiency
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xing, B.; Vecitis, C.D.; Senesi, N. (Eds.) Engineered Nanoparticles and the Environment: Biophysicochemical Processes and Toxicity; John Wiley & Sons: Hoboken, NJ, USA, 2016; Volume 4. [Google Scholar]
- Lv, H.; Cui, S.; Yang, Q.; Song, X.; Wang, D.; Hu, J.; Zhou, Y.; Liu, Y. AgNPs-Incorporated Nanofiber Mats: Relationship between AgNPs Size/Content, Silver Release, Cytotoxicity, and Antibacterial Activity. Mater. Sci. Eng. C 2021, 118, 111331. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jiang, G. (Eds.) Silver Nanoparticles in the Environment; Springer: Heidelberg, Germany, 2015; Available online: https://www.springerprofessional.de/en/silver-nanoparticles-in-the-environment/4399802 (accessed on 18 May 2023).
- Kalwar, K.; Shen, M. Electrospun Cellulose Acetate Nanofibers and Au@AgNPs for Antimicrobial Activity—A Mini Review. Nanotechnol. Rev. 2019, 8, 246–257. [Google Scholar] [CrossRef]
- Velgosova, O.; Mudra, E.; Vojtko, M. Preparing, Characterization and Anti-Biofilm Activity of Polymer Fibers Doped by Green Synthesized AgNPs. Polymers 2021, 13, 605. [Google Scholar] [CrossRef] [PubMed]
- Khoshnamvand, M.; Hao, Z.; Fadare, O.O.; Hanachi, P.; Chen, Y.; Liu, J. Toxicity of Biosynthesized Silver Nanoparticles to Aquatic Organisms of Different Trophic Levels. Chemosphere 2020, 258, 127346. [Google Scholar] [CrossRef]
- Lapresta-Fernández, A.; Fernández, A.; Blasco, J. Nanoecotoxicity Effects of Engineered Silver and Gold Nanoparticles in Aquatic Organisms. TrAC Trends Anal. Chem. 2012, 32, 40–59. [Google Scholar] [CrossRef]
- Zhangabay, Z.; Berillo, D. Antimicrobial and antioxidant activity of AgNPs stabilized with Calendula officinalis flower extract. Results Surf. Interfaces 2023, 11, 100109. [Google Scholar] [CrossRef]
- Balachandar, R.; Navaneethan, R.; Biruntha, M.; Ashok Kumar, K.K.; Govarthanan, M.; Karmegam, N. Antibacterial Activity of Silver Nanoparticles Phytosynthesized from Glochidion Candolleanum Leaves. Mater. Lett. 2022, 311, 131572. [Google Scholar] [CrossRef]
- Cohen, M.S.; Stern, J.M.; Vanni, A.J.; Kelley, R.S.; Baumgart, E.; Field, D.; Libertino, J.A.; Summerhayes, I.C. In Vitro Analysis of a Nanocrystalline Silver-Coated Surgical Mesh. Surg. Infect. 2007, 8, 397–404. [Google Scholar] [CrossRef]
- Kiani, M.; Rabiee, N.; Bagherzadeh, M.; Ghadiri, A.M.; Fatahi, Y.; Dinarvand, R.; Webster, T.J. Improved green biosynthesis of chitosan decorated Ag-and Co3O4-nanoparticles: A relationship between surface morphology, photocatalytic and biomedical applications. Nanomed. Nanotechnol. Biol. Med. 2021, 32, 102331. [Google Scholar] [CrossRef]
- Consumer Products Inventory. Available online: https://www.nanotechproject.tech/cpi/browse/nanomaterials/silver-nanoparticle/ (accessed on 18 May 2023).
- Wen, L.-S.; Santschi, P.H.; Gill, G.A.; Paternostro, C.L.; Lehman, R.D. Colloidal and Particulate Silver in River and Estuarine Waters of Texas. Environ. Sci. Technol. 1997, 31, 723–731. [Google Scholar] [CrossRef]
- Gomez-Caballero, J.A.; Villasenor-Cabral, M.G.; Santiago-Jacinto, P.; Ponce-Abad, F. hypogene Ba rich todokite and associated nanometric native silver in the San Miguel Tenango mining area, Zacatlan, Puebla, Mexico. Can. Mineral. 2010, 48, 1237–1253. [Google Scholar] [CrossRef]
- Yin, Y.; Liu, J.; Jiang, G. Sunlight-Induced Reduction of Ionic Ag and Au to Metallic Nanoparticles by Dissolved Organic Matter. ACS Nano 2012, 6, 7910–7919. [Google Scholar] [CrossRef]
- Silver: Supply and Demand. Available online: https://www.silverinstitute.org/silver-supply-demand/ (accessed on 18 May 2023).
- Syafiuddin, A.; Salmiati, S.; Hadibarata, T.; Kueh, A.B.H.; Salim, M.R.; Zaini, M.A.A. Silver Nanoparticles in the Water Environment in Malaysia: Inspection, Characterization, Removal, Modeling, and Future Perspective. Sci. Rep. 2018, 8, 986. [Google Scholar] [CrossRef]
- Olkhovych, O.; Svietlova, N.; Konotop, Y.; Karaushu, O.; Hrechishkina, S. Removal of metal nanoparticles colloidal solutions by water plants. Nanoscale Res. Lett. 2016, 11, 518. [Google Scholar] [CrossRef]
- Khalaf, N.; Ahamad, T.; Naushad, M.; Al-Hokbany, N.; Al-Saeedi, S.I.; Almotairi, S.; Alshehri, S.M. Chitosan polymer complex derived nanocomposite (AgNPs/NSC) for electrochemical non-enzymatic glucose sensor. Int. J. Biol. Macromol. 2020, 146, 763–772. [Google Scholar] [CrossRef]
- Wu, Z.; Zhou, W.; Deng, W.; Xu, C.; Cai, Y.; Wang, X. Antibacterial and hemostatic thiol-modified chitosan-immobilized AgNPs composite sponges. ACS Appl. Mater. Interfaces 2020, 12, 20307–20320. [Google Scholar] [CrossRef]
- Yang, J.; Chen, Y.; Zhao, L.; Feng, Z.; Peng, K.; Wei, A.; Wang, Y.; Tong, Z.; Cheng, B. Preparation of a chitosan/carboxymethyl chitosan/AgNPs polyelectrolyte composite physical hydrogel with self-healing ability, antibacterial properties, and good biosafety simultaneously, and its application as a wound dressing. Compos. Part B Eng. 2020, 197, 108139. [Google Scholar] [CrossRef]
- Jiang, Y.; Huang, J.; Wu, X.; Ren, Y.; Li, Z.; Ren, J. Controlled release of silver ions from AgNPs using a hydrogel based on konjac glucomannan and chitosan for infected wounds. Int. J. Biol. Macromol. 2020, 149, 148–157. [Google Scholar] [CrossRef]
- Polinarski, M.A.; Beal, A.L.; Silva, F.E.; Bernardi-Wenzel, J.; Burin, G.R.; de Muniz, G.I.; Alves, H.J. New Perspectives of Using Chitosan, Silver, and Chitosan–Silver Nanoparticles against Multidrug-Resistant Bacteria. Part. Part. Syst. Charact. 2021, 38, 2100009. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, R.; Li, Q.; Dai, F.; Lan, G.; Shang, S.; Lu, F. A self-adapting hydrogel based on chitosan/oxidized konjac glucomannan/AgNPs for repairing irregular wounds. Biomater. Sci. 2020, 8, 1910–1922. [Google Scholar] [CrossRef]
- Priya, K.; Vijayakumar, M.; Janani, B. Chitosan-mediated synthesis of biogenic silver nanoparticles (AgNPs), nanoparticle characterisation and in vitro assessment of anticancer activity in human hepatocellular carcinoma HepG2 cells. Int. J. Biol. Macromol. 2020, 149, 844–852. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Tian, R.; Zhou, J.; Liu, Y. Multifunctional chitosan/grape seed extract/silver nanoparticle composite for food packaging application. Int. J. Biol. Macromol. 2022, 207, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Kibeche, A.; Dionne, A.; Brion-Roby, R.; Gagnon, C.; Gagnon, J. Simple and green technique for sequestration and concentration of silver nanoparticles by polysaccharides immobilized on glass beads in aqueous media. Chem. Cent. J. 2015, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Li, Y.; Yue, R.; Zhang, X.; Huang, Y. Removal of silver nanoparticles by mussel-inspired Fe3O4@ polydopamine core-shell microspheres and its use as efficient catalyst for methylene blue reduction. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef]
- Dhandayuthapani, B.; Mallampati, R.; Sriramulu, D.; Dsouza, R.F.; Valiyaveettil, S. PVA/gluten hybrid nanofibers for removal of nanoparticles from water. ACS Sustain. Chem. Eng. 2014, 2, 1014–1021. [Google Scholar] [CrossRef]
- Kumar, J.; Mallampati, R.; Adin, A.; Valiyaveettil, S. Functionalized carbon spheres for extraction of nanoparticles and catalyst support in water. ACS Sustain. Chem. Eng. 2014, 2, 2675–2682. [Google Scholar] [CrossRef]
- Savina, I.N.; Otero-Gonzalez, L.; Berillo, D. Macroporous Cryogel-Based Systems for Water Treatment Applications and Safety: Nanocomposite-Based Cryogels and Bacteria-Based Bioreactors. In Biomedical Applications and Toxicity of Nanomaterials; Mohanan, P.V., Kappalli, S., Eds.; Springer: Singapore, 2023. [Google Scholar] [CrossRef]
- Dinu, I.A.; Ghimici, L.; Raschip, I.E. Macroporous 3D Chitosan Cryogels for Fastac 10EC Pesticide Adsorption and Antibacterial Applications. Polymers 2022, 14, 3145. [Google Scholar] [CrossRef]
- Dinu, M.V.; Humelnicu, I.; Ghiorghita, C.A.; Humelnicu, D. Aminopolycarboxylic Acids-Functionalized Chitosan-Based Composite Cryogels as Valuable Heavy Metal Ions Sorbents: Fixed-Bed Column Studies and Theoretical Analysis. Gels 2022, 8, 221. [Google Scholar] [CrossRef]
- Yin, M.; Li, X.; Liu, Y.; Ren, X. Functional Chitosan/Glycidyl Methacrylate-Based Cryogels for Efficient Removal of Cationic and Anionic Dyes and Antibacterial Applications. Carbohydr. Polym. 2021, 266, 118129. [Google Scholar] [CrossRef]
- Bratskaya, S.; Privar, Y.; Slobodyuk, A.; Shashura, D.; Marinin, D.; Mironenko, A.; Zheleznov, V.; Pestov, A. Cryogels of Carboxyalkylchitosans as a Universal Platform for the Fabrication of Composite Materials. Carbohydr. Polym. 2019, 209, 1–9. [Google Scholar] [CrossRef]
- Moustafa, M.; Abu-Saied, M.; Taha, T.; Elnouby, M.; El-Shafeey, M.; Alshehri, A.G.; Alamri, S.; Shati, A.; Alrumman, S.; Alghamdii, H.; et al. Chitosan functionalized AgNPs for efficient removal of Imidacloprid pesticide through a pressure-free design. Int. J. Biol. Macromol. 2021, 168, 116–123. [Google Scholar] [CrossRef]
- El Shahawy, A.; Mubarak, M.F.; El Shafie, M.; Abdulla, H.M. Fe (III) and Cr (VI) ions’ removal using AgNPs/GO/chitosan nanocomposite as an adsorbent for wastewater treatment. RSC Adv. 2022, 12, 17065–17084. [Google Scholar] [CrossRef]
- Chaplin, J.D.; Berillo, D.; Purkis, J.M.; Byrne, M.L.; Tribolet, A.D.C.C.M.; Warwick, P.E.; Cundy, A.B. Effective 137Cs+ and 90Sr2+ Immobilisation from Groundwater by Inorganic Polymer Resin Clevasol® Embedded within a Macroporous Cryogel Host Matrix. Mater. Today Sustain. 2022, 19, 100190. [Google Scholar] [CrossRef]
- Berillo, D. Gold Nanoparticles Incorporated into Cryogel Walls for Efficient Nitrophenol Conversion. J. Clean. Prod. 2020, 247, 119089. [Google Scholar] [CrossRef]
- VanLoon, G.W.; Duffy, S.J. Environmental Chemistry: A Global Perspective, 3rd ed.; Oxford University Press: Oxford, NY, USA, 2011. [Google Scholar]
- Yuh-Shan, H. Citation Review of Lagergren Kinetic Rate Equation on Adsorption Reactions. Scientometrics 2004, 59, 171–177. [Google Scholar] [CrossRef]
- Gerente, C.; Lee, V.K.C.; Cloirec, P.L.; McKay, G. Application of Chitosan for the Removal of Metals from Wastewaters by Adsorption—Mechanisms and Models Review. Crit. Rev. Environ. Sci. Technol. 2007, 37, 41–127. [Google Scholar] [CrossRef]
- Thomas, H.C. Heterogeneous ion exchange in a flowing system. J. Am. Chem. Soc. 1944, 66, 1664–1666. [Google Scholar] [CrossRef]
- Futalan, C.M.; Kan, C.C.; Dalida, M.L.; Pascua, C.; Wan, M.W. Fixed-bed column studies on the removal of copper using chitosan immobilized on bentonite. Carbohydr. Polym. 2011, 83, 697–704. [Google Scholar] [CrossRef]
- Meneses, I.P.; Novaes, S.D.; Dezotti, R.S.; Oliveira, P.V.; Petri, D.F.S. CTAB-modified carboxymethyl cellulose/bagasse cryogels for the efficient removal of bisphenol A, methylene blue and Cr (VI) ions: Batch and column adsorption studies. J. Hazard. Mater. 2022, 421, 126804. [Google Scholar] [CrossRef]
- Chowdhury, Z.Z.; Zain, S.M.; Rashid, A.K.; Rafique, R.F.; Khalid, K. Breakthrough Curve Analysis for Column Dynamics Sorption of Mn(II) Ions from Wastewater by Using Mangostana garcinia Peel-Based Granular-Activated Carbon. J. Chem. 2013, 2013, 959761. [Google Scholar] [CrossRef]
- Gao, C.; Wang, Y.; Shi, J.; Wang, Y.; Huang, X.; Chen, X.; Chen, Z.; Xie, Y.; Yang, Y. Superamphiphilic Chitosan Cryogels for Continuous Flow Separation of Oil-In-Water Emulsions. ACS Omega 2022, 7, 5937–5945. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, M.; Nguyen, V.; Mori, Y.; Nakamura, S.; Hattori, H. Adsorption of Silver Nanoparticles onto Different Surface Structures of Chitin/Chitosan and Correlations with Antimicrobial Activities. Int. J. Mol. Sci. 2015, 16, 13973–13988. [Google Scholar] [CrossRef] [PubMed]
- Agnihotri, S.; Mukherji, S.; Mukherji, S. Antimicrobial Chitosan–PVA Hydrogel as a Nanoreactor and Immobilizing Matrix for Silver Nanoparticles. Appl. Nanosci. 2012, 2, 179–188. [Google Scholar] [CrossRef]
- Bundjaja, V.; Santoso, S.P.; Angkawijaya, A.E.; Yuliana, M.; Soetaredjo, F.E.; Ismadji, S.; Ayucitra, A.; Gunarto, C.; Ju, Y.-H.; Ho, M.-H. Fabrication of Cellulose Carbamate Hydrogel-Dressing with Rarasaponin Surfactant for Enhancing Adsorption of Silver Nanoparticles and Antibacterial Activity. Mater. Sci. Eng. C 2021, 118, 111542. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Zhang, X.; Li, S.; Huang, Y. Nitrogen rich core–shell magnetic mesoporous silica as an effective adsorbent for removal of silver nanoparticles from water. J. Hazard. Mater. 2017, 337, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yaychi, R.F.; Manteghian, M. Investigation of effect of electric field on silver nanoparticles in order to separation of them from aqueous medium using electrocoagulation process. Sep. Purif. Technol. 2018, 193, 155–164. [Google Scholar] [CrossRef]
- Sousa, V.S.; Teixeira, M.R. Silver nanoparticles separation from the water using nanofiltration membranes: The role of mono-divalent salts and NOM. Sep. Purif. Technol. 2015, 149, 165–173. [Google Scholar] [CrossRef]
- Wan Ngah, W.S.; Ariff, N.F.M.; Hanafiah, M.A.K.M. Preparation, characterization, and environmental application of crosslinked chitosan-coated bentonite for tartrazine adsorption from aqueous solutions. Water Air Soil Pollut. 2010, 206, 225–236. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, C.; Wang, C.; Pu, F.; Ren, J.; Qu, X. Silver nanoprobe for sensitive and selective colorimetric detection of dopamine via robust Ag-catechol interaction. Chem. Commun. 2011, 47, 1181–1183. [Google Scholar] [CrossRef]
- Berillo, D.A.; Savina, I.N. Cryogels with Noble Metal Nanoparticles as Catalyst for “Green” Decomposition of Chlorophenols. Inorganics 2023, 11, 23. [Google Scholar] [CrossRef]







| Langmuir Linear | Langmuir Non-Linear | Freundlich | ||||||
|---|---|---|---|---|---|---|---|---|
| b | KL | n | KF | |||||
| 47.25 | 5.441 | 0.92 | 55 | 3.3398 | 0.951 | 4.511 | 37.4125 | 0.934 |
| Type of AgNPs | Removal Efficiency or Adsorption Capacity | Method | Reference |
|---|---|---|---|
| Negatively charged sodium citrate AgNPs, neutral AgNPs, and polyvinylpyrrolidone AgNPs | 99.91%in the sol | Electrocoagulation process in electric field for 90 min | [52] |
| AgNPs not specified | 92% of AgNPs and Ag+ released from NPs | Separation from water using nanofiltration membranes | [53] |
| Negatively charged gum arabic-capped AgNPs | Equilibrium of sorption process for Fe3O4@PDA at about 26 h with capacity for AgNPs of 169.5 mg/g | Fe3O4@polydopamine nanocomposite | [28] |
| Citrate-stabilized AgNPs and AuNPs | Adsorption capacity of 31.8 and 36.5 mg/g | PVA/gluten hybrid nanofibers, filtration | [29] |
| In situ immobilized AgNPs | Loading capacity of 1 mg/g | Chitosan–PVA gel for antimicrobial purposes | [49] |
| AgNPs not specified | Mechanical treatment of AgNPs led to a decrease in wastewater samples with an average removal efficiency of 35%, with a subsequent bioremediation treatment increasing removal to 72% | [3] | |
| Glucose-stabilized negatively charged AgNPs | Adsorption capacity of 2.2–26 mg/g | Chitin/chitosan nano/micro powders 10–31 μm, batch experiment | [48] |
| Plant-stabilized negatively charged AgNPs | Adsorption capacity of 48.3 mg/g with 94% efficiency | Rapid filtration via cryogel at flowrate of 0.8 mL/min | Current study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berillo, D.; Arysbek, A. Chitosan Glutaraldegyde Cryogels for Wastewater Treatment and Extraction of Silver Nanoparticles. Processes 2023, 11, 1661. https://doi.org/10.3390/pr11061661
Berillo D, Arysbek A. Chitosan Glutaraldegyde Cryogels for Wastewater Treatment and Extraction of Silver Nanoparticles. Processes. 2023; 11(6):1661. https://doi.org/10.3390/pr11061661
Chicago/Turabian StyleBerillo, Dmitriy, and Aknazar Arysbek. 2023. "Chitosan Glutaraldegyde Cryogels for Wastewater Treatment and Extraction of Silver Nanoparticles" Processes 11, no. 6: 1661. https://doi.org/10.3390/pr11061661
APA StyleBerillo, D., & Arysbek, A. (2023). Chitosan Glutaraldegyde Cryogels for Wastewater Treatment and Extraction of Silver Nanoparticles. Processes, 11(6), 1661. https://doi.org/10.3390/pr11061661